Abstract
Background
The majority of early preterm births are associated with intrauterine infections, which are thought to occur when microbes traffic into the uterus from the lower genital tract and seed the placenta. Bacterial vaginosis (BV) is associated with heterogeneous bacterial communities in the vagina and is linked to preterm birth. The extent to which trafficking into the uterus of normal and BV-associated vaginal bacteria occurs is unknown. The study objective was to characterize in parallel the distribution and quantities of bacteria in the vagina, uterus, and placental compartments.
Methods
Pregnant women at term (≥ 37 weeks) presenting for delivery were recruited prospectively. Swabs were collected in parallel from the vagina, chorioamnion. Choriodecidual swabs were collected if a Cesarean section was performed. Samples were analyzed by culture, broad-range 16S rRNA gene PCR, and bacterial species-specific quantitative PCR (qPCR) for DNA from Lactobacillus and a panel of BV-associated bacteria. Results were correlated with placental histopathology.
Results
Of the 23 women enrolled, 15 were delivered by Cesarean section (N=10 without labor; N=5 in labor) and 8 were delivered vaginally. BV was diagnosed in two women not in labor. Placental histopathology identified chorioamnionitis or funisitis in 6 cases [1/10 (10%) not in labor; 5/13 (38%) in labor]. Among non-laboring women, broad-range 16S qPCR detected bacteria in the chorioamnion and the choriodecidua (4/10; 40%). Among laboring women, Lactobacillus species were frequently detected in the chorioamnion by qPCR (4/13; 31%). In one case, mild chorioamnionitis was associated with qPCR detection of similar microbes in the chorioamnion and vagina (e.g. Leptotrichia/Sneathia, Megasphaera), along a quantitative gradient.
Conclusions
Microbial trafficking of lactobacilli and fastidious bacteria into the chorioamniotic membranes and choriodecidua occurs at term in normal pregnancies. In one case, we demonstrated a quantitative gradient between multiple bacterial species in the lower genital tract and placenta. Not all bacterial colonization is associated with placental inflammation and clinical sequelae. Further studies of the role of placental colonization with Lactobacillus in normal pregnancy and fastidious bacteria in chorioamnionitis may improve prevention and treatment approaches for preterm labor.
Keywords: intraamniotic infection, chorioamnionitis, amniotic fluid colonization, microbiome, microbial invasion of the amniotic cavity, intraamniotic infection, bacterial vaginosis, fastidious bacteria, preterm labor, preterm birth
Introduction
Infection and inflammation in the pregnant uterus is associated with preterm birth, fetal inflammatory response, chronic lung disease and cerebral palsy.[1] The majority of early preterm births result from intrauterine infections, which are thought to arise from microbes trafficking into the uterus from the lower genital tract and ultimately seeding the placenta.[1] Infection-associated preterm birth is commonly polymicrobial and includes vaginal microbes associated with bacterial vaginosis (BV), such as Gardnerella vaginalis and Prevotella species. The microbiology of BV is highly heterogeneous and dynamic with a shift away from a lactobacillus-dominated microbiota to one dominated by anaerobes.[2, 3, 4] The use of molecular microbiological methods has facilitated the identification of new taxa of fastidious (cultivation-resistant) bacteria in the vagina of women with BV. Many BV-associated microorganisms have not been previously cultured by standard microbiological techniques.[4] The role of BV as a risk factor for microbial trafficking and preterm labor has been controversial, in part because BV-associated microbial communities are dynamic and complex.[2, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] Further, the extent to which microbial trafficking occurs during normal pregnancy remains unknown.
Characterization of the distribution and quantities of bacteria in the vagina, uterus, and placental compartments could suggest a microbial “path” into the uterus. Several fastidious BV-associated species, including Leptotrichia/Sneathia, have been identified in the amniotic fluid of women in preterm labor, but whether these bacteria routinely colonize the chorioamnion (placental membranes) or choriodecidual space (between uterus and placenta) is unknown.[12, 15, 16, 17] Cesarean section at term provides an ideal opportunity to aseptically obtain samples from these compartments to assess colonization. Although microbial communities tend to become less diverse during pregnancy as gestation advances, some women have BV and a highly diverse vaginal microbiota even in the third trimester.[18, 19, 20]
We hypothesized that microbial trafficking occurs in normal pregnancies independent of labor, as demonstrated by the detection of similar microbial species in the vagina and intrauterine compartment. A quantitative gradient of Lactobacillus species and BV-associated microbes along the anatomical “path” of ascending infection (vagina to choriodecidual space to chorioamnion) would provide important evidence of microbial trafficking between vaginal and intrauterine compartments. The objective of this study was to compare the quantity and distribution of Lactobacillus species and specific BV-associated bacteria along the continuum of vaginal, chorioamnion and choriodecidual spaces in healthy, term pregnant women before and after labor.
Methods
Study Design
Pregnant women at term (≥ 37 weeks) presenting for delivery at the University of Washington Medical Center were recruited prospectively and provided informed consent (UW IRB #34004). Women were excluded for multiple gestation and known infection. Women not in labor presented for a scheduled cesarean delivery without painful contractions. Women in labor were defined as having regular painful contractions with cervical change in dilation.
Sampling of the vaginal fluid, choriodecidual and chorioamnion
Upon enrollment and prior to delivery, two polyurethane foam swabs (Epicentre Biotechnologies, Madison, WI) were inserted into the vagina blindly, and rotated for 15 seconds, one swab was used for Gram stain and one for PCR. Swabs (polyurethane foam and a DacronTM tip) were used to collect samples from the choriodecidua and chorioamnion. To sample the choriodecidual space, obstetricians performing the cesarean delivery paused after incision of the myometrium in the lower uterine segment and visualization of the bulging amniotic membranes. Swabs were then rotated in the choriodecidual space between the myometrium and the chorioamnion for approximately 5 seconds. Swabbing of the chorioamnion was performed in between the membranes after careful separation of the amnion from the chorion, using a sterile technique either in the operating room, the delivery room or in a laminar flow biosafety cabinet by the primary author (S.L.). Swabs were placed into the chorioamnion space and rolled for 30 seconds to sample a ~10 cm2 space. Polyurethane swabs for DNA extraction and PCR were re-sheathed and frozen immediately at −20° C and subsequently held at −80° C. DacronTM swabs were immediately placed in Port-A-CulTM tubes for culture by plating within 72 hours.
Placental histopathology
Placenta and umbilical cord samples underwent tissue fixation in 10% neutral buffered formalin. For histologic examination, full-thickness umbilical cord and fetal membrane rolls were stained with hematoxylin and eosin (H&E) by standard protocols. A board-certified pediatric pathologist (RPK) blinded to group assignment examined the stained samples. Chorioamnionitis was diagnosed by the presence of a neutrophilic infiltrate at the chorion-decidua junction (mild) or amniochorion junction (moderate or severe). Funisitis denoted neutrophils in the umbilical vessels and/or surrounding connective tissue.
BV diagnosis and Nugent scoring
BV was diagnosed by a Gram stain Nugent score of 7–10.[21, 22] Nugent scoring was performed by an experienced microbiologist (K.A.). Inability to assess for BV occurred in a few cases due to gel on the slide (n=3) or no sample (n=5).
Cultures
Chorioamnion and choriodecidual samples were cultured for aerobic and anaerobic bacteria as well as Mycoplasma hominis and Ureaplasma urealyticum. Columbia agar with 5% sheep blood and chocolate agar plates were incubated for up to 72 hours at 35–37° C in 5–10% CO2. Brucella agar plates and chopped meat carbohydrate broth were incubated for 5–7 days in anaerobe jar at 35–37°C. A8 plates and Ureaplasma and Mycoplasma broths were incubated for 48–72 hours at 35–37°C in 5–10% CO2, then subcultured to a fresh A8 plate and incubated for an additional 5 days. Coagulase negative staphylococci were identified by Gram stain, and being catalase positive and coagulase negative. Gardnerella vaginalis was identified by Gram stain and by being catalase negative. Anaerobic Gram-positive cocci and anaerobic Gram-negative rods were identified by Gram stain after growth in anaerobic conditions.
DNA extraction and quality control
Swabs were thawed, washed with 1-mL filtered saline solution, and centrifuged at 10,000g for 10 minutes. DNA was extracted from the resulting cell pellets using the Ultra Clean Soil DNA Isolation Kit (Mobio, Carlsbad, CA) and eluted in 100–300 μl buffer. Two μl of diluted DNA was used in each qPCR assay. Sham digests from swabs without human contact were used to assess contamination from DNA extraction reagents or collection swabs. Human 18S rRNA gene qPCR measured human DNA levels, verifying that the swab contacted a human tissue surface and documenting successful extraction. All DNA samples were tested for PCR inhibition using a sensitive qPCR approach targeting a segment of exogenously added jellyfish DNA at a concentration suitable for detection of low levels of inhibitors.[23] PCR Inhibition was defined as a delay in the threshold cycle by ≥2 cycles compared to the no-DNA-template controls.
Quantitative PCR
Total bacterial load was measured by qPCR targeting a highly conserved region of the 16S ribosomal RNA gene for all vaginal, choriodecidual and chorioamnion samples. In addition, 8 species-specific qPCR assays targeting key vaginal bacteria were performed including assays for G. vaginalis, Lactobacillus genus, Leptotrichia/Sneathia species (single assay), Megasphaera-like bacterium (type 1 & type 2), Atopobium vaginae, and three Clostridiales order bacteria designated as bacterial vaginosis associated bacterium 1 (BVAB1), bacterial vaginosis associated bacterium 2 (BVAB2) and Mageeibacillus indolicus (formerly BVAB3) with methods as described previously.[2, 24, 25] The qPCR assays were performed on all choriodecidual and chorioamnion samples; if any sample was positive, the same species-specific qPCR was performed on the woman’s vaginal fluid. Assays underwent 45 cycles of amplification on the Applied Biosystems ABI 7500 (Waltham, MA) or the Eppendorf Mastercycler® ep realplex Thermal cycler (Hamburg, Germany). Standards were run in duplicate from 10 to 107 copies (broad range 16S assay) and 2.5 to 106 to copies (species-specific assays) for quantitation. Values are reported as 16S rRNA gene copies/swab. Samples with undetectable levels of bacteria were assigned the value of the lower limit of the assay.
Statistical Analysis
Bacterial quantities were log transformed for analysis and presented in copies per swab. Median quantities of bacteria among laboring and non-laboring women are reported with standard deviations.
Results
Study Participants, BV and Chorioamnionitis
We enrolled a total of 23 pregnant women in this study (Table 1, N=10 not in labor, N=13 in labor). Table 2 presents a comprehensive description of each case including demographics, delivery mode, antibiotic use, BV by Nugent’s score, culture results, qPCR results, and histopathology results. Cesarean was performed on all women not in labor (N=10) and 5 women in labor. An additional 8 women in labor delivered vaginally. Prophylactic antibiotics were administered prior to all Cesarean deliveries per standard practice and in two women delivering vaginally for either Group B streptococcus colonization (N=1) or presumed chorioamnionitis (N=1). BV was diagnosed in two women not in labor (Nugent score=8, each). Placental histopathology identified chorioamnionitis or funisitis in 6 cases [1/10 (10%) not in labor; 5/13 (38%) in labor]. The severity of chorioamnionitis or funisitis was mild in all cases except in one patient, from whom Group B Streptococcus was cultured in the chorioamnion (Case 18, Table 1).
Table 1.
16S rRNA PCR Quantities from the Vagina, Choriodecidua and the Chorioamnion
| Case | Vagina before rupture | Vagina after rupture | Choriodecidua | Chorioamnion |
|---|---|---|---|---|
| Women Not in Labor (N=10) | ||||
| 1 | 5.6 × 108 | - | 1.6 × 103 | |
| 2 | 6.0 × 109 | 2.3 x104 | - | |
| 3 | 9.8 × 108 | 3.9 × 104 | 4.0 × 104 | |
| 4 | 4.5 × 109 | 4.3 × 104 | 3.4 × 104 | |
| 5 | 5.4 × 108 | - | - | |
| 6 | 7.2 × 108 | - | - | |
| 7 | 1.4 × 108 | - | - | |
| 8 | 2.6 × 109 | - | - | |
| 9 | 9.4 × 107 | - | - | |
| 10 | 3.4 × 108 | - | - | |
| Women in Labor (N=13) | ||||
| 11 | 2.2 × 109 | - | - | |
| 12 | 6.2 × 109 | 1.4x 109 | - | - |
| 13 | 1.7 × 108 | - | ||
| 14 | 2.6 × 109 | - | ||
| 15 | 2.7 × 107 | 7.6 × 106 | - | |
| 16 | 4.2 × 106 | 2.6 × 105 | - | |
| 17 | 8.2 × 108 | 2.0 × 109 | 4.8 × 103 | |
| 18 | 5.5 × 108 | 3.7 × 106 | - | |
| 19 | 4.1 × 108 | 7.2 × 107 | 2.0 × 105 | |
| 20 | 1.1 × 108 | 9.1 × 107 | 1.3 × 103 | |
| 21 | 3.8 × 108 | 1.78x 108 | - | |
| 22 | - | 4.0 × 103 | ||
| 23 | 1.6 × 105 | 1.0 × 103 | ||
- Indicates below the threshold of detection.
Choriodecidual swabs were only obtained from women in labor if they underwent Cesarean section.
Data presented as 16S rRNA gene copies/swab.
Table 2.
Placental Cultures, Histopathology and Species-Specific qPCR
| Case | Maternal Age | Gestational Age | Delivery Route | Chorioamnion and Choriodecidual Culture | Species-Specific qPCR | Histopathology | |||
|---|---|---|---|---|---|---|---|---|---|
| Chorioamnion | Vagina | Chorioamnionitis | Funisitis | ||||||
| BV-associated species+ | Lactobacillus | ||||||||
| Women Not in Labor (N=10) | |||||||||
| 1* | 37 | 39.6 | Cesarean | No growth | Negative | 9.3 × 107 | No | No | |
| 2 | 24 | 39.1 | Cesarean | No growth | Negative | 7.0 × 104 BVAB-1 | 1.1 × 108 | No | No |
| 3 | 35 | 39 | Cesarean | No growth | Negative | 1.4 × 106 Megasphaera | 6.2 × 109 | No | No |
| 4 | 29 | 39 | Cesarean | No growth | Negative | 2.7 × 104 BVAB-1 | 7.0 × 107 | No | No |
| 5 | 35 | 39.3 | Cesarean | No growth | Negative | 1.8 × 107 | No | Mild | |
| 6* | 34 | 37.6 | Cesarean | No growth | Negative | 7.7 × 107 | No | No | |
| 7 | 29 | 38.7 | Cesarean | CA: <102
Propionibacterium acnes CD: 5x102 diphtheroids, 102 coagulase-negative staphylococci |
Negative | 1.6 × 107 | No | No | |
| 8 | 37 | 36 | Cesarean | CA: <102 anaerobic Gram-positive cocci | Negative | 3.4 × 107 | No | No | |
| 9 | 42 | 37.7 | Cesarean | No growth | Negative | 4.4 × 106 | No | No | |
| 10 | 26 | 39.1 | Cesarean | CD: <102 coagulase-negative staphylococci | Negative | 8.2 × 103 Leptotrichia/ Sneathia | No | No | |
| Women in Labor (N=13) | |||||||||
| 11 | 31 | 36.6 | Cesarean | No growth | Negative | 1.5 × 107 | No | No | |
| 12 | 34 | 38.1 | Cesarean | No growth | Negative | 1.9 × 107 | No | No | |
| 13 | 33 | 39.1 | Cesarean | CA: 2x102 Propionibacterium acnes | Negative | 2.2 × 106 | No | No | |
| 14 | 29 | 41.4 | Cesarean | No growth | Negative | 6.9 × 107 | No | No | |
| 15 | 28 | 41.4 | Cesarean | No growth | Negative | Negative | Mild | Mild | |
| 16 | 38 | 40.9 | Vaginal | CA: <102 anaerobic Gram-positive cocci | Negative | 7.0 × 105 | No | Mild | |
| 17 | 28 | 38.9 | Vaginal | CA: 106 Enterococci and coagulase-negative staphylococci | 5.4 × 104 Lactobacillus | 5.0 × 107 | No | No | |
| 18 | 19 | 38.6 | Vaginal | CA: <102 (beta strep) Group B Streptococcus and coagulase-negative staphylococci | Negative | 4.9 × 106 | Moderate | Severe | |
| 19^ | 20 | 39.4 | Vaginal | Multiple microbes (see Fig. 1) | Mild | Mild | |||
| 20 | 32 | 41.6 | Vaginal | CA: <102 coagulase-negative staphylococci, anaerobic Gram-positive rods, Lactobacillus species | 2.2 × 104 Lactobacillus | Negative | 2.3 × 107 | No | No |
| 21^ | 38 | 38.4 | Vaginal | CA: 105 Acinetobacter baumannii and Enterococcus species | Negative | 1.9 × 105 | No | No | |
| 22 | 31 | 40.7 | Vaginal | 102 Gardnerella vaginalis, <102 coagulase-negative staphylococci, anaerobic Gram-positive cocci | 9.4 × 103 Lactobacillus | Negative | Mild | No | |
| 23 | 26 | 39.6 | Vaginal | <102 coagulase-negative staphylococci | Negative | 7.6 × 103 | No | No | |
Only evaluated when BV-associated species detected in the chorioamnion or choriodecidua, with the exception of Case 3, which was incidentally run for all selected BV-associated species.
Bacterial vaginosis diagnosed by Gram stain. Nugent score = 8 (both cases).
CA: chorioamnion, CD: choriodecidual space.
Indicates the two women delivering vaginally that received antibiotics prior to delivery. Note, that all women undergoing cesarean also received antibiotics.
Broad-range 16S qPCR of the Vagina, Choriodecidua, and Chorioamnion
To determine if the total quantity of vaginal bacterial DNA might be altered during labor, we compared 16S rRNA gene copies (bacterial load by quantitative PCR) for laboring and non-laboring groups. The median quantity of total vaginal bacterial DNA in the laboring group was similar to the non-laboring group (Not in labor: 7.2 × 107 ± 2.1× 108 copies/swab; Labor: 4.8 × 107 ± 1.9 × 108 copies/swab).
We performed broad-range 16S rRNA gene qPCR to determine bacterial load in the chorioamnion and choriodecidua. We hypothesized that microbial trafficking from the vagina into the pregnant uterus would be facilitated by labor, but that occasionally bacteria may also be detected in low levels in the choriodecidua and chorioamnion of women not in labor. In all women, we obtained swabs using sterile technique from within the placental membranes (chorioamnion) and, if a cesarean was performed, from the space between the uterus and placenta (choriodecidual space). Bacteria were detected in the choriodecidua and/or chorioamnion for 4/10 (40%) women prior to the onset of labor and 5/13 (38%) women after the onset of labor (Table 1). Bacterial detection did not correspond to chorioamnionitis or funisitis, as none of the pre-labor cases and only 2/5 laboring cases were noted to have placental or umbilical cord inflammation. These results suggest that bacteria may colonize the chorioamnion in late pregnancy even in the absence of labor or placental inflammation.
Lactobacillus and BV-Associated Bacterial Species in the Vagina, Choriodecidua and Chorioamnion
To identify bacteria found by 16S rRNA qPCR in the chorioamnion and choriodecidua, we chose to assay for Lactobacillus species and specific BV-associated bacterial species using a panel of validated qPCR assays. We assayed for the same bacterial species in the vagina when the selected species or genus was found in the chorioamnion or choriodecidua. We identified Lactobacillus in either the chorioamnion or choriodecidua by a genus-specific 16S qPCR in 4 of 5 women in labor undergoing Cesarean section, but not prior to labor. Regardless of mode of delivery, Lactobacillus species were detected in the chorioamnion of 31% of women in labor (4/13) by qPCR (Table 2). Interestingly, our selected species-specific qPCR assays did not identify bacteria in the chorioamnion or choriodecidua prior to labor, suggesting that BV-associated microbes rarely inhabit these spaces in term pregnancies prior to labor.
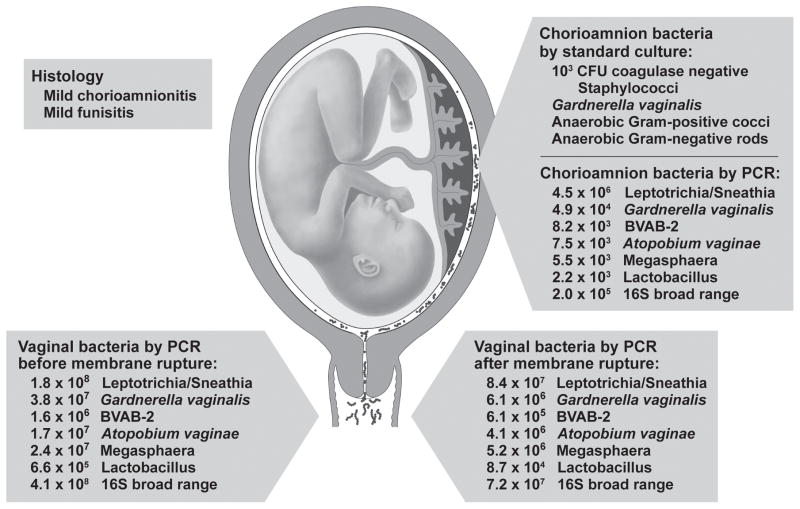
For one woman in labor with mild chorioamnionitis, DNA from several BV-associated species was detected in the chorioamnion, including Leptotrichia/Sneathia, G. vaginalis, BVAB-2, Atopobium vaginae, Megasphaera, and Lactobacillus (case 19, Table 1, Fig. 1). DNA for each of these species was also detected on vaginal swabs collected during labor. Each of these species demonstrated a gradient of bacterial load, with highest quantities in the vagina prior to rupture of membranes, followed by lower quantity in the vagina after rupture of membranes, and lowest quantities in the chorioamnion (Fig. 1). A similar gradient from vagina to chorioamanion and choriodecidua was noted among women prior to the onset of labor using 16S rRNA for bacterial detection. Detection of a quantitative gradient between bacterial species in the vagina and chorioamnion is consistent with microbial trafficking between these compartments.
Figure 1.
Placental Cultures, Histopathology and Species-Specific qPCR for a Woman in Labor. Standard cultures and species-specific qPCR reveal the complexity of a polymicrobial infection in a woman in labor (case 19, Table 1). In this case, multiple BV-associated bacteria were detected in both the chorioamnion and vagina in the setting of mild chorioamnionitis and funisitis before and after rupture of the chorioamniotic membranes. The combination of parallel detection of the same bacterial species in the chorioamnion and vagina with a quantitative gradient (higher vaginal levels, lower chorioamnion levels) suggests microbial trafficking of these bacteria from the vagina and into the placenta. Copy numbers in the figure are per swab.
Discussion
A prevailing model in the obstetrical literature for the etiology of infection-associated preterm birth has involved ascension of bacteria from the lower genital tract into the uterus. As the earliest events in this model occur within hidden compartments of asymptomatic pregnant women, this hypothesis has not been practical to prove in humans. Murine models have been developed only recently to demonstrate preterm birth after bacterial trafficking from the vagina into the uterus.[26] In this study, we demonstrate for the first time that Lactobacillus and fastidious BV-associated bacteria can be identified in the chorioamnion and vagina along a quantitative gradient, which provides supportive evidence for the model of bacterial ascension. The strength of this study is in the detailed description of the microbiota in parallel between the vagina, chorioamnion and choriodecidual compartments at term. Our results suggest that vaginal bacteria can migrate into the chorioamniotic membranes during labor, and may not always be associated with clinical sequelae.
Existence of a “placental microbiome” remains controversial,[27, 28, 29, 30] but use of sensitive and specific qPCR assays in our study to detect bacterial species in both the vagina and chorioamnion provides important evidence suggesting microbial trafficking contributes to a placental and intrauterine microbiome during labor at term. Recently, the chorioamniotic membranes have been profiled using 16S rRNA broad-range PCR, which also demonstrates the common detection of bacterial DNA in placental tissues and spatial variation in the microbial signature.[31, 32] However, these studies did not evaluate the relation of placental bacteria to those found in the vagina. Evidence for microbial trafficking from the lower genital tract into the placenta in pregnant animal models has been demonstrated in rabbits and recently in mice[26, 33, 34, 35, 36, 37], but not definitively in pregnant women due to ethical reasons. Our results support the existence of a human placental microbiome in labor, but indicate that detection of microbes in the chorioamniotic membranes at term prior to labor by qPCR is infrequent.[38, 39]
The relative frequency with which Lactobacillus species were detected in the chorioamnion of women in labor suggests that these bacteria may be part of the normal microbial community that develops in the chorioamnion during labor. Gram-positive Lactobacillus species are the most prevalent bacteria in the vagina and are thought to promote a healthy vaginal environment. Several studies using in vitro and in vivo models suggest that Lactobacillus species can inhibit growth of other bacteria[40, 41], their infectivity[42], and adhesion to vaginal epithelial cells[43]. Two recent reports suggest that bacteria, including Lactobacillus species, can be recovered from umbilical cord blood[44] or the early meconium from neonates[45], also suggesting an intrauterine microbiome originating from vaginal microbiota. In contrast, BV-associated microbes were found in the chorioamnion and choriodecidua of only one case in our study (Figure 1). This case was complicated by clinical and histopathological diagnosis of chorioamnionitis. BV-associated microbes were detected in the vagina and chorioamnion in a quantitatively decreasing gradient. Several of the identified species in this case have also been associated with preterm birth when identified in the amniotic fluid.[12] These findings also support the current theory that BV-associated microbes in the chorioamnion or amniotic fluid are pathologic.
The clear strength of the study is in the parallel detection of bacteria in the vagina and chorioamnion at term as prior studies in pregnancy have reported detection of fastidious bacteria in a single compartment (e.g. amniotic fluid, vagina) or tissue (e.g. chorioamniotic membranes). Our collection technique of sampling the choriodecidua during Cesarean section and swabbing between the chorioamniotic membranes, limits the risk of contamination when compared to collection directly from placental tissues exposed to the lower genital tract during vaginal birth. While the result of bacteria in the choriodecidua and chorioamnion could still represent contamination, this risk seems unlikely due to the detection of 16s qPCR bacterial DNA in the chorioamnion and choriodecidua of women prior to the onset of labor and at the time of Cesarean section. Another study strength was the use of an expanded number of species-specific quantitative assays to include eight vaginal bacterial species and Lactobacillus species. Species-specific assays are more sensitive than broad range 16S rRNA gene PCR for bacterial detection, with detection thresholds of a few molecules per reaction. Limitations of this study include the modest number of subjects enrolled, but this is tempered by the detailed analysis of the microbial quantities in each subject’s vaginal fluid and chorioamnion. The impact of antibiotic administration on the data derived from women delivering by cesarean is unknown, but likely diminished our ability to detect placental bacterial DNA.
Collectively, these findings indicate that microbial trafficking of fastidious bacteria and lactobacilli from the vagina into the uterus occurs during term labor in normal pregnancies, which may or may not lead to chorioamnionitis. Detection of Lactobacillus species occurs commonly in the chorioamnion during labor and may provide protection to the neonate if a Lactobacillus dominant microbiota impedes microbial trafficking of more pathogenic bacteria. In one case of clinical chorioamnionitis, detection of a quantitative gradient between BV-associated bacterial species in the lower genital tract and placenta supports the theory that ascension of these microbes are pathologic in labor. Further studies of the role of Lactobacillus in normal pregnancy and fastidious bacteria in chorioamnionitis may improve prevention and treatment approaches for preterm labor.
Acknowledgments
We thank Jan Hamanishi for technical assistance with the figure. Research reported in this publication was supported by the University of Washington Department of Obstetrics & Gynecology, Washington State Obstetrical Association and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Allergy and Infectious Diseases, and National Center for Research Resources of the National Institute of Health under award numbers [R01AI061628, R01AI100989, R21AI125907, R01AI33976]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.
Footnotes
The authors report no conflict of interest.
References
- 1.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014 Aug 15;345(6198):760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5(4):e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012 May 02;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005 Nov 3;353(18):1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 5.Martius J, Krohn MA, Hillier SL, et al. Relationships of vaginal Lactobacillus species, cervical Chlamydia trachomatis, and bacterial vaginosis to preterm birth. Obstet Gynecol. 1988 Jan;71(1):89–95. eng. [PubMed] [Google Scholar]
- 6.Hitti J, Hillier SL, Agnew KJ, et al. Vaginal indicators of amniotic fluid infection in preterm labor. Obstet Gynecol. 2001 Feb;97(2):211–9. doi: 10.1016/s0029-7844(00)01146-7. S0029-7844(00)01146-7 [pii]eng. [DOI] [PubMed] [Google Scholar]
- 7.Hillier SL, Krohn MA, Cassen E, et al. The role of bacterial vaginosis and vaginal bacteria in amniotic fluid infection in women in preterm labor with intact fetal membranes. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1995 Jun;20(Suppl 2):S276–8. doi: 10.1093/clinids/20.supplement_2.s276. [DOI] [PubMed] [Google Scholar]
- 8.Meis PJ, Goldenberg RL, Mercer B, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995 Oct;173(4):1231–5. doi: 10.1016/0002-9378(95)91360-2. 0002-9378(95)91360-2[pii]eng. [DOI] [PubMed] [Google Scholar]
- 9.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000 Feb 24;342(8):534–40. doi: 10.1056/nejm200002243420802. [DOI] [PubMed] [Google Scholar]
- 10.Joesoef MR, Hillier SL, Wiknjosastro G, et al. Intravaginal clindamycin treatment for bacterial vaginosis: effects on preterm delivery and low birth weight. Am J Obstet Gynecol. 1995 Nov;173(5):1527–31. doi: 10.1016/0002-9378(95)90644-4. 0002-9378(95)90644-4 [pii]eng. [DOI] [PubMed] [Google Scholar]
- 11.McGregor JA, French JI, Jones W, et al. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. Am J Obstet Gynecol. 1994 Apr;170(4):1048–59. doi: 10.1016/s0002-9378(94)70098-2. discussion 1059–60 eng. [DOI] [PubMed] [Google Scholar]
- 12.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson DB, Hanlon A, Hassan S, et al. Preterm labor and bacterial vaginosis-associated bacteria among urban women. J Perinat Med. 2009;37(2):130–4. doi: 10.1515/JPM.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson DB, Hanlon A, Nachamkin I, et al. Early pregnancy changes in bacterial vaginosis-associated bacteria and preterm delivery. Paediatr Perinat Epidemiol. 2014 Mar;28(2):88–96. doi: 10.1111/ppe.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiGiulio DB. Diversity of microbes in amniotic fluid [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Review] Semin Fetal Neonatal Med. 2012 Feb;17(1):2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Menard JP, Mazouni C, Salem-Cherif I, et al. High vaginal concentrations of Atopobium vaginae and Gardnerella vaginalis in women undergoing preterm labor [Research Support, Non-U.S. Gov’t] Obstet Gynecol. 2010 Jan;115(1):134–40. doi: 10.1097/AOG.0b013e3181c391d7. [DOI] [PubMed] [Google Scholar]
- 17.Han YW, Shen T, Chung P, et al. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth [Comparative Study Research Support, N.I.H., Extramural] J Clin Microbiol. 2009 Jan;47(1):38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review] Am J Obstet Gynecol. 1989 Sep;161(3):817–24. doi: 10.1016/0002-9378(89)90409-2. eng. [DOI] [PubMed] [Google Scholar]
- 19.Stout MJ, Zhou Y, Wylie KM, et al. Early pregnancy vaginal microbiome trends and preterm birth. Am J Obstet Gynecol. 2017 Sep;217(3):356 e1–356 e18. doi: 10.1016/j.ajog.2017.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freitas AC, Chaban B, Bocking A, et al. The vaginal microbiome of pregnant women is less rich and diverse, with lower prevalence of Mollicutes, compared to non-pregnant women. Sci Rep. 2017 Aug 23;7(1):9212. doi: 10.1038/s41598-017-07790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of clinical microbiology. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klebanoff MA, Hillier SL, Nugent RP, et al. Is bacterial vaginosis a stronger risk factor for preterm birth when it is diagnosed earlier in gestation? Am J Obstet Gynecol. 2005 Feb;192(2):470–7. doi: 10.1016/j.ajog.2004.07.017. S0002937804007756 [pii]eng. [DOI] [PubMed] [Google Scholar]
- 23.Khot PD, Ko DL, Hackman RC, et al. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC Infect Dis. 2008;8:73. doi: 10.1186/1471-2334-8-73. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fredricks DN, Fiedler TL, Thomas KK, et al. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR [Research Support, N.I.H., Extramural] J Clin Microbiol. 2009 Mar;47(3):721–6. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis. 2018 doi: 10.1016/S1473-3099(18)30058-6. [DOI] [PMC free article] [PubMed]
- 26.Vornhagen J, Quach P, Boldenow E, et al. Bacterial Hyaluronidase Promotes Ascending GBS Infection and Preterm Birth. MBio. 2016 Jun 28;7(3) doi: 10.1128/mBio.00781-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aagaard K, Ma J, Antony KM, et al. The placenta harbors a unique microbiome. Sci Transl Med. 2014 May 21;6(237):237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kliman HJ. Comment on “the placenta harbors a unique microbiome”. Sci Transl Med. 2014 Sep 17;6(254):254le4. doi: 10.1126/scitranslmed.3009864. [DOI] [PubMed] [Google Scholar]
- 29.Aagaard KM. Author response to comment on “the placenta harbors a unique microbiome”. Sci Transl Med. 2014 Sep 17;6(254):254lr3. doi: 10.1126/scitranslmed.3010007. [DOI] [PubMed] [Google Scholar]
- 30.Lauder AP, Roche AM, Sherrill-Mix S, et al. Comparison of placenta samples with contamination controls does not provide evidence for a distinct placenta microbiota. Microbiome. 2016 Jun 23;4(1):29. doi: 10.1186/s40168-016-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doyle RM, Harris K, Kamiza S, et al. Bacterial communities found in placental tissues are associated with severe chorioamnionitis and adverse birth outcomes. PLoS One. 2017;12(7):e0180167. doi: 10.1371/journal.pone.0180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parnell LA, Briggs CM, Cao B, et al. Microbial communities in placentas from term normal pregnancy exhibit spatially variable profiles. Sci Rep. 2017 Sep 11;7(1):11200. doi: 10.1038/s41598-017-11514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDuffie RS, Gibbs RS. Animal models of ascending genital-tract infection in pregnancy. Infectious diseases in obstetrics and gynecology. 1994;2(2):60–70. doi: 10.1155/S1064744994000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racicot K, Cardenas I, Wunsche V, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013 Jul 15;191(2):934–41. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randis TM, Gelber SE, Hooven TA, et al. Group B Streptococcus beta-hemolysin/cytolysin breaches maternal-fetal barriers to cause preterm birth and intrauterine fetal demise in vivo. The Journal of infectious diseases. 2014 Jul 15;210(2):265–73. doi: 10.1093/infdis/jiu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gendrin C, Vornhagen J, Ngo L, et al. Mast cell degranulation by a hemolytic lipid toxin decreases GBS colonization and infection. Sci Adv. 2015 Jul 17;1(6):e1400225. doi: 10.1126/sciadv.1400225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kothary V, Doster RS, Rogers LM, et al. Group B Streptococcus Induces Neutrophil Recruitment to Gestational Tissues and Elaboration of Extracellular Traps and Nutritional Immunity. Front Cell Infect Microbiol. 2017;7:19. doi: 10.3389/fcimb.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steel JH, Malatos S, Kennea N, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005 Mar;57(3):404–11. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 39.Jones HE, Harris KA, Azizia M, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4(12):e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breshears LM, Edwards VL, Ravel J, et al. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015 Dec 09;15:276. doi: 10.1186/s12866-015-0608-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang SE, Jeong JJ, Choi SY, et al. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 Attenuate Gardnerella vaginalis-Infected Bacterial Vaginosis in Mice. Nutrients. 2017 May 23;9(6) doi: 10.3390/nu9060531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nardini P, Nahui Palomino RA, Parolin C, et al. Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci Rep. 2016 Jun 29;6:29024. doi: 10.1038/srep29024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osset J, Bartolome RM, Garcia E, et al. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. The Journal of infectious diseases. 2001 Feb 01;183(3):485–91. doi: 10.1086/318070. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez E, Fernandez L, Marin ML, et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005 Oct;51(4):270–4. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 45.Ardissone AN, de la Cruz DM, Davis-Richardson AG, et al. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS One. 2014;9(3):e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]