Abstract
In recent years, lymphangiogenesis, the process of lymphatic vessel formation from existing lymph vessels, has been demonstrated to have a significant role in diverse pathologies, including cancer metastasis, organ graft rejection, and lymphedema. Our understanding of the mechanisms of lymphangiogenesis has advanced on the heels of studies demonstrating vascular endothelial growth factor C (VEGF-C) as a central pro-lymphangiogenic regulator and others identifying multiple lymphatic endothelial biomarkers. Despite these breakthroughs and a growing appreciation of the signaling events that govern the lymphangiogenic process, there are no FDA-approved drugs that target lymphangiogenesis. In this review, we reflect on the lessons available from the development of anti-angiogenic therapies (26 FDA-approved drugs to date), review current lymphangiogenesis research including nanotechnology in therapeutic drug delivery and imaging, and discuss molecules in the lymphangiogenic pathway that are promising therapeutic targets.
Keywords: Angiogenesis, Lymphangiogenesis
1 Introduction
Considered the “forgotten” circulation in health and systemic diseases [1], the lymphatic system is often perceived as an extensive, passive conduit of fluid and cells that regulates fluid homeostasis, nutrient absorption in the gastrointestinal tract, and immune surveillance throughout the body. [2] Utilizing knowledge of lymphatic endothelial cell (LEC) markers, including lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1), prospero-related homeobox 1 (Prox-1), and podoplanin, investigators characterized an otherwise invisible lymphatic network in animal models and humans and came to understand the complex role of lymphangiogenesis in the context of various pathologies. [3,4] For example, tumor cells can release pro-angiogenic and pro-lymphangiogenic factors that direct the formation of lymphatic and blood vessels, via which metastasis occurs. In chronic inflammatory conditions like psoriasis, lymphatic vessels suppress inflammation by promoting the drainage of inflammatory mediators and fluids. [3]
885
In 1971, Judah Folkman proposed that tumorigenesis often advances due to the co-option of angiogenesis. [5] Since then the clinical use of anti-angiogenic therapies has rapidly developed from preclinical stages. Studies in the 1990’s used monoclonal antibodies against vascular endothelial growth factor (VEGF) to demonstrate that tumor growth can be suppressed by neutralizing VEGF. [6] In 2004, the U.S. Food and Drug Administration (FDA) approved a first-in-class anti-VEGF monoclonal antibody, bevacizumab (brand name Avastin), for the treatment of metastatic carcinoma of the colon and rectum, and later approved the drug for other indications including non-small cell lung cancer [7] and other cancers (Figure I). Bevacizumab acts by binding to VEGF-A preventing it from activating VEGF receptor 2 (VEGFR2), which is a key player in the pro-angiogenic pathway. [8] Aflibercept, sorafenib, sunitinib, axitinib, nintedanib, regorafenib, pazobanib, cabozantinib, vandetaniband thalidomide are among the 26 anti-angiogenic agents that are now approved for the treatment of various cancers (Table I). Another anti-angiogenic compound, endostatin, which is FDA-approved in the US and other countries, is important due to its widespread anti-angiogenic effects. All of these drugs target various members of signaling pathways that are involved in angiogenesis, and they do this is a variety of ways. [9] For example, while bevacizumab targets the ligand to prevent ligand-dependent activation of a key receptor tyrosine kinase, targeting the receptor to prevent either ligand binding (ramucirumab) or receptor dimerization (pertuzumab) is an additional therapeutically beneficial strategy. On the other hand, endostatin, which is a 20-kDa C-terminal fragment of type-XVIII collagen, causes tumor regression by suppressing endothelial cell migration, thereby inducing apoptosis in cells. Altered endostatin levels have been observed in a variety of diseases, indicating that it acts through several other mechanisms, both anti-angiogenic and anti-lymphangiogenic, that are not yet completely understood, and thus, further research is required to better understand its various effects. [10]
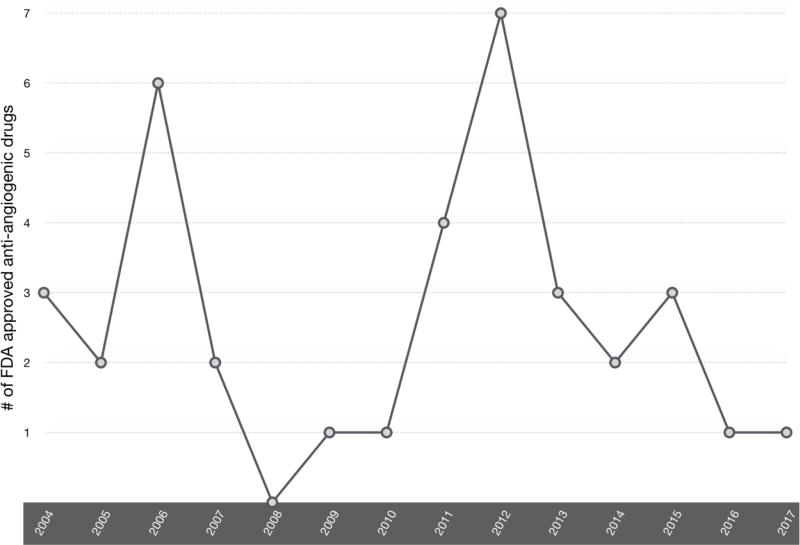
Figure I. Trend in FDA approval of anti-angiogenic therapies.
In 2004, bevacizumab became the first anti-angiogenic therapy approved for the treatment of metastatic colorectal cancer, and the approval of erlotinib and pegaptanib followed shortly after in the same year. Regorafenib, a treatment for hepatocellular carcinoma, is the most recently approved anti-angiogenic therapy. We observe that from 2008 to 2012 there was a steady increase of the number of FDA-approved drugs. A total of 7 anti-angiogenic drugs were approved by the FDA. This steady increase reflects the growing interest in using anti-angiogenic drugs in cancer treatment. There are 26 molecules approved for anti-angiogenic therapy, some with multiple, unique indications. Drugs were counted once per approved indication (e.g., Afatinib is counted for one approval in 2013 and one approval for a separate indication in 2016.)
Table I.
FDA-approved anti-angiogenic therapeutics.
| Anti-angiogenic agent | Targeted Pathway | Clinical Indications (year approved) |
|---|---|---|
| Afatinib | Receptor tyrosine kinase inhibitor (epidermal growth factor receptor [EGFR], human epidermal growth factor receptor 2 [HER2]) | Non-small cell lung cancer (2013); squamous cell lung carcinoma (2016) |
| Aflibercept | VEGFR1/VEGR2/IgG chimeric protein (VEGF-A, VEGF-B, and placental growth factor [PIGF]) | Wet age-related macular degeneration (2011); metastatic colorectal cancer (2012) |
| Axitinib | Receptor tyrosine kinase inhibitor (VEGFR, platelet-derived growth factor receptor [PDGFR], c-KIT) | Renal cell carcinoma (2012) |
| Bevacizumab | Humanized monoclonal antibody (VEGF-A) | Metastatic colorectal cancer (2004); lung cancer (2006) |
| Cabozantinib | Receptor tyrosine kinase inhibitor (c-MET, VEGFR2, AXL, RET) | Metastatic medullary thyroid cancer (2012) |
| Cetuximab | Chimeric monoclonal antibody (EGFR) | Metastatic colorectal cancer (2012); recurrent or metastatic head and neck cancer (2011) |
| Erlotinib | Receptor tyrosine kinase inhibitor (EGFR) | Lung cancer (2004) |
| Gefitinib | Receptor tyrosine kinase inhibitor (EGFR) | Metastatic non-small cell lung cancer (2015) |
| Lapatinib | Receptor tyrosine kinase inhibitor (EGFR, HER2) | HER2+ breast cancer (2007) |
| Lenalidomide | Small molecule that binds to cerublon and thereby reduces the level of certain transcription factors | Myelodysplastic syndrome (2005) |
| Necitumumab | human IgG1 monoclonal antibody (EGFR) | Metastatic squamous non-small cell lung cancer, in combination with gemcitabine and cisplatin (2015) |
| Nintedanib | Receptor tyrosine kinase inhibitor (VEGFR, fibroblast growth factor receptor [FGFR], PDGFR) | Idiopathic pulmonary fibrosis (2014) |
| Panitumumab | Human IgG2 monoclonal antibody (EGFR) | Colorectal cancer (2006) |
| Pazopanib | Receptor tyrosine kinase inhibitor (c-KIT, FGFR, PDGFR, VEGFR) | Renal cell carcinoma (2009); soft tissue sarcoma (2012) |
| Pegaptanib | RNA aptamer (VEGF-A165 isoform) | Wet age-related macular degeneration (2004) |
| Pertuzumab | Humanized monoclonal antibody (HER2) | Neoadjuvant treatment of HER2+ breast cancer in combination with Trastuzamab and Docetaxel (2012) |
| Pomalidomide | Small molecule that binds to cerublon and thereby reduces the level of certain transcription factors | Multiple myeloma (2013) |
| Ramucirumab | IgG1 monoclonal antibody (VEGFR2) | Metastatic gastric or gastroesophageal junction adenocarcinoma and platinum-resistant metastatic non-small cell lung cancer (2014); metastatic colorectal cancer (2015) |
| Ranibizumab | Fab fragment of bevacizumab (VEGF-A) | Neovascular age-related macular degeneration (2006) |
| Regorafenib | Receptor tyrosine kinase inhibitor (VEGFR2, TIE2) | Metastatic colorectal cancer (2012); GIST (2013); and hepatocellular carcinoma (2017) |
| Sorafenib | Kinase inhibitor (VEGFR, PDGFR, RAF family) | Renal cell carcinoma (2005); hepatocellular carcinoma (2007) |
| Sunitinib | Receptor tyrosine kinase inhibitor (PDGFR, VEGFR, c-KIT, RET, granulocyte colony-stimulating factor receptor [G-CSFR], FLT3) | Metastatic renal cell carcinoma and gastrointestinal stromal tumor (2006); pancreatic neuroendocrine tumors (2011) |
| Temsirolimus | Small molecule inhibitor (mammalian target of rapamycin [mTOR]) | Multiple myeloma in combination with dexamethasone (2006) |
| Thalidomide | Small molecule that binds to cerublon and thereby reduces the level of certain transcription factors | Multiple myeloma (2003) |
| Trastuzumab | Humanized monoclonal antibody (HER2) | HER2+ breast cancer (2006); HER2-overexpressing metastatic gastric or gastroesophageal (GE) junction adenocarcinoma (2010) |
| Vandetanib | Receptor tyrosine kinase inhibitor (VEGFR, EGFR, RET) | Unresectable, locally advanced, or metastatic medullary thyroid cancer (2011) |
Interest in targeting lymphangiogenesis in pathologies was delayed compared to that related to angiogenesis, due to difficulties in characterizing and imaging lymphatic vessels. The discovery of pro-lymphangiogenic factors VEGF-C in 1996 [11] and VEGF-D in 1997[12] led to the initial interest in lymphangiogenesis. Then the characterization of LYVE-1, [13] Prox-1, [14] podoplanin, [15] VEGFR3 [16] and other lymphatic-specific biomarkers in the late 1990s brought more attention to this space (Figure II). Akin to the enthusiasm for anti-angiogenic therapies, the potential for anti-lymphangiogenic therapies created considerable excitement. Despite a growing understanding of lymphangiogenesis biology, there has yet to be a single drug on the market that targets lymphangiogenesis for treatment of conditions that involve lymphatic vessels, including cancer metastasis and organ graft rejection.
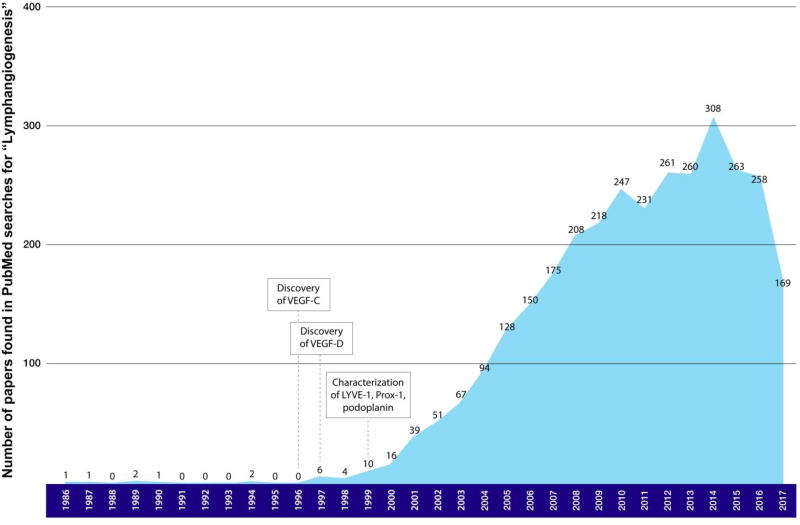
Figure II. Number of papers found in PubMed searches for “lymphangiogenesis” per year since 1986.
The published literature devoted to lymphangiogenesis research has grown dramatically since 1996, when VEGF-C and -D were discovered to be lymphangiogenic factors. The number of published studies steadily increased from 1997 to 2013 from less than 10 to more than 300. The volume of research on lymphangiogenesis increased by more than 50 times after the discovery of VEGF-C, VEGF-D, and the characterization of three lymphatic vessel biomarkers—LYVE-1, Prox-1, and podoplanin. These events proved to be ground-breaking for research into lymphangiogenesis.
Unfortunately, despite promising results in murine models for the treatment of cancer, organ graft rejection, and other conditions, many of the 26 approved drugs targeting angiogenesis have fallen short of expectations in the clinic. For example, bevacizumab is largely used only in combination with other drugs. While the progression-free survival (PFS) of patients treated with bevacizumab as first-line therapy has shown marked improvement in many phase III studies, overall survival (OS) failed to differ significantly between experimental and control groups [17–24]. Even as a second-line treatment, bevacizumab has failed to show improvement in PFS. [25–29] Many groups have challenged the efficacy of anti-angiogenic drugs, [30–35] urging the scientific community to find alternative targets for diseases that involve vasculature and lymphangiogenesis has become a key topic in such discussions. [36–40]
The growing appreciation for the potential therapeutic benefits of anti-lymphangiogenic drugs has not yet been translated clinically. A search for clinical trials using the key words “angiogenesis” and “cancer” returned nearly 317 studies, whereas a search of “lymphangiogenesis” and “cancer” returned only 3 studies (Figure III). We reported in 2016 that a PubMed search for “angiogenesis” returned nearly 80,000 reports at that time, whereas a search for “lymphangiogenesis” returned approximately 2,635 entries at that time. [4] A more recent (2017) similar search showed that angiogenesis research continues to accelerate beyond the pace of lymphangiogenesis research (a search for “angiogenesis” yielded 94,542 results, whereas a search for “lymphangiogenesis” yielded a total of 3,163 results to date). This review describes the challenges encountered in the course of developing anti-angiogenic therapies, summarizes the principles of lymphangiogenesis, and identifies potential drug targets for lymphangiogenesis-related diseases.
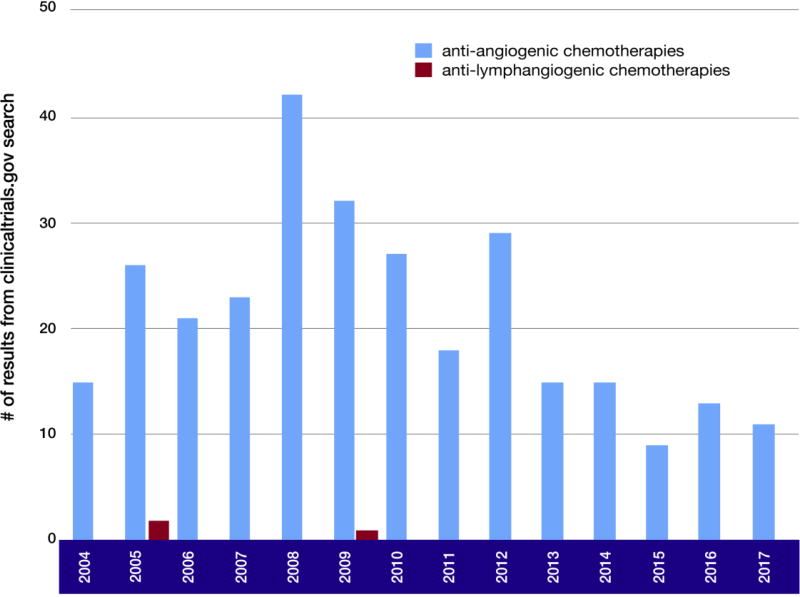
Figure III. Clinical trials of anti-angiogenic chemotherapy and anti-lymphangiogenic chemotherapy.
An advanced search of NIH U.S National Library of Medicine ClinicalTrials.gov shows a steady approval rate of clinical trials for anti-angiogenic therapies. In contrast, clinical trials for therapies targeting lymphangiogenesis are sparse. The only observable approvals of anti-lymphangiogenic therapies occurred in 2005 and 2010, with less than 10 approvals in total. While the number of anti-angiogenic chemotherapies approved in 2008 was well beyond 40, the number of anti-lymphangiogenic therapies was far fewer.
2 Anti-angiogenesis therapies: Successes and lessons learned
As Folkman predicted in 1971,[5] inhibition of angiogenesis, which deprives cells within a tumor of oxygen and other nutrients, is a viable anti-cancer therapy. Signal transduction of VEGFs and their receptors activate the formation of new blood vessels that supply oxygen and nutrients to tumors and are the target of 50% of FDA-approved anti-angiogenic therapies (Table 1).[41] Bevacizumab remains the most successful anti-angiogenic therapy developed to date. This drug is an anti-VEGF-A monoclonal antibody (mAb) that does not interfere with other signaling pathways. [42] Clinical trials of bevacizumab concluded that it is well tolerated with less toxicity and can be used in combination with chemotherapy without any synergistic toxicity. [43,44] Previously known as rhuMAb, it binds to VEGF-A. [45] Notably, even with the success of mAb therapies such as bevacizumab, the next stage of anti-angiogenic mAb therapy may take the form of nanobodies, small antigen-binding fragments derived from mAbs that can overcome the limited diffusion capabilities of their larger counterparts and thus better access various tissues including dense tumors. [46] Sunitinib, another approved anti-angiogenic drug, targets the tyrosine kinases VEGFRs and platelet-derived growth factor receptor (PDGFR) to reduce tumor neovascularization. Sunitinib, unlike bevacizumab, has a broad spectrum of activity against multiple tyrosine kinase pathways. [47] It is particularly effective in treating renal cell carcinoma and gastrointestinal stromal tumors. [48] Sunitinib is an oxindol molecule that binds with the intracellular ATP-binding sites of a wide range of receptor tyrosine kinases including VEGFR, PDGFR, KIT, and FLT3. [48,49] In this way, it inhibits angiogenesis by interfering with the signaling pathway of VEGF.
These VEGF pathway-targeting drugs represent, in theory, a promising therapeutic approach, because they block a well-characterized, major regulatory pathway for blood vessel growth. Favorable results for anti-angiogenic therapies in many murine models, which often demonstrate reduced metastatic activity and increased survival rates, have boosted investment into the angiogenesis space. However, most of these drugs have failed to meet expectations in human patients, supporting the notion that animal models and/or the clinical trial process may not accurately predict the efficacy of a given therapy in the “real world” setting. [50] The anti-angiogenic therapies developed so far show the maximal benefit for patients when used in a combination with agents that also target the cancer itself. [51–55] Thus, targeting the VEGF-A/VEGFR2 pathway alone is not enough, and there is a demand for additional therapies against other molecular pathways contributing to angiogenesis. This is outside the scope of this paper. For a more comprehensive review on the molecular mechanisms of angiogenesis and the limitations of current anti-angiogenic therapies, readers should consult the following reference [56]. More importantly, patients may benefit from anti-angiogenic and lymphangiogenesis-targeting combination therapies, since lymphangiogenesis is known to be involved in the pathobiology of several diseases (discussed later in this review) that also present with aberrant angiogenesis for which single VEGF-A/VEGFR2 inhibitors have been shown to be insufficient. Likewise, as is most likely the case for VEGF-A– and VEGFR2-targeting therapies that have shown limited clinical efficacy [56], lymphangiogenesis-targeting therapies developed against VEGF-C or VEGFR3 alone may be likely to face the same problems such as resistance. [57]
Nevertheless, the development and application of anti-angiogenic therapies has provided key insights into the relevant pathogenesis, particularly of tumors. For instance, preventing angiogenesis is likely to intensify and/or prolong hypoxia, which triggers a plethora of alternative strategies for tumor cells to survive, including vascular mimicry, vascular co-option, and metastasis.[50,58–65] Mathematical modeling of the consequences of suppressing oxygen availability predicts this very outcome, due to the natural selection of cells that can tolerate blood deficiency and are then driven to escape nutrient-starved regions. [66] Such responses are driven at least in part by hypoxia-induced stabilization of hypoxia-inducible factor 1α (HIF-1α), a transcription factor that increases the expression of many genes, including VEGF, matrix metalloproteinases (MMPs), fibroblast growth factor 2 (FGF2), angiopoietin-1, and TIE2. [67,68] Increased levels of VEGF enable the tumor to overwhelm anti-VEGF–based therapies and/or engage alternative pathways to initiate the angiogenesis program (e.g., FGF2). A better understanding of the vascularization of cancers as well as evasion mechanisms will be crucial to the future development of anti-angiogenic therapies. [69]
3 Biomarkers
The paucity of biomarkers of the angiogenic status of a vascular bed contributes to our inability to effectively design and administer anti-angiogenic therapy. The ideal biomarker would not only indicate the disease status (similar to how abnormally elevated body temperature indicates infection), but also provides information on the drivers of pathological angiogenesis (analogous to a strep throat test which indicates the presence of group A streptococcus). Profiling the changes in signaling networks associated with and responsible for the resolution of pathological angiogenesis is one approach to identifying such biomarkers.
Just as how one biomarker for evaluating the effectiveness of anti-angiogenic therapies in metastatic cancer patients will not likely explain heterogeneity,[69] a single biomarker for pathological lymphangiogenesis will be insufficient. As discussed by Vasudev and Reynolds, the use of predictive algorithms in the future will help inform doctors and patients of the most appropriate anti-angiogenic therapies based on individual patients’ parameters. These parameters may include single nucleotide polymorphisms (SNPs), serum markers, and imaging. [69] Moreover, markers characterizing the angiogenic and lymphangiogenic status of patients’ tumor vasculatures would be invaluable tools.
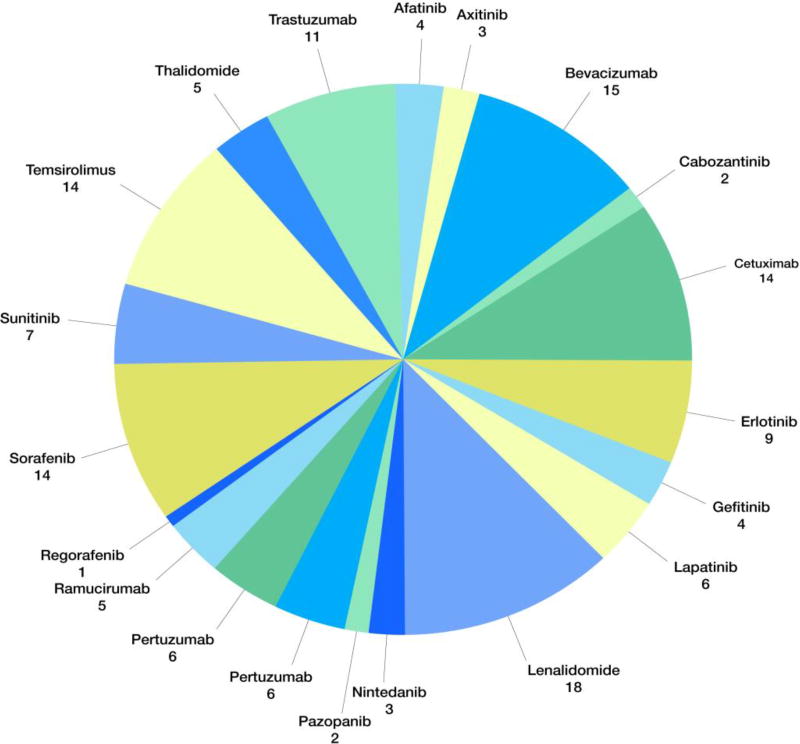
There are currently approximately 149 phase III clinical trials studying anti-angiogenic cancer therapies (Figure IV). Among these are 20 phase II/III trials of anti-angiogenic therapies specifically for treatment of colorectal cancers only (Table II). In stark contrast, there are no ongoing phase II or III clinical trials of lymphangiogenesis-targeting therapies for colorectal cancer, or any type of cancer, despite a wealth of literature indicating the importance of aberrant lymphangiogenesis in the progression of colorectal cancer. [36] However, Nagahashi et al. state that the National Institutes of Health may not explicitly mention lymphangiogenesis in trial descriptions, and as such, there are in fact many currently ongoing or completed clinical trials studying potential anti-lymphangiogenic drugs. [36] Much needed research on the lymphangiogenic effects of not only novel but current cancer therapeutics may help build more robust data on the correlation between lymphatic interference and survival in cancer patients.
Figure IV. Active Phase III clinical trials for anti-angiogenic therapies (as of August, 2017).
Lenalidomide and bevacizumab have been tested in the highest numbers of clinical trials among all anti-angiogenic therapies. Sorafenib has been tested in the most clinical trials for receptor tyrosine kinase inhibitors. Sunitinib and other receptor tyrosine kinase inhibitors account for around 40% of all related clinical trials, while monoclonal antibody (mAb) drugs account for around 42%, of which more than 25% trials tested bevacizumab. The rest of the trials were for immunomodulatory drugs like thalidomide and lenalidomide.
Table II.
Phase II/III clinical trials of anti-angiogenic drugs for patients with metastatic colon cancers (modified from Nandikolla and Rajdev 2016) [35]
| Angiogenesis inhibitor |
Identification number |
Clinical trial | Primary Outcome | Phase |
|---|---|---|---|---|
| Afilbercept | NCT02331927 | PERMAD: Personalized Marker-driven Early Switch to Aflibercept in Patients With Metastatic Colorectal Cancer (PERMAD) | PFS | II |
| NCT02384759 | Aflibercept +/− LV5FU2 in First Line of Non-resectalbe Metastatic Colorectal Cancers (FOLFA) | Radiological PFS | II | |
|
| ||||
| Bevacizumab | NCT01640405 | Study of 5-Fluorouracil/Leucovorin/Oxaliplatin (FOLFOX) + Bevacizumab Versus 5-Fluorouracil/Leucovorin/Oxaliplatin/Irinotecan (FOLFOXIRI) + Bevacizumab as First Line Treatment of Patients With Metastatic Colorectal Cancer Not Previously Treated and With Three or More Circulating Tumoral Cells (VISNU-1) | PFS | III |
| NCT01718873 | Optimization of Bevacizumab Scheduling With Chemotherapy for Metastatic Colorectal Cancer (OBELICS) | No. of objective responses | III | |
| NCT01249638 | Combination of capecitabine and bevacizumab versus the combination of capecitabine, bevacizumab and irinotecan. | Time of failure strategy | III | |
| NCT02339116 | Folfoxiri Plus Bev Followed by Reintroduction of Folfoxiri Plus Bev at Progression Versus Folfox Plus Bev Followed by Folfiri Plus Bev in mCRC (TRIBE2) | PFS | III | |
| NCT02141295 | A Study Comparing the Efficacy and Safety of Vanucizumab and FOLFOX With Bevacizumab and FOLFOX in Participants With Untreated Metastatic Colorectal Cancer (McCAVE) | PFS | II | |
| NCT01206530 | FOLFOX/Bevacizumab/Hydroxychloroquine (HCQ) in Colorectal Cancer | Response rate | II | |
| NCT00200200 | Hepatic Arterial Infusion With Floxuridine and Dexamethasone Combination With Chemotherapy With/Without Bevacizumab for Hepatic Metastases From Colorectal Cancer | TTP | II | |
| NCT00544700 | Bevacizumab in Treating Patients Who Have Undergone First-Line Therapy for Metastatic Colorectal Cancer | TTP | III | |
| NCT00217737 | Oxaliplatin, Leucovorin Calcium, and Fluorouracil With or Without Bevacizumab in Treating Patients Who Have Undergone Surgery for Stage II Colon Cancer | Disease-free survival | III | |
| NCT00952029 | Combination Chemotherapy and Bevacizumab With or Without Bevacizumab Maintenance Therapy in Treating Patients With Metastatic Colorectal Cancer | Disease-control duration | III | |
| NCT00098787 | Bevacizumab and Oxaliplatin Combined With Irinotecan or Leucovorin and Fluorouracil in Treating Patients With Metastatic or Recurrent Colorectal Cancer | Objective response rate | II | |
| NCT01279681 | Combination Chemotherapy Plus Bevacizumab With or Without Oxaliplatin in Treating Older Patients With Metastatic Colorectal Cancer | PFS | III | |
|
| ||||
| Famitinib | NCT02390947 | Safety and Efficacy Study of Famitinib in Patients with Advanced Colorectal Adenocarcinoma (FACT) | OS | III |
|
| ||||
| Nintedanib | NCT02149108 | Nintedanib (BIBF 1120) vs Placebo in Refractory Metastatic Colorectal Cancer (LUME-Colon 1) | OS | III |
|
| ||||
| Panitumumab | NCT01814501 | Panitumumab and Chemotherapy in Patients With Advanced Colorectal Cancer After Prior Therapy With Bevacizumab | PFS | II |
|
| ||||
| Ramucirumab | NCT01079780 | Irinotecan Hydrochloride and Cetuximab With or Without Ramucirumab in Treating Patients With Advanced Colorectal Cancer With Progressive Disease After Treatment With Bevacizumab-Containing Chemotherapy | PFS | II |
|
| ||||
| Regorafenib | NCT02368886 | Lower or Standard Dose Regorafenib in Treating Patients With Refractory Metastatic Colorectal Cancer | Proportion of patients in each arm who completed 2 courses of treatment | II |
|
| ||||
| Sorafenib | NCT00826540 | Sorafenib and Bevacizumab in Treating Patients With Metastatic Colorectal Cancer | PFS | II |
4 Lymphangiogenesis in health and disease: why we should focus on lymphangiogenesis-targeting treatments
The lymphatic system is blind-ended and unidirectional, lacking a central pump unlike the cardiovascular system, and it serves to regulate fluid homeostasis, nutrient absorption in the gastrointestinal tract, and immune surveillance throughout the body. [2] Physiological lymphangiogenesis in adults is rare, with few exceptions including in the ovaries and mammary tissues during the female reproductive cycle. [39,70] In contrast, pathologic lymphangiogenesis is found in many disease-related processes, including organ graft rejection, cancer metastasis, lymphedema, and injury-induced corneal edema. [71] The molecular and cellular mechanisms of lymphangiogenesis involved in organ graft rejection, [72] cancer metastasis, [2,38,39,73] and lymphedema [74] are discussed in detail elsewhere. Moreover, lymphangiogenesis has an active and complex role in pathological circumstances such as inflammation, possibly including allergies and asthma. [75,76] A common factor in these diverse lymphangiogenic-related conditions is activation of the VEGF pathway, which includes ligands (VEGF-C, VEGF-D), receptors (VEGFR2 and VEGFR3), intracellular signaling enzymes (PI3K, mitogen-activated protein kinases [MAPKs]), and transcription factors (Prox-1). Lymphatic-deficient tissue like the cornea is avascular and easily accessible. This makes the cornea, in particular, an excellent platform for lymphangiogenesis research. Recently, groups have studied the corneal lymphatic response to injury and transplantation using real-time intravital imaging techniques on fluorescent reporter-tagged transgenic mice,[77,78] identified novel factors that regulate lymphangiogenesis such as aqueous humor-derived vasoactive intestinal peptide and α-melanocyte stimulating hormone,[79] and studied the role of lymphatic vessel growth in modulating the adaptive immune response to HSV-1 infection.[80]
4.1 Metastasis
Lymphatic metastasis is a major prognostic factor for different types of cancer. While there are various means for tumor cells to spread, intravasation into the lymphatic or blood circulation has been observed as the principle method by which metastases arise. In fact, due to structural advantages of the lymphatic route, tumor cell intravasation preferably occurs via the lymphatic system rather than the bloodstream.[81] Interestingly, it was once thought that almost no lymphatic vessels exist within tumors.[82] Many studies have since demonstrated the role of lymphangiogenic growth factors in tumor dissemination to regional lymph nodes. Furthermore, the lymphatic system was commonly thought to facilitate metastasis simply as a passive channel, providing a route for tumor cells to travel. However, recent advances in lymphatics research have revealed more complex and active mechanisms by which the lymphatic network interacts with cancer cells and induce tolerance of anti-tumor immune cells.[73,83] Therefore, this network has emerged as a major point of interest in oncological research.
In the current paradigm of the role of lymphangiogenesis in cancer, there are two distinct ways by which lymph vessels grow during metastasis. Intratumoral and peritumoral lymphatic vessel growth is known as “tumoral lymphangiogenesis,”[84,85] whereas “lymph node lymphangiogenesis” occurs from sentinel nodes[86,87] and is the main passage through which tumor cells travel to regional lymph nodes via the lymphatics.[2] In fact, tumor cells induce lymph node lymphangiogenesis even before their metastasis, allowing them to build their own paths to regional node sites. [88,89] Metastasis is a reliable prognostic factor for survival and cancer staging, involving evaluation of regional lymph node invasion and the severity of disease, and is important for therapeutic decision making. Evidence of lymph node lymphangiogenesis suggests that lymph nodes are primed to serve as a supportive environment for the arrival of cancer cells.[90–92] There is also good reason to pursue lymphangiogenesis targets for therapeutic applications, as some cancer patients have shown increased lymphangiogenic activity and subsequent recurrence of secondary tumors despite anti-angiogenic therapy. It is theorized that the regression of blood vessels leads to hypoxia-induced over-expression of lymphangiogenic factors that counteractively heighten the risk of tumor metastasis.[93] Preliminary studies in mouse models show promising results by knockdown of VEGFC or sVEGFR2 in mammary cancer[94] and lung cancer.[95] To date, however, no anti-lymphangiogenic cancer therapies have been approved by the FDA due to our limited knowledge of the complex interactions between anti-angiogenic drugs, lymphangiogenesis, and cancer metastasis.
4.2 Organ graft rejection
Despite improved human leukocyte antigen (HLA)-matching criteria and modern immunosuppressive treatments, the incidence of immune-mediated graft rejection in organ transplantation remains high. Lymphangiogenesis is a central mechanism generating lymphatic vessels, which act as the “afferent” highway for antigen-presenting cells (APCs) and soluble antigenic material to travel to regional lymph nodes, while blood vessels act as “efferent” highways by which effector cells travel from lymph nodes to reject allografts. [96] In fact, every solid organ transplantation is accompanied by angiogenesis and lymphangiogenesis to varying extents, and lymphangiogenesis-mediated graft rejection has been observed after corneal, kidney, lung, and heart transplantations.
Among the various organs that are routinely transplanted, the cornea serves as an excellent model for understanding vascular growth in the pre- and post-operative periods due to its baseline clarity and avascular state. The lack of vasculature is vital to maintaining corneal transparency. The 2-year rejection rate for corneal transplantation cases is about 10% in low-risk patients who demonstrate little to no vasculature in the cornea prior to surgery. [97] However, this rate of rejection is 50% if the host corneal bed is vascularized. [98] Furthermore, it has been experimentally shown in mice that even low-risk transplantations can end in rejection when post-operative vascularization is induced. While the mechanisms remain unclear, it is hypothesized that newly formed blood and lymphatic vessels play a collaborative role in recruiting mononuclear phagocytes to promote an immune response. In one study, sorafenib, a multi-tyrosine kinase inhibitor with anti-angiogenic and anti-lymphangiogenic properties, showed promise for improving corneal graft survival in a high-risk corneal transplantation mouse model. [99] Investigations of anti-lymphangiogenic molecules have only recently picked up momentum and have already demonstrated their clinical potential in lymphatics-related pathologies. However, while much lymphangiogenesis research has been conducted in the cornea, lymphangiogenic mechanisms in other organ systems remain less clear. For example, some studies purport the favorability of cortical and perivascular lymphatic vessels in renal allograft survival and function, suggesting that lymphatic vessels may initially play a role in clearing inflammatory cytokines and cells that would otherwise lead to fibrosis and compromise allograft kidney function [100,101]. However, long-term renal allograft fibrosis caused by chronic inflammation that ultimately leads to rejection may be mediated by increased lymphangiogenesis. [102,103]
4.3 Lymphedema
A disruption in the lymphatic structure can result in the accumulation of protein and biochemical metabolites in the interstitial space, causing an increase in the tissue colloid osmotic pressure and accumulation of fluid. This condition is known as lymphedema, and severe forms are characterized by fibroadipose deposition. [104] Lymphedema can lead to significantly decreased quality of life due to physical disfigurement and disablement. Furthermore, chronic lymphedema increases the risk of angiosarcoma, also called Stewart-Treves Syndrome.[1,36] The pathophysiology is proposed to be a result of impaired lymphangiogenesis; despite this, therapeutic options for the management of lymphedema are relegated to mainly surgical or physiotherapeutic procedures with limited efficacy. This may be because our understanding of the biological mechanisms underlying lymphangiogenesis is still lacking, although it is increasing quickly, and only recently have we been able to identify candidates for therapeutic targets in the lymphatic network.
Primary lymphedema arises from dysfunctional development of the lymphatic system, often manifesting at birth but also in adolescence or later in life. [104] On the other hand, more common is secondary lymphedema, which occurs as a consequence of surgery, trauma, parasitic infection or radiotherapy. [105] Surgery and radiation cancer therapies are the leading cause of secondary lymphedema in industrialized nations. Approximately 30% of breast cancer patients experience lymphedema of the upper limb, due to the disruption of lymphatic passage to the axillary nodes. [106] Chronic inflammation with a prominent T helper 2 cell response in lymphedematous limbs is a common finding in both animal models and humans, in which the inflammatory milieu of cytokines inhibits lymphangiogenesis, impairs lymphatic function, and promotes fibrosis. [104,107,108] This complex interplay between inflammation and impaired lymphatic function is an attractive focus for research, since in such cases, treatment with the pro-lymphangiogenic factor VEGF-C, which may already be over-expressed in lymphedematous tissues, may further increase the risks of relapse and metastasis in these patients. [104,109–111] Some studies have also experimentally demonstrated that pro-inflammatory cytokines, including interleukin-8 (IL-8), can reduce lymphedema by causing inflammation and promoting lymphangiogenesis. [112]
5 Lymphangiogenesis mechanisms: major target sites
5.1 VEGF pathway
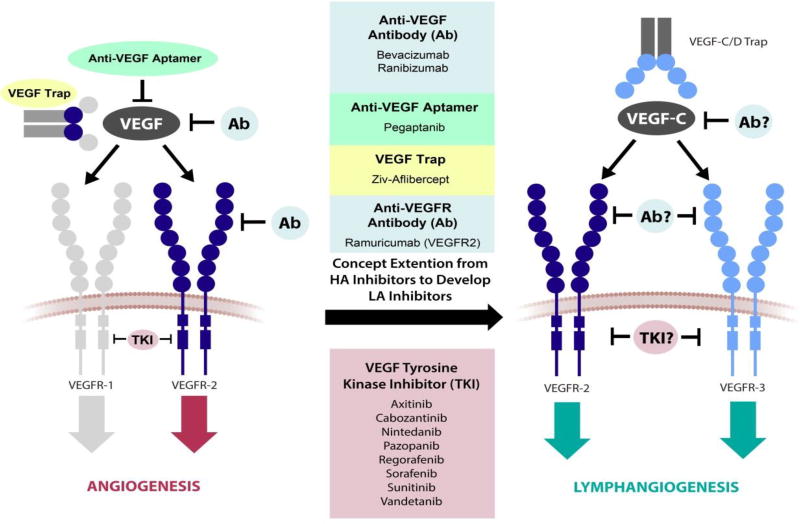
Lymphangiogenesis is regulated mainly by the VEGF/VEGFR system (Figure V), specifically ligands, VEGF-C and VEGF-D, and receptor tyrosine kinases, VEGFR2 and VEGFR3. VEGFR3 was the first to be discovered and was instrumental in the isolation of VEGF-C [11,113]. As VEGFR2 is for hemangiogenesis, VEGFR3 is the main receptor modulating lymphangiogenesis and is highly selectively expressed on endothelial cells committed to the lymphatic lineage; an exception, however, are endothelial cells of fenestrated blood vessels in some endocrine organs that also express VEGFR3. [2] VEGF-A triggers angiogenesis through its interaction with VEGFR1 and VEGFR2. Lymphangiogenesis is driven by VEGF-C and VEGF-D, which activate VEGFR3; VEGF-C also engages VEGFR2. [11] The interplay between complex factors such as the structural differences between VEGF-C and VEGF–D and their expression levels [114], the proteolytic cleavage of these factors [39,115], and the tissue of origin all impact the binding affinities of VEGF-C and VEGF-D to VEGFR2 and VEGFR3 and thus impact the regulation of lymphangiogenesis. [114,116] Because VEGF-C is considered the main ligand for lymphangiogenesis, this review will continue discussing the role of VEGF-C; however, the role of VEGF-D in human disease is just beginning to be better understood. [117]
Figure V. Schematic of VEGF pathway target sites of FDA-approved anti-angiogenesis drugs and potential VEGF pathway target sites for future anti-lymphangiogenesis drugs.
Axitinib, Bevacizumab, Cabozantinib, Nintedanib, Pazopanib, Pegaptanib, Ramuricumab, Ranibizumab, Regorafenib, Sorafenib, Sunitinib, and Vandetanib are approved anti-angiogenic therapies that target VEGF-mediated angiogenesis. Inhibition of a VEGF ligand, a VEGFR binding site, or VEGFR tyrosine kinase activity leads to a reduction in angiogenesis. This strategy can potentially be translated for lymphangiogenesis-targeted drug design. Development of anti-lymphangiogenic therapies may follow the design of anti-angiogenic therapies by interrupting the lymphangiogenic factor VEGF-C, its receptors VEGFR2 and VEGFR3, or the tyrosine kinase activity of VEGFR2 and VEGFR3. HA, hemangiogenic; LA, lymphangiogenic.
In a recent study, Zhong et al. introduced a Prox1-GFP/Flt1-DsRed (PGFD) mouse model that allows for live imaging of angiogenic and lymphangiogenic processes in the cornea and other organs. They found that lymphangiogenesis occurred even when angiogenesis did not, in concordance with previous studies reporting the occurrence of corneal lymphangiogenesis independently of hemangiogenesis. [118–123] Conditional deletion of VEGFR2 in endothelial cells markedly reduced lymphangiogenesis in the mouse cornea in response to VEGF-A and VEGF-C pellets, compared to that in mice without this conditional deletion, suggesting a role for blood endothelial cell VEGFR2 in lymphatic vessel formation and growth. [78] There seem to be various combinations of ligand binding affinities and kinase activities that produce a spectrum of downstream effects, both angiogenic and lymphangiogenic. Thus, before a therapeutic target can be determined, it is crucial to further characterize the specific mechanisms of the VEGF signaling system.
All VEGFRs are expressed on vascular endothelial cells at differing levels. [124] VEGFR1 expression is documented in macrophages, osteoclasts, dendritic cells, placental trophoblasts and pericytes, although its functions in these contexts have yet to be determined. [125–127] VEGFR2 is expressed mainly during early embryogenesis and re-expressed under certain pathological conditions, including tumor-induced angiogenesis. [128] Expression of VEGFR2 has also been observed in osteoblasts, neuronal cells, retinal progenitor cells, pancreatic ductal cells, megakaryocytes [128], and mesenchymal stem cells. [129] This does not include the upregulation of VEGFR3 seen in certain diseases. While VEGFR1 and VEGFR2 have shown diverse expression profiles, VEGFR3 has only been observed in a small set of cell types, including LECs, fenestrated vessels in endocrine organs, macrophages [130], osteoblasts [131], and bone-marrow mesenchymal stem cells. [129] The importance of VEGFR2 in VEGF-C–dependent responses has been investigated using VEGF-C156S, a mutant form of VEGF-C specific for VEGFR3. [132–134] VEGFR3 stimulation may induce an anti-inflammatory gene expression profile in LECs, consistent with previous findings of reduced inflammation following VEGF-C/VEGFR3 signaling. [135]
Blockade of VEGFR3 or VEGF-C may help or hinder the progression of certain pathologies, indicating that the beneficial or detrimental role of VEGFR3 signaling depends on the disease in question. Blockade of the VEGFR3 signaling pathway mitigates inflammation and thus ameliorates the phenotypes in certain animal models of disease such as graft versus host disease (GVHD), [136] corneal graft rejection, [137] dry eye disease, [138] allergic eye disease, [139] obesity, [140] the tumorigenesis of squamous cell carcinoma, [141] and pulmonary arterial hypertension (PAH). [142,143] Interestingly, in the clinic, PAH has been found to be associated with increased levels of the anti-angiogenic chemokine CXCL4 [144] and the anti-angiogenic isoform of VEGF VEGF165b, [145] suggesting the role of suppressed angiogenesis in the etiology of PAH. Also, one preclinical study reports that VEGFR3 inhibition early in the course of disease reduces inflammation, lung arterial obliteration, and pulmonary hypertension by offsetting the increased VEGFR3 signaling that contributes to pulmonary remodeling in PAH. [143] Thus, hypothetically, treatment of PAH could involve a combination pro-angiogenic and anti-lymphangiogenic therapies.
Conversely, blockade of VEGFR3 may be detrimental in the treatment of certain diseases and exacerbate inflammation or inhibit therapeutic immune responses. For example, VEGFR3 inhibition enhances inflammation of the colon in a mouse model of inflammatory bowel disease (IBD). [146] An increased density of abnormally dilated lymphatic vessels is found in the colons of these mice, similar to findings in IBD patients.[147,148] In the aforementioned study, anti-VEGFR3 mAb therapy impaired lymphatic vessel function important for the clearance of inflammatory mediators. [146] Similarly, due to defective drainage of inflammatory cells and cytokines, VEGFR3 inhibition also exacerbates rheumatoid arthritis [149,150] and chronic airway inflammation. [151] In the context of melanoma treatment, VEGFR3 blockade interferes with the beneficial effect of checkpoint blockade immunotherapy, [152] This suggests that VEGF-C is beneficial in combination with immunotherapy through the induction of CCL21 expression in tumor-associated lymphatic vessels, which recruits naïve T cells and dendritic cells to the tumor microenvironment. At this point, it is unclear whether this is the case with other cancers. However, the latter example illustrates that potential anti-lymphangiogenic cancer therapies, especially if they are to be used with cancer immunotherapies in the future, may need to be designed to balance the competing processes of inhibiting lymphangiogenesis-associated metastasis and facilitating the recruitment of tumor-killing immune cells. All together, these studies indicate that pro- and anti-lymphangiogenic therapies that must be applied only after understanding whether lymphangiogenesis in certain diseases is an instigator of inflammation or a necessary sequela for its resolution (for recent reviews on inflammation-associated and the immunomodulatory effects of lymphangiogenesis, refer to the following references.[153,154]
An example of an inflammatory pathway encroaching on the VEGF pathway to induce lymphangiogeneis is well demonstrated by the role of TNF-α in lymphatic metastasis. Ji et al. shed light on this mechanism in which TNF-α directly stimulates lymphatic endothelial cell migration independently of VEGFR3. [155] Lymphatic vessel development is triggered by TNF-α binding to TNFR1, but TNF-α–induced lymphangiogenesis can only occur after lymphatic endothelial cell tip formation, which can only be induced by VEGFR3-mediated signaling. Hence, if VEGFR3 is blocked, TNF-α–TNFR1 signaling-induced lymphangiogenesis is also precluded. TNF-α also indirectly stimulates lymphangiogenesis by activating inflammatory macrophages that produce VEGF-C, inducing persistent stimulation of lymphangiogenesis and ultimately, tumor metastasis. [155]
5.2 EGF pathway
The epidermal growth factor receptor (EGFR) family is a group of receptor tyrosine kinases that are over-expressed in many cancer types (Figure VI) [156,157] Of the four receptors in the family, EGFR2 (also often called human epidermal growth factor receptor 2 [HER2] or NEU) and EGFR3 lack the ability to bind EGF or show intrinsic tyrosine kinase activity, respectively, and therefore, form heterodimers with EGFR1 or EGFR3 to initiate downstream MAPK signaling. [158,159] Research has demonstrated that over-expression of EGFRs promotes cancer cell proliferation, migration, and invasion, and over-expression of VEGFs, induced by EGFR signaling in tumors, promotes angiogenesis to expand the tumor vasculature. [160–162]
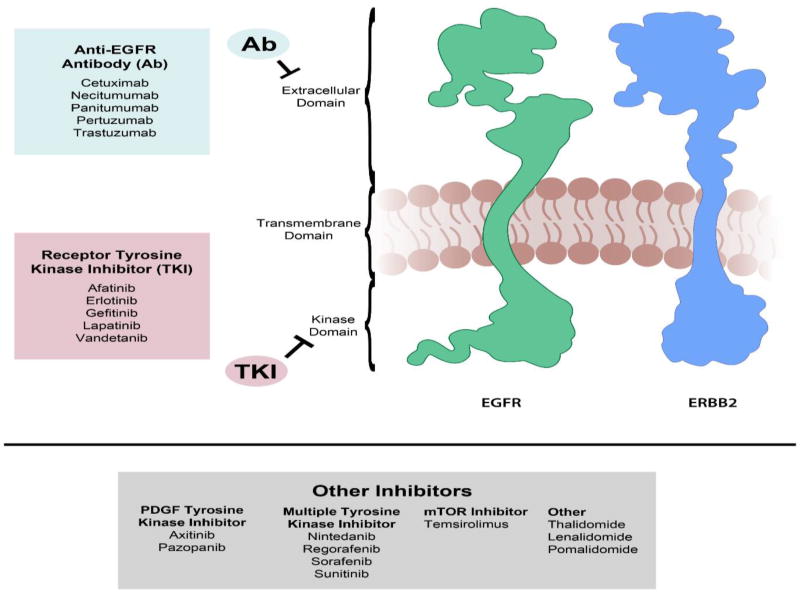
Figure VI. Schematic of EGF pathway target sites of FDA-approved anti-angiogenesis drugs.
EGFR (also known as ERBB1 and HER1) and ERBB2 (also known as HER2/neu) are the main therapeutic targets of currently available anti-angiogenic drugs. ERBB2 lacks the ability to bind growth factor ligands and forms heterodimers with EGFR, ERBB3, or ERBB4. Monoclonal antibodies targeting the extracellular domain such as trastuzumab and pertuzumab prevent heterodimer formation, while others such as cetuximab, necitumumab, and panitumumab competitively inhibit EGFR ligand binding. Receptor tyrosine kinase inhibitors target the intracellular kinase domains and prevent signaling downstream of ligand-binding and dimer formation.
This supports an intimate interplay between the VEGF and EGF pathways in promoting cancer progression through angiogenesis. A novel sorafenib derivative that targets both VEGFRs and EGFRs has been shown to be efficacious in vitro against prostate tumors. [163] In addition, in animal xenograft models of non-small cell lung cancer, therapies targeting both VEGFR and EGFR for tumor treatment are more effective than those targeting either receptor family alone [164,165]. Therefore, monoclonal antibodies such as cetuximab and receptor tyrosine kinase inhibitors such as erlotinib that target EGFRs represent a two-pronged approach to cancer therapy by inhibiting tumor growth directly and indirectly by blocking proliferation of the tumor vasculature (Figure VI).
Compared to that of normal endothelial cells, tumor-associated endothelial cell expression of EGFRs is abnormal. [166–168] The transfer of oncogenic EGFR produced by tumors via extracellular vesicles to endothelial cells in pathologic angiogenesis has also been demonstrated. [169,170] Despite preclinical evidence of the efficacy of EGFR inhibitors for treating angiogenesis-driven cancer progression and metastasis, clinical evidence shows only limited efficacy of EGFR-targeting anti-angiogenic therapies in humans. [161,171–175] Lymphatics have a well-supported role in tumor metastasis, and thus, understanding the role of EGFR signaling in lymphangiogenesis and the effects of EGFR inhibitors may advance the development of potentially effective anti-lymphangiogenic therapies for the prevention or treatment of metastatic cancers.
Signaling events involving EGFRs and the processes governing lymphangiogenesis are closely intertwined. EGFR and HER2 are expressed on human LECs associated with skin [176,177], while EGFR has been shown to be expressed in the intra- and peritumoral lymphatic vessels of oral squamous cell carcinoma [178] and the peritumoral lymphatics of colon cancer. [179] In addition, anti-EGFR therapy improved lung phenotypes in mice models of lymphangioleiomyomatosis [180], a destructive cystic lung disease characterized by neoplastic lesions that express lymphatic endothelial cell markers Prox-1, LYVE-1 and podoplanin [181] and producing lymphangiogenic factors VEGF-C and VEGF-D. [182] In vitro, EGF-treated human LECs exhibit enhanced migration and tube formation, while in vivo, EGF treatment leads to increased lymphatic vessel area and size, albeit to a lesser extent than VEGF-C treatment. [176] In a xenograft melanoma mouse model, EGF produced by tumors induces lymphangiogenesis, with knockdown of EGF expression decreasing tumor-associated lymphatic vessel density but not blood vessel density. [183] Frequently, tumors shed extracellular portions of transmembrane proteins through the over-expression of sheddases to modulate cell-to-cell communication [184] and increase ectodomain shedding of membrane-bound EGFs. [185] For example, A disintegrin and metalloprotease 17 (ADAM17) increases heparin-binding epidermal growth factor (HB-EGF) shedding by LECs, resulting in increased LEC invasion, migration, and tube sprouting. [177] Silencing of ADAM17 in LECs and treatment with an EGFR inhibitor resulted in both decreased motility and sprouting. [177] Finally, dual inhibition of transforming growth factor beta (TGF-β) and EGFR/HER2 using lapatinib suppresses the growth and metastasis of pancreatic ductal adenocarcinoma, which expresses many lymphangiogenic factors. [186] Although there are promising results on the interaction between EGFR signaling and lymphangiogenesis, the detailed mechanisms have yet to be elucidated, and thus far, there have been no clinical trials specifically studying the pro-/anti-lymphangiogenic effects of EGFR inhibitors on lymphangiogenesis-related disorders or cancer metastasis.
5.3 FGF and PDGF pathways
FGFs and platelet-derived growth factors (PDGFs) induce both angiogenesis and lymphangiogenesis. [187] In the cornea, FGF2 (also known as basic fibroblast growth factor, bFGF) increases expression of VEGF-A, VEGF-C, and VEGF-D, thereby indirectly increasing angiogenesis via VEGFR2 signaling and lymphangiogenesis via VEGFR3 signaling. [188,189] One study showed that while both FGF2 and VEGFs induce blood and lymphatic vessel growth consistent with previous findings, FGF2 more potently induces lymphatic vessel growth in the cornea. [190] FGF2 is the main driver of corneal neovascularization after herpes simplex virus 1 (HSV-1) infection, and consequently, neutralization of FGF2 reduces both corneal angiogenesis and lymphangiogenesis. [191] In the tumor microenvironment, FGF2 indirectly promotes LEC proliferation and migration via FGFR1 signaling. [192] This interaction may be fortified by LYVE-1, as glycosylated LYVE-1 binds FGF2 tightly, and in fact, soluble LYVE-1 inhibits FGF2-mediated lymphangiogenesis. [193] However, inhibition of FGF2 in targeted therapies must be executed carefully to avoid possible toxicities; for example, FGF2 is involved in neurogenesis and its inhibition may impair nerve regeneration. [80,194] Furthermore, FGF2 and VEGF-C were shown to work synergistically to promote tumor growth and metastasis to the lung via blood vessels and to sentinel lymph nodes via lymphatic vessels in a mouse model of fibrosarcoma. [192] Together, these studies support the advantage of combination therapies that inhibit both FGFR and VEGFR-3 signaling to therapeutically target pathologic lymphangiogenesis.
The FGF/FGFR pathway may also be an attractive target for anti-angiogenic cancer therapies, as it may be a potential escape mechanism for cancers refractory to anti-VEGF-A monotherapies. [195] Therefore, potential VEGF-C/VEGFR3 anti-lymphangiogenesis therapies for metastatic cancers can also benefit from anti-FGF2/FGFR compounds. In addition, while the family of PDGF molecules, PDGF-AA, PDGF-BB, and PDGF-AB, can all induce angiogenesis equally, PDGF-BB and PDGF-AB more potently stimulate lymphangiogenesis by binding to PDGFRβ compared with PDGF-AA, which engages PDGFRα exclusively. [196,197] PDGF-BB–mediated lymphangiogenesis in the cornea occurs independently of VEGFR3 and TIE2 and facilitates tumor growth and metastasis in vivo. [197] Interestingly, FGF2 upregulates PDGFRα and PDGFRβ and potentiates angiogenic signaling of PDGF-BB in a mouse model of corneal angiogenesis. [198] Conversely, stimulating both FGF and PDGF-BB signaling pathways results in a more potent angiogenic effect than VEGF stimulation alone, for these molecules may act as chemoattractants for macrophages that facilitate blood endothelial tip migration and anastomosis. [199] It is well documented that macrophages can induce lymphangiogenesis both directly through trans-differentiation and indirectly via the production of VEGF-C and VEGF-D. [118–121] However, there are no recent studies investigating the potential synergistic effect of PDGF-BB and FGF2 in lymphangiogenesis, despite plenty of research validating their pro-lymphangiogenic effects alone. Interestingly, a recent study by Liu et al. showed that metastasis of tumor cells is enhanced by PDGF-BB and FGF2 treatment of cancer-associated fibroblasts. [200] Such findings provide additional motivation for studies on the interaction between blood endothelial cells, LECs, fibroblasts, other stromal cells, and tumor cells within the tumor microenvironment to understand their multifaceted interactions in cancer metastasis. Therefore, potential VEGF-C/VEGFR3 anti-lymphangiogenesis therapies for metastatic cancers may also benefit from anti-FGF-2/FGFR and anti-PDGF-BB/PDGFRβ compounds.
6 Current lymphangiogenesis research
The biological regulation of angiogenesis and lymphangiogenesis is vast, involving hundreds of regulatory molecules. Thus, much effort is focused on studying molecules that act beyond the aforementioned growth factors and could have clinical applicability. For instance, therapeutic agents that target the angiopoietin (Ang)-TIE pathway are currently in clinical development for oncological and ophthalmological applications. [201] Ang-1 and Ang-2 have a direct promoting effect on cervical cancer migration and invasion, and their down-regulation has predictably attenuated tumor growth. [202] The same study found that TIE2 expression in cervical tumor cells is associated with significantly poor prognosis. Furthermore, treatment with Ang-2 inhibitors significantly improves outcomes of corneal grafts. [203]
A recent report indicates that fatty acid β-oxidation is required for lymphangiogenesis. Lymphangiogenesis is impaired in mice that lack carnitine palmitoyltransferase 1A (CPT1A), a rate-controlling enzyme in fatty acid oxidation. [204] A decrease in acetyl coenzyme A, a consequence of CPT1A knockout, leads to decreased acetylation of histones at lymphangiogenic genes. Furthermore, CPT1A is upregulated by Prox-1. Wong et al. suggested that there may be underappreciated therapeutic potential in lowering fatty acid oxidation to suppress these genes. In the case of lymphedema, supplementation with metabolites such as acetate may promote lymphangiogenesis instead. [204]
One study demonstrated that toluquinol, a marine fungus metabolite, suppresses lymphangiogenesis by interfering with VEGF-C–induced VEGFR3 phosphorylation in vivo, ex vivoand in vitro. [205] In these experiments, toluquinol is a more potent inhibitor of lymphangiogenesis than of angiogenesis. Several other drugs have been shown previously to reduce lymphangiogenesis by blocking VEGFR3, including deguelin [206,207], liposomal honokiol [208], Ki23057 [209], etodolac [210], MMI270 [211], CSDA [212], and norcantharidin [213]. More recently, fucoidan, a fucose-enriched sulfated polysaccharide, was shown to have dose-dependent depressive-effects on Prox-1 and VEGFR3 in murine models, demonstrating anti-lymphangiogenic capabilities. [214] Additionally, the VEGFR3 human immunoglobulin G subclass 1 monoclonal antibody LY3022856 was studied in a phase 1 dose-escalation study to measure its anti-tumor effects. [215] Although the study concluded that the antibody had insignificant anti-tumor activity, this phase 1 trial demonstrates that investigators are gradually bringing attention to lymphangiogenesis mechanisms as targets for treating pathologies.
Nanoparticles have additionally emerged as a controlled gene delivery system that suppresses lymphangiogenesis sustainably through targeted delivery and then continuous release of genes to tumorigenic areas. For instance, small interfering RNA (siRNA) targeting VEGF-C suppresses tumor lymphangiogenesis in subcutaneous xenografts particularly when the siRNA is complexed with calcium carbonate (CaCO3) nanoparticles. [216] Divalent calcium ions of the CaCO3 nanoparticles stabilize the DNA structure by forming ionic complexes with the nucleic acid backbone. This not only protects the siRNA from degradation but also allows the CaCO3 nanoparticle–siRNA complex to cross the cancer cell membrane via ion channel-mediated endocytosis. In a similar context, VEGFR3-targeting siRNA molecules are complexed with polyethylenimine (PEI)-alginate nanoparticles with an average diameter of 139 nm. [217] Alternatively, Flt23k, an intraceptor inhibitor of VEGF, is loaded in biodegradable poly-(L-lactic-co-glycolic acid) (PLGA) nanoparticles. [218] The delivery of these nanoparticles to the murine cornea significantly decreases neovascularization and lymphangiogenesis.
In addition to therapeutic applications, nanoparticles have been applied to imaging lymphatics in cancer. Various magnetic resonance imaging (MRI) contrast agents are injected into the interstitial space for visualization of lymphatic vessels and lymph nodes. For example, gadolinium (Gd)-labeled nanoparticles with a polyamidoamine dendrimer core and an average diameter of 10 nm are used to differentiate intralymphatic- from extralymphatic-involved lymphoma. [219] Alternatively, poly(ethylene glycol) (PEG)-conjugated nanoparticles consisting of an ultra-small superparamagnetic iron oxide (USPIO) core and a gold shell as well as dextran-coated SPIO nanoparticles are used to image lymphangiogenesis within tumors. [220] Notably, dextran-coated SPIO nanoparticles have been approved for diagnostic use in certain European countries. [221]
7 Conclusion
Over the last 15 years, lymphangiogenesis has been implicated in numerous pathologies including metastasis, organ graft rejection, and lymphedema. Consequently, gate keepers of the lymphangiogenic process have emerged as a novel class of therapeutic targets. This constitutes a fertile and untapped opportunity; while there are 26 clinically approved approaches to treat pathological angiogenesis, none exist for lymphangiogenesis. Identification of governors of the lymphangiogenic process, along with the experience obtained in the process of developing anti-angiogenic modalities, has set the stage for rapid development of biomarkers and therapeutics for lymphangiogenesis.
Acknowledgments
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Publication of this article was supported by National Institutes of Health grants EY10101 (D.T.A.), EY023691, EY021886, I01 BX002386, the Eversight, Midwest Eye Bank Award (J.H.C), EY01792, EY027912 (MIR), and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Nonstandard Abbreviations
- ADAM17
A disintegrin and metalloprotease 17
- Ang
angiopoietin
- APCs
antigen-presenting cells
- bFGF
basic fibroblast growth factor
- CPT1A
carnitine palmitoyltransferase IA
- EGFR
epidermal growth factor receptor
- ERBB
homologous to erythroblastoma viral gene product, v-erbB
- FGFR
fibroblast growth factor receptor
- Gd
gadolinium
- GVHD
graft versus host disease
- HB-EGF
heparin-binding epidermal growth factor
- HER1
human epidermal growth factor receptor 1
- HER2
human epidermal growth factor receptor 2
- HIF-1alpha
hypoxia-inducible factor 1-alpha
- HLA
human leukocyte antigen
- HSV-1
herpes simplex virus 1
- IBD
inflammatory bowel disease
- IL-8
interleukin-8
- LECs
lymphatic endothelial cells
- LYVE-1
lymphatic vessel endothelial hyaluronan receptor 1
- MAPKs
mitogen-activated protein kinases
- MRI
magnetic resonance imaging
- MMPs
matrix metalloproteinases
- micro-MRL
micromagnetic resonance lymphangiography
- mAb
monoclonal antibody
- OS
overall survival
- PlGF
placental growth factor
- PDGFR
platelet-derived growth factor receptor
- PEI
polyethylenimine
- PFS
progression-free survival
- PGFD
Prox1-GFP/Flt1-DsRed
- PEG
poly(ethylene glycol)
- Prox-1
prospero-related homeobox 1
- PAH
pulmonary arterial hypertension
- SNPs
single nucleotide polymorphisms
- siRNA
small interfering RNA
- sVEGFR
soluble vascular endothelial growth factor receptor
- TGF-β
transforming growth factor beta
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Biographies
Michael Yamakawa received his bachelor's degree in Biophysics from Johns Hopkins University. His research there utilized x-ray crystallography to characterize ligand-binding promiscuity and to advance knowledge on the binding mechanisms of multi-drug recognizing transcription factors. He received a grant to evaluate didactic methods in biophysics using an open-source Java viewer of macromolecular structures. He received a second grant to evaluate a set of adjusted eligibility criteria for heart transplantation and study the effectiveness of third-generation ventricular assist devices as a destination treatment for heart failure. He also worked at Northwestern University to determine the structure of PDR3, a transcription factor within the yeast pleiotropic drug resistance network and elucidate its DNA-binding mechanisms. After graduating, he was an intern at a start-up pharmaceutical company in Baltimore where he helped a life-saving pediatric orphan drug achieve FDA approval. He is currently a second-year medical student at the University of Illinois at Chicago (UIC) College of Medicine with a research focus on understanding the development of pathological blood and lymphatic vasculatures in the cornea through the study of vascular endothelial growth factors and their receptors. He also takes part in a selective, specialized program called Innovation Medicine (IMED), which allows him to bridge technology with medical education and practices.
Susan J. Doh is an M.D. student at the University of Illinois at Chicago (UIC) College of Medicine. She received her B.A. in Biology at the University of Chicago, where she conducted research on the human immunoglobulin response to the anthrax vaccine. She also co-founded and led the University of Chicago’s inaugural synthetic biology research team to the 2013 International Genetically Engineered Machines (iGEM) contest held in Toronto. After graduating, she was an Intramural Research Training Award Fellow at the National Institutes of Health in Bethesda, MD, generating antigen-specific cytotoxic T cells against renal cell carcinoma to develop novel cellular cancer immunotherapies through a gene therapy technique called adoptive cell transfer. Currently, she studies the role of vascular endothelial growth factor receptors in pathologic angiogenesis and lymphangiogenesis and is one of fourteen members of the UIC College of Medicine’s Innovation Medicine (IMED) program through which she synthesizes her passions for human biology and medicine, service, and technology.
Samuel Mikha Santosa, M.D.obtained his M.D. degree from Universitas Padjadjaran, Indonesia. Currently, he is a visiting research scholar in the Department of Ophthalmology, University of Illinois at Chicago. He is working on research that focuses on in-vivo fluorescence imaging of corneal blood and lymph vessels in special in-house bred Prox1-GFP/Flt1-DsRed (PGFD) mice and understanding the interactions between VEGF-A and -C with VEGFR-2 in inducing corneal angiogenesis and lymphangiogenesis with various conditional VEGFR knockout mice.
Mario Montana, M.D.is currently a visiting research scholar at the University of Illinois at Chicago. He received his M.D. degree from Universitas Pelita Harapan in 2012. His current research interest is in the characterization of murine corneal blood and lymphatic vessel growth. More specifically, his research focuses on the effects of limbal deficiency induced by limbal epithelial scraping on Prox1-GFP/Flt1-DsRed (PGFD) mice with or without conditional knockout of lymphatic or angiogenic VEGFR-2. He has also contributed in writing a review paper entitle Angiogenesis and Lymphangiogenesis in Corneal Transplantation, which is currently pending review.
Ellen C. Qin is currently a Ph.D. student in Materials Science and Engineering at the University of Illinois, Urbana-Champaign, under the mentorship of Prof. Hyunjoon Kong. Her current research focuses on the synthesis and characterization of biomaterials for tissue regeneration. She received her Bachelor of Science degrees in Chemical Engineering and in Biochemistry (summa cum laude) from Arizona State University.
Hyunjoon Kong, PhD.is a professor in the Department of Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign (UIUC). He also holds affiliations with the Department of Bioengineering, Department of Pathobiology, Center for Biophysics and Computational Biology, and Neuroscience Program. He received his engineering education from the University of Michigan at Ann Arbor (Ph.D. 2001) and performed post-doctoral research at the University of Michigan and Harvard University. He joined the University of Illinois in 2007. He has been developing a series of soft matters that can regulate molecular and cell transport for diagnosis and treatment of vascular and pro-inflammatory diseases.
Kyu-yeon Han, PhD.is a Research Assistant Professor in Opthalmology and Visual Sciences at the University of Illinois at Chicago. He received his Ph.D. from Kyunghee University in Korea, Seoul. His current research is focused on corneal wound healing and neovascularization dependent on matrix metalloproteinases (MMPs), specifically concerning the regulation of receptor tyrosine kinase by MMP enzyme activity. Another major field of his research is the study of intercellular communications via exosomes and their relevance for the mechanism of corneal wound healing and neovascularization. He has published 30 articles in international peer-reviewed journals and has been a reviewer for more than 10 papers in international peer-reviewed journals.
Charles Yu, MD received undergraduate training at the University of California Berkeley. He is a fellowship-trained cornea, cataract, and refractive physician and surgeon. He went to the University of California Davis Medical School and completed his residency at Stanford University. He received his HHMI Research Fellowship from Weill Cornell Medical College and also completed a Fellowship in Corneal and Refractive Surgery there. His clinical interests are primarily diseases of the external eye, cornea, ocular surface, and corneal replacement. He is engaged in research endeavors to overcome corneal blindness through the development of keratoprostheses and an artificial cornea. He is a recipient of an NIH Mentored Clinical Scientist Research Career Development Award (K08).
Mark I. Rosenblatt, MD, PhD, MBAis Professor and Head of the Department of Ophthalmology and Visual Sciences at the Illinois Eye and Ear Infirmary of University of Illinois at Chicago (UIC). He is a corneal surgeon and physician-scientist, with a National Institutes of Health-sponsored research program exploring mechanisms to regenerate corneal nerves following corneal injury. Dr. Rosenblatt is also the Illinois Lions/Charles I. Young Chair, as well as Associate Director of MSTP at UIC’s College of Medicine. Prior to joining the UIC Faculty in October, 2014, Dr. Rosenblatt was Director of the Margaret M. Dyson Vision Research Institute and Vice Chair of Ophthalmology at Weill Cornell Medical College. Dr. Rosenblatt has made significant contributions to our understanding of corneal nerve regeneration, as well as to the establishment of innovative models for investigating corneal nerves. At Weill Cornell Medical College, Dr. Rosenblatt expanded his research program to not only include regenerative issues related to corneal nerves, but also regeneration of the ocular surface. He is currently exploring novel ways to promote trans-differentiation of skin stem cells into corneal epithelial stem cells for the treatment of corneal epithelial stem cell deficiency.
Andrius Kazlauskas, PhDreceived his BS (1982) and PhD (1986) degrees in chemistry from Cleveland State University. His graduate studies focused on elucidating the signaling events responsible for growth factor-driven proliferation of cells. He continued in this area of basic research at the Fred Hutchinson Cancer Research Center in Seattle (1986–1991) and then in his own research lab at the National Jewish Center in Denver (1991–1996). He joined the Schepens Eye Research Institute and Department of Ophthalmology at Harvard Medical School in 1996 to investigate the signaling events driving the pathogenesis of blinding eye diseases such as proliferative diabetic retinopathy, age-related macular degeneration, and proliferative vitreoretinopathy. In 2015, Dr. Kazlauskas transitioned to F. Hoffman-La Roche in Basel, Switzerland in order to fully engage in translational research leading to new therapies for patients with sight-threatening conditions. In 2017, Dr. Kazlauskas transitioned back to academia, with the Departments of Physiology and Biophysics and Ophthalmology and Visual Sciences at University of Illinois at Chicago, where he is investigating signaling events that govern angiogenic homeostasis.
Jin-Hong Chang, Ph.D.is Research Associate Professor and the Director of the Angiogenesis Research Laboratory at the Department of Ophthalmology and Visual Sciences, University of Illinois at Chicago (UIC). He obtained his B.S. degree from National Taiwan Ocean University and Ph.D degree in Biochemistry from University of Mississippi. He received his postdoctoral fellowship in microbiology from University of Virginia and instructorship in pediatric oncology from Dana Farber Cancer Institute and Massachusetts Eye and Ear infirmary, Harvard Medical School. His team’s current research focus is on understanding the role of VEGF receptors and other factors in growth factor-, suture- and hemi-limbal injury-induced corneal hemangiogenesis (HA) and lymphangiogenesis (LA) by using their own developed Prox1-GFP/Flt1-dsRed (PGFD) mouse. With the use of this technique, he developed several conditional VEGFR1, 2 and 3 KO mice and conducts various in vivo, ex vivoand in vitro assays to understand the growth factor- and injury-induced HA and LA development. His research aims to understand the molecular processes of HA and LA and hence to develop therapeutic treatments such as anti-angiogenic and anti-lymphangiogenic treatments for preventing transplant rejection, limbal stem cell deficiency, as well as angiogenesis- and lymphangiogenesis-related diseases.
Dimitri Azar, MD, MBA.is Dean of the College of Medicine, Professor of Ophthalmology Bioengineering and Pharmacology, and B.A. Field Chair in Ophthalmologic Research at University of Illinois at Chicago (UIC). Dr. Azar is an internationally recognized ophthalmic surgeon who brings experience in administration, research, education and clinical practice to his position as Dean of the College of Medicine at University of Illinois. He was also a tenured Professor of Ophthalmology at Harvard Medical School, Director of the Cornea Service at Massachusetts Eye and Ear Infirmary, Senior Scientist at Schepens Eye Institute, and a faculty member at Johns Hopkins School of Medicine. He earned an executive MBA from the University of Chicago. Dr. Azar is board certified in ophthalmology. He is a leader in basic science and clinically related vision research, making significant contributions to the treatment of corneal diseases, angiogenesis-related disorders, and advances in refractive surgery through mathematical analyses and applications of advanced optics. His basic science research on matrix metalloproteinases in corneal wound healing and angiogenesis has been continually funded by a National Eye Institute R01 award since 1993.
Footnotes
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Yamakawa, Doh, Santosa, Montana, Qin, Kong, Han, Yu, Rosenblatt, Kazlauskas, Chang, Azar.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
References
- 1.Adamczyk LA, Gordon K, Kholova I, Meijer-Jorna LB, Telinius N, Gallagher PJ, van der Wal AC, Baandrup U. Lymph e: the forgotten second circulation in health and disease. Virchows Arch. 2016;469(1):3–17. doi: 10.1007/s00428-016-1945-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140(4):460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 3.Dieterich LC, Seidel CD, Detmar M. Lymphatic vessels: new targets for the treatment of inflammatory diseases. Angiogenesis. 2014;17(2):359–371. doi: 10.1007/s10456-013-9406-1. [DOI] [PubMed] [Google Scholar]
- 4.Yang JF, Walia A, Huang YH, Han KY, Rosenblatt MI, Azar DT, Chang JH. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv Ophthalmol. 2016;61(3):272–296. doi: 10.1016/j.survophthal.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MH, Gootenberg J, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) plus Carboplatin and Paclitaxel as first-line treatment of advanced/metastatic recurrent nonsquamous non-small cell lung cancer. Oncologist. 2007;12(6):713–718. doi: 10.1634/theoncologist.12-6-713. [DOI] [PubMed] [Google Scholar]
- 8.Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies - A review. J Adv Res. 2017;8(6):591–605. doi: 10.1016/j.jare.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quesada AR, Munoz-Chapuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26(4):483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 10.Walia A, Yang JF, Huang YH, Rosenblatt MI, Chang JH, Azar DT. Endostatin's emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim Biophys Acta. 2015;1850(12):2422–2438. doi: 10.1016/j.bbagen.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15(7):1751. [PMC free article] [PubMed] [Google Scholar]
- 12.Yamada Y, Nezu J, Shimane M, Hirata Y. Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics. 1997;42(3):483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- 13.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144(4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wigle JT, Chowdhury K, Gruss P, Oliver G. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet. 1999;21(3):318–322. doi: 10.1038/6844. [DOI] [PubMed] [Google Scholar]
- 15.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154(2):385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92(8):3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gray R, Bhattacharya S, Bowden C, Miller K, Comis RL. Independent review of E2100: a phase III trial of bevacizumab plus paclitaxel versus paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2009;27(30):4966–4972. doi: 10.1200/JCO.2008.21.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miles DW, Chan A, Dirix LY, Cortes J, Pivot X, Tomczak P, Delozier T, Sohn JH, Provencher L, Puglisi F, Harbeck N, Steger GG, Schneeweiss A, Wardley AM, Chlistalla A, Romieu G. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28(20):3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 19.Robert NJ, Dieras V, Glaspy J, Brufsky AM, Bondarenko I, Lipatov ON, Perez EA, Yardley DA, Chan SY, Zhou X, Phan SC, O'Shaughnessy J. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29(10):1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 20.Gianni L, Romieu GH, Lichinitser M, Serrano SV, Mansutti M, Pivot X, Mariani P, Andre F, Chan A, Lipatov O, Chan S, Wardley A, Greil R, Moore N, Prot S, Pallaud C, Semiglazov V. AVEREL: a randomized phase III Trial evaluating bevacizumab in combination with docetaxel and trastuzumab as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer. J Clin Oncol. 2013;31(14):1719–1725. doi: 10.1200/JCO.2012.44.7912. [DOI] [PubMed] [Google Scholar]
- 21.Martin M, Loibl S, von Minckwitz G, Morales S, Martinez N, Guerrero A, Anton A, Aktas B, Schoenegg W, Munoz M, Garcia-Saenz JA, Gil M, Ramos M, Margeli M, Carrasco E, Liedtke C, Wachsmann G, Mehta K, De la Haba-Rodriguez JR. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: the letrozole/fulvestrant and avastin (LEA) study. J Clin Oncol. 2015;33(9):1045–1052. doi: 10.1200/JCO.2014.57.2388. [DOI] [PubMed] [Google Scholar]
- 22.Dickler MN, Barry WT, Cirrincione CT, Ellis MJ, Moynahan ME, Innocenti F, Hurria A, Rugo HS, Lake DE, Hahn O, Schneider BP, Tripathy D, Carey LA, Winer EP, Hudis CA. Phase III Trial Evaluating Letrozole As First-Line Endocrine Therapy With or Without Bevacizumab for the Treatment of Postmenopausal Women With Hormone Receptor-Positive Advanced-Stage Breast Cancer: CALGB 40503 (Alliance) J Clin Oncol. 2016;34(22):2602–2609. doi: 10.1200/JCO.2015.66.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochlitz C, Bigler M, von Moos R, Bernhard J, Matter-Walstra K, Wicki A, Zaman K, Anchisi S, Kung M, Na KJ, Bartschi D, Borner M, Rordorf T, Rauch D, Muller A, Ruhstaller T, Vetter M, Trojan A, Hasler-Strub U, Cathomas R, Winterhalder R, Swiss Group for Clinical Cancer R SAKK 24/09: safety and tolerability of bevacizumab plus paclitaxel vs. bevacizumab plus metronomic cyclophosphamide and capecitabine as first-line therapy in patients with HER2-negative advanced stage breast cancer - a multicenter, randomized phase III trial. BMC Cancer. 2016;16(1):780. doi: 10.1186/s12885-016-2823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miles D, Cameron D, Bondarenko I, Manzyuk L, Alcedo JC, Lopez RI, Im SA, Canon JL, Shparyk Y, Yardley DA, Masuda N, Ro J, Denduluri N, Hubeaux S, Quah C, Bais C, O'Shaughnessy J. Bevacizumab plus paclitaxel versus placebo plus paclitaxel as first-line therapy for HER2-negative metastatic breast cancer (MERiDiAN): A double-blind placebo-controlled randomised phase III trial with prospective biomarker evaluation. Eur J Cancer. 2017;70:146–155. doi: 10.1016/j.ejca.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23(4):792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 26.Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O'Neill V, Rugo HS. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29(32):4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 27.Gligorov J, Doval D, Bines J, Alba E, Cortes P, Pierga JY, Gupta V, Costa R, Srock S, de Ducla S, Freudensprung U, Mustacchi G. Maintenance capecitabine and bevacizumab versus bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(12):1351–1360. doi: 10.1016/S1470-2045(14)70444-9. [DOI] [PubMed] [Google Scholar]
- 28.von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, Villanueva C, Romieu G, Lang I, Ciruelos E, De Laurentiis M, Veyret C, de Ducla S, Freudensprung U, Srock S, Gligorov J. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1269–1278. doi: 10.1016/S1470-2045(14)70439-5. [DOI] [PubMed] [Google Scholar]
- 29.Tredan O, Follana P, Moullet I, Cropet C, Trager-Maury S, Dauba J, Lavau-Denes S, Dieras V, Beal-Ardisson D, Gouttebel M, Orfeuvre H, Stefani L, Jouannaud C, Burki F, Petit T, Guardiola E, Becuwe C, Blot E, Pujade-Lauraine E, Bachelot T. A phase III trial of exemestane plus bevacizumab maintenance therapy in patients with metastatic breast cancer after first-line taxane and bevacizumab: a GINECO group study. Ann Oncol. 2016;27(6):1020–1029. doi: 10.1093/annonc/mdw077. [DOI] [PubMed] [Google Scholar]
- 30.Qin L, Bromberg-White JL, Qian CN. Opportunities and challenges in tumor angiogenesis research: back and forth between bench and bed. Adv Cancer Res. 2012;113:191–239. doi: 10.1016/B978-0-12-394280-7.00006-3. [DOI] [PubMed] [Google Scholar]
- 31.Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett. 2012;320(2):130–137. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Hong S, Tan M, Wang S, Luo S, Chen Y, Zhang L. Efficacy and safety of angiogenesis inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2015;141(5):909–921. doi: 10.1007/s00432-014-1862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Zhu S, Hong C, Cai H. Angiogenesis inhibitors for patients with ovarian cancer: a meta-analysis of 12 randomized controlled trials. Curr Med Res Opin. 2016;32(3):555–562. doi: 10.1185/03007995.2015.1131152. [DOI] [PubMed] [Google Scholar]
- 34.Lin Z, Zhang Q, Luo W. Angiogenesis inhibitors as therapeutic agents in cancer: Challenges and future directions. Eur J Pharmacol. 2016;793:76–81. doi: 10.1016/j.ejphar.2016.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Nandikolla AG, Rajdev L. Targeting angiogenesis in gastrointestinal tumors: current challenges. Transl Gastroenterol Hepatol. 2016;1:67. doi: 10.21037/tgh.2016.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagahashi M, Ramachandran S, Rashid OM, Takabe K. Lymphangiogenesis: a new player in cancer progression. World J Gastroenterol. 2010;16(32):4003–4012. doi: 10.3748/wjg.v16.i32.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J Oncol. 2012;2012:204946. doi: 10.1155/2012/204946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14(3):159–172. doi: 10.1038/nrc3677. [DOI] [PubMed] [Google Scholar]
- 39.Dieterich LC, Detmar M. Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev. 2016;99(Pt B):148–160. doi: 10.1016/j.addr.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimatsu Y, Miyazaki H, Watabe T. Roles of signaling and transcriptional networks in pathological lymphangiogenesis. Adv Drug Deliv Rev. 2016;99(Pt B):161–171. doi: 10.1016/j.addr.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis. 2010;13(1):1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao Z, Shang B, Zhang G, Miele L, Sarkar FH, Wang Z, Zhou Q. Tumor cell-mediated neovascularization and lymphangiogenesis contrive tumor progression and cancer metastasis. Biochim Biophys Acta. 2013;1836(2):273–286. doi: 10.1016/j.bbcan.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Margolin K, Gordon MS, Holmgren E, Gaudreault J, Novotny W, Fyfe G, Adelman D, Stalter S, Breed J. Phase Ib trial of intravenous recombinant humanized monoclonal antibody to vascular endothelial growth factor in combination with chemotherapy in patients with advanced cancer: pharmacologic and long-term safety data. J Clin Oncol. 2001;19(3):851–856. doi: 10.1200/JCO.2001.19.3.851. [DOI] [PubMed] [Google Scholar]
- 44.Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol. 2001;19(14):3312–3322. doi: 10.1200/JCO.2001.19.14.3312. [DOI] [PubMed] [Google Scholar]
- 45.Kim KJ, Li B, Houck K, Winer J, Ferrara N. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7(1):53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- 46.Arezumand R, Alibakhshi A, Ranjbari J, Ramazani A, Muyldermans S. Nanobodies As Novel Agents for Targeting Angiogenesis in Solid Cancers. Front Immunol. 2017;8:1746. doi: 10.3389/fimmu.2017.01746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Motzer RJ, Hutson TE, McCann L, Deen K, Choueiri TK. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med. 2014;370(18):1769–1770. doi: 10.1056/NEJMc1400731. [DOI] [PubMed] [Google Scholar]
- 48.Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6(9):734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 49.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 50.Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S. Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev. 2017 doi: 10.1002/med.21452. [DOI] [PubMed] [Google Scholar]
- 51.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF, 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 53.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 54.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 55.Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, Mazier MA, Canon JL, Georgoulias V, Peeters M, Bridgewater J, Cunningham D, First Bi. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol. 2009;20(11):1842–1847. doi: 10.1093/annonc/mdp233. [DOI] [PubMed] [Google Scholar]
- 56.Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest. 2013;123(8):3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zarrin B, Zarifi F, Vaseghi G, Javanmard SH. Acquired tumor resistance to antiangiogenic therapy: Mechanisms at a glance. J Res Med Sci. 2017;22:117. doi: 10.4103/jrms.JRMS_182_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15(3):167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 59.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Bock K, Mazzone M, Carmeliet P. Antiangiogenic therapy, hypoxia, and metastasis: risky liaisons, or not? Nat Rev Clin Oncol. 2011;8(7):393–404. doi: 10.1038/nrclinonc.2011.83. [DOI] [PubMed] [Google Scholar]
- 61.Giuliano S, Pages G. Mechanisms of resistance to anti-angiogenesis therapies. Biochimie. 2013;95(6):1110–1119. doi: 10.1016/j.biochi.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Johannessen TC, Wagner M, Straume O, Bjerkvig R, Eikesdal HP. Tumor vasculature: the Achilles' heel of cancer? Expert Opin Ther Targets. 2013;17(1):7–20. doi: 10.1517/14728222.2013.730522. [DOI] [PubMed] [Google Scholar]
- 63.Dufies M, Giuliano S, Ambrosetti D, Claren A, Ndiaye PD, Mastri M, Moghrabi W, Cooley LS, Ettaiche M, Chamorey E, Parola J, Vial V, Lupu-Plesu M, Bernhard JC, Ravaud A, Borchiellini D, Ferrero JM, Bikfalvi A, Ebos JM, Khabar KS, Grepin R, Pages G. Sunitinib Stimulates Expression of VEGFC by Tumor Cells and Promotes Lymphangiogenesis in Clear Cell Renal Cell Carcinomas. Cancer Res. 2017;77(5):1212–1226. doi: 10.1158/0008-5472.CAN-16-3088. [DOI] [PubMed] [Google Scholar]
- 64.Cao Y. Future options of anti-angiogenic cancer therapy. Chin J Cancer. 2016;35:21. doi: 10.1186/s40880-016-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70(2):93–102. doi: 10.1016/j.critrevonc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Wang D, Tan C, Xiao F, Zou L, Wang L, Wei Y, Yang H, Zhang W. The "inherent vice" in the anti-angiogenic theory may cause the highly metastatic cancer to spread more aggressively. Sci Rep. 2017;7(1):2365. doi: 10.1038/s41598-017-02534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol. 2006;59(1):15–26. doi: 10.1016/j.critrevonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 68.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 69.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ran S, Wilber A. Novel role of immature myeloid cells in formation of new lymphatic vessels associated with inflammation and tumors. J Leukoc Biol. 2017 doi: 10.1189/jlb.1MR1016-434RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hos D, Bukowiecki A, Horstmann J, Bock F, Bucher F, Heindl LM, Siebelmann S, Steven P, Dana R, Eming SA, Cursiefen C. Transient Ingrowth of Lymphatic Vessels into the Physiologically Avascular Cornea Regulates Corneal Edema and Transparency. Sci Rep. 2017;7(1):7227. doi: 10.1038/s41598-017-07806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hos D, Cursiefen C. Lymphatic vessels in the development of tissue and organ rejection. Adv Anat Embryol Cell Biol. 2014;214:119–141. doi: 10.1007/978-3-7091-1646-3_10. [DOI] [PubMed] [Google Scholar]
- 73.Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124(3):922–928. doi: 10.1172/JCI71606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ly CL, Kataru RP, Mehrara BJ. Inflammatory Manifestations of Lymphedema. Int J Mol Sci. 2017;18(1) doi: 10.3390/ijms18010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abouelkheir GR, Upchurch BD, Rutkowski JM. Lymphangiogenesis: fuel, smoke, or extinguisher of inflammation's fire? Exp Biol Med (Maywood) 2017;242(8):884–895. doi: 10.1177/1535370217697385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moldobaeva A, Jenkins J, Zhong Q, Wagner EM. Lymphangiogenesis in rat asthma model. Angiogenesis. 2017;20(1):73–84. doi: 10.1007/s10456-016-9529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang GJ, Ecoiffier T, Truong T, Yuen D, Li G, Lee N, Zhang L, Chen L. Intravital Imaging Reveals Dynamics of Lymphangiogenesis and Valvulogenesis. Sci Rep. 2016;6:19459. doi: 10.1038/srep19459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhong W, Gao X, Wang S, Han K, Ema M, Adams S, Adams RH, Rosenblatt MI, Chang JH, Azar DT. Prox1-GFP/Flt1-DsRed transgenic mice: an animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis. 2017 doi: 10.1007/s10456-017-9572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bock F, Onderka J, Braun G, Schneider AC, Hos D, Bi Y, Bachmann BO, Cursiefen C. Identification of Novel Endogenous Anti(lymph)angiogenic Factors in the Aqueous Humor. Invest Ophthalmol Vis Sci. 2016;57(15):6554–6560. doi: 10.1167/iovs.15-18526. [DOI] [PubMed] [Google Scholar]
- 80.Gurung HR, Carr MM, Carr DJ. Cornea lymphatics drive the CD8+ T-cell response to herpes simplex virus-1. Immunol Cell Biol. 2017;95(1):87–98. doi: 10.1038/icb.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2(8):573–583. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- 82.Leu AJ, Berk DA, Lymboussaki A, Alitalo K, Jain RK. Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res. 2000;60(16):4324–4327. [PubMed] [Google Scholar]
- 83.Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res. 2014;2(8):701–707. doi: 10.1158/2326-6066.CIR-14-0115. [DOI] [PubMed] [Google Scholar]
- 84.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62(5):1315–1320. [PubMed] [Google Scholar]
- 85.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161(3):947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201(7):1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109(3):1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18(9):1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 89.Wissmann C, Detmar M. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res. 2006;12(23):6865–6868. doi: 10.1158/1078-0432.CCR-06-1800. [DOI] [PubMed] [Google Scholar]
- 90.Preynat-Seauve O, Contassot E, Schuler P, Piguet V, French LE, Huard B. Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res. 2007;67(10):5009–5016. doi: 10.1158/0008-5472.CAN-06-4494. [DOI] [PubMed] [Google Scholar]
- 91.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1(3):191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 92.Pereira ER, Jones D, Jung K, Padera TP. The lymph node microenvironment and its role in the progression of metastatic cancer. Semin Cell Dev Biol. 2015;38:98–105. doi: 10.1016/j.semcdb.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Garcia-Caballero M, Paupert J, Blacher S, Van de Velde M, Quesada AR, Medina MA, Noel A. Targeting VEGFR-3/-2 signaling pathways with AD0157: a potential strategy against tumor-associated lymphangiogenesis and lymphatic metastases. J Hematol Oncol. 2017;10(1):122. doi: 10.1186/s13045-017-0484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shibata MA, Ambati J, Shibata E, Yoshidome K, Harada-Shiba M. Mammary cancer gene therapy targeting lymphangiogenesis: VEGF-C siRNA and soluble VEGF receptor-2, a splicing variant. Med Mol Morphol. 2012;45(4):179–184. doi: 10.1007/s00795-012-0576-5. [DOI] [PubMed] [Google Scholar]
- 95.Maehana S, Nakamura M, Ogawa F, Imai R, Murakami R, Kojima F, Majima M, Kitasato H. Suppression of lymphangiogenesis by soluble vascular endothelial growth factor receptor-2 in a mouse lung cancer model. Biomed Pharmacother. 2016;84:660–665. doi: 10.1016/j.biopha.2016.09.083. [DOI] [PubMed] [Google Scholar]
- 96.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17(11):1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 97.Niederkorn JY, Larkin DF. Immune privilege of corneal allografts. Ocul Immunol Inflamm. 2010;18(3):162–171. doi: 10.3109/09273948.2010.486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Naacke HG, Borderie VM, Bourcier T, Touzeau O, Moldovan M, Laroche L. Outcome of Corneal transplantation rejection. Cornea. 2001;20(4):350–353. doi: 10.1097/00003226-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 99.Cho YK, Shin EY, Uehara H, Ambati BK. Effect of sorafenib in a murine high risk penetrating keratoplasty model. Int J Ophthalmol. 2017;10(6):834–839. doi: 10.18240/ijo.2017.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stuht S, Gwinner W, Franz I, Schwarz A, Jonigk D, Kreipe H, Kerjaschki D, Haller H, Mengel M. Lymphatic neoangiogenesis in human renal allografts: results from sequential protocol biopsies. Am J Transplant. 2007;7(2):377–384. doi: 10.1111/j.1600-6143.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 101.Tsuchimoto A, Nakano T, Hasegawa S, Masutani K, Matsukuma Y, Eriguchi M, Nagata M, Nishiki T, Kitada H, Tanaka M, Kitazono T, Tsuruya K. The potential role of perivascular lymphatic vessels in preservation of kidney allograft function. Clin Exp Nephrol. 2017;21(4):721–731. doi: 10.1007/s10157-016-1338-9. [DOI] [PubMed] [Google Scholar]
- 102.Hamar P, Kerjaschki D. Blood capillary rarefaction and lymphatic capillary neoangiogenesis are key contributors to renal allograft fibrosis in an ACE inhibition rat model. Am J Physiol Heart Circ Physiol. 2016;311(4):H981–H990. doi: 10.1152/ajpheart.00320.2016. [DOI] [PubMed] [Google Scholar]
- 103.Phillips S, Kapp M, Crowe D, Garces J, Fogo AB, Giannico GA. Endothelial activation, lymphangiogenesis, and humoral rejection of kidney transplants. Hum Pathol. 2016;51:86–95. doi: 10.1016/j.humpath.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dayan JH, Ly CL, Kataru RP, Mehrara BJ. Lymphedema: Pathogenesis and Novel Therapies. Annu Rev Med. 2017 doi: 10.1146/annurev-med-060116-022900. [DOI] [PubMed] [Google Scholar]
- 105.Saito Y, Nakagami H, Kaneda Y, Morishita R. Lymphedema and therapeutic lymphangiogenesis. Biomed Res Int. 2013;2013:804675. doi: 10.1155/2013/804675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 107.Avraham T, Zampell JC, Yan A, Elhadad S, Weitman ES, Rockson SG, Bromberg J, Mehrara BJ. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27(3):1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Savetsky IL, Ghanta S, Gardenier JC, Torrisi JS, Garcia Nores GD, Hespe GE, Nitti MD, Kataru RP, Mehrara BJ. Th2 cytokines inhibit lymphangiogenesis. PLoS One. 2015;10(6):e0126908. doi: 10.1371/journal.pone.0126908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7(2):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 110.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72(3):161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hartiala P, Saarikko AM. Lymphangiogenesis and Lymphangiogenic Growth Factors. J Reconstr Microsurg. 2016;32(1):10–15. doi: 10.1055/s-0035-1544179. [DOI] [PubMed] [Google Scholar]
- 112.Choi I, Lee YS, Chung HK, Choi D, Ecoiffier T, Lee HN, Kim KE, Lee S, Park EK, Maeng YS, Kim NY, Ladner RD, Petasis NA, Koh CJ, Chen L, Lenz HJ, Hong YK. Interleukin-8 reduces post-surgical lymphedema formation by promoting lymphatic vessel regeneration. Angiogenesis. 2013;16(1):29–44. doi: 10.1007/s10456-012-9297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aprelikova O, Pajusola K, Partanen J, Armstrong E, Alitalo R, Bailey SK, McMahon J, Wasmuth J, Huebner K, Alitalo K. FLT4, a novel class III receptor tyrosine kinase in chromosome 5q33-qter. Cancer Res. 1992;52(3):746–748. [PubMed] [Google Scholar]
- 114.Davydova N, Harris NC, Roufail S, Paquet-Fifield S, Ishaq M, Streltsov VA, Williams SP, Karnezis T, Stacker SA, Achen MG. Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D. J Biol Chem. 2016;291(53):27265–27278. doi: 10.1074/jbc.M116.736801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bui HM, Enis D, Robciuc MR, Nurmi HJ, Cohen J, Chen M, Yang Y, Dhillon V, Johnson K, Zhang H, Kirkpatrick R, Traxler E, Anisimov A, Alitalo K, Kahn ML. Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest. 2016;126(6):2167–2180. doi: 10.1172/JCI83967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karnezis T, Shayan R, Fox S, Achen MG, Stacker SA. The connection between lymphangiogenic signalling and prostaglandin biology: a missing link in the metastatic pathway. Oncotarget. 2012;3(8):893–906. doi: 10.18632/oncotarget.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stacker SA, Achen MG. Emerging Roles for VEGF-D in Human Disease. Biomolecules. 2018;8(1) doi: 10.3390/biom8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113(7):1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115(9):2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Riabov V, Gudima A, Wang N, Mickley A, Orekhov A, Kzhyshkowska J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front Physiol. 2014;5:75. doi: 10.3389/fphys.2014.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alishekevitz D, Gingis-Velitski S, Kaidar-Person O, Gutter-Kapon L, Scherer SD, Raviv Z, Merquiol E, Ben-Nun Y, Miller V, Rachman-Tzemah C, Timaner M, Mumblat Y, Ilan N, Loven D, Hershkovitz D, Satchi-Fainaro R, Blum G, J PS, Vlodavsky I, Shaked Y. Macrophage-Induced Lymphangiogenesis and Metastasis following Paclitaxel Chemotherapy Is Regulated by VEGFR3. Cell Rep. 2016;17(5):1344–1356. doi: 10.1016/j.celrep.2016.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goyal S, Chauhan SK, El Annan J, Nallasamy N, Zhang Q, Dana R. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. 2010;128(7):819–824. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang H, Hu X, Tse J, Tilahun F, Qiu M, Chen L. Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol Vis Sci. 2011;52(1):334–338. doi: 10.1167/iovs.10-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312(5):549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 125.Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico MG, Terman BI, Alitalo K. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med. 1993;178(6):2077–2088. doi: 10.1084/jem.178.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nomura M, Yamagishi S, Harada S, Hayashi Y, Yamashima T, Yamashita J, Yamamoto H. Possible participation of autocrine and paracrine vascular endothelial growth factors in hypoxia-induced proliferation of endothelial cells and pericytes. J Biol Chem. 1995;270(47):28316–28324. doi: 10.1074/jbc.270.47.28316. [DOI] [PubMed] [Google Scholar]
- 127.Dikov MM, Ohm JE, Ray N, Tchekneva EE, Burlison J, Moghanaki D, Nadaf S, Carbone DP. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174(1):215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 128.Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001(112):re21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- 129.Murakami J, Ishii M, Suehiro F, Ishihata K, Nakamura N, Nishimura M. Vascular endothelial growth factor-C induces osteogenic differentiation of human mesenchymal stem cells through the ERK and RUNX2 pathway. Biochem Biophys Res Commun. 2017;484(3):710–718. doi: 10.1016/j.bbrc.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 130.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004;25(7):387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 131.Orlandini M, Spreafico A, Bardelli M, Rocchigiani M, Salameh A, Nucciotti S, Capperucci C, Frediani B, Oliviero S. Vascular endothelial growth factor-D activates VEGFR-3 expressed in osteoblasts inducing their differentiation. J Biol Chem. 2006;281(26):17961–17967. doi: 10.1074/jbc.M600413200. [DOI] [PubMed] [Google Scholar]
- 132.Cui Y, Liu K, Monzon-Medina ME, Padera RF, Wang H, George G, Toprak D, Abdelnour E, D'Agostino E, Goldberg HJ, Perrella MA, Forteza RM, Rosas IO, Visner G, El-Chemaly S. Therapeutic lymphangiogenesis ameliorates established acute lung allograft rejection. J Clin Invest. 2015;125(11):4255–4268. doi: 10.1172/JCI79693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Visuri MT, Honkonen KM, Hartiala P, Tervala TV, Halonen PJ, Junkkari H, Knuutinen N, Yla-Herttuala S, Alitalo KK, Saarikko AM. VEGF-C and VEGF-C156S in the pro-lymphangiogenic growth factor therapy of lymphedema: a large animal study. Angiogenesis. 2015;18(3):313–326. doi: 10.1007/s10456-015-9469-2. [DOI] [PubMed] [Google Scholar]
- 134.Wang XL, Zhao J, Qin L, Cao JL. VEGFR-3 blocking deteriorates inflammation with impaired lymphatic function and different changes in lymphatic vessels in acute and chronic colitis. Am J Transl Res. 2016;8(2):827–841. [PMC free article] [PubMed] [Google Scholar]
- 135.Dieterich LC, Ducoli L, Shin JW, Detmar M. Distinct transcriptional responses of lymphatic endothelial cells to VEGFR-3 and VEGFR-2 stimulation. Sci Data. 2017;4:170106. doi: 10.1038/sdata.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mertlitz S, Shi Y, Kalupa M, Grotzinger C, Mengwasser J, Riesner K, Cordes S, Elezkurtaj S, Penack O. Lymphangiogenesis is a feature of acute GVHD, and VEGFR-3 inhibition protects against experimental GVHD. Blood. 2017;129(13):1865–1875. doi: 10.1182/blood-2016-08-734210. [DOI] [PubMed] [Google Scholar]
- 137.Dietrich T, Bock F, Yuen D, Hos D, Bachmann BO, Zahn G, Wiegand S, Chen L, Cursiefen C. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010;184(2):535–539. doi: 10.4049/jimmunol.0903180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goyal S, Chauhan SK, Dana R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. 2012;130(1):84–89. doi: 10.1001/archophthalmol.2011.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee HS, Hos D, Blanco T, Bock F, Reyes NJ, Mathew R, Cursiefen C, Dana R, Saban DR. Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease. Invest Ophthalmol Vis Sci. 2015;56(5):3140–3148. doi: 10.1167/iovs.14-16186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Karaman S, Hollmen M, Robciuc MR, Alitalo A, Nurmi H, Morf B, Buschle D, Alkan HF, Ochsenbein AM, Alitalo K, Wolfrum C, Detmar M. Blockade of VEGF-C and VEGF-D modulates adipose tissue inflammation and improves metabolic parameters under high-fat diet. Mol Metab. 2015;4(2):93–105. doi: 10.1016/j.molmet.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alitalo AK, Proulx ST, Karaman S, Aebischer D, Martino S, Jost M, Schneider N, Bry M, Detmar M. VEGF-C and VEGF-D blockade inhibits inflammatory skin carcinogenesis. Cancer Res. 2013;73(14):4212–4221. doi: 10.1158/0008-5472.CAN-12-4539. [DOI] [PubMed] [Google Scholar]
- 142.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15(2):427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 143.Al-Husseini A, Kraskauskas D, Mezzaroma E, Nordio A, Farkas D, Drake JI, Abbate A, Felty Q, Voelkel NF. Vascular endothelial growth factor receptor 3 signaling contributes to angioobliterative pulmonary hypertension. Pulm Circ. 2015;5(1):101–116. doi: 10.1086/679704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, Farina GA, Stifano G, Mathes AL, Cossu M, York M, Collins C, Wenink M, Huijbens R, Hesselstrand R, Saxne T, DiMarzio M, Wuttge D, Agarwal SK, Reveille JD, Assassi S, Mayes M, Deng Y, Drenth JP, de Graaf J, den Heijer M, Kallenberg CG, Bijl M, Loof A, van den Berg WB, Joosten LA, Smith V, de Keyser F, Scorza R, Lunardi C, van Riel PL, Vonk M, van Heerde W, Meller S, Homey B, Beretta L, Roest M, Trojanowska M, Lafyatis R, Radstake TR. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med. 2014;370(5):433–443. doi: 10.1056/NEJMoa1114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Suzuki S, Yoshihisa A, Yokokawa T, Misaka T, Sakamoto N, Sugimoto K, Yamaki T, Kunii H, Nakazato K, Saitoh S, Takeishi Y. Association between levels of anti-angiogenic isoform of vascular endothelial growth factor A and pulmonary hypertension. Int J Cardiol. 2016;222:416–420. doi: 10.1016/j.ijcard.2016.07.277. [DOI] [PubMed] [Google Scholar]
- 146.Jurisic G, Sundberg JP, Detmar M. Blockade of VEGF receptor-3 aggravates inflammatory bowel disease and lymphatic vessel enlargement. Inflamm Bowel Dis. 2013;19(9):1983–1989. doi: 10.1097/MIB.0b013e31829292f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jurisic G, Sundberg JP, Bleich A, Leiter EH, Broman KW, Buechler G, Alley L, Vestweber D, Detmar M. Quantitative lymphatic vessel trait analysis suggests Vcam1 as candidate modifier gene of inflammatory bowel disease. Genes Immun. 2010;11(3):219–231. doi: 10.1038/gene.2010.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rahier JF, De Beauce S, Dubuquoy L, Erdual E, Colombel JF, Jouret-Mourin A, Geboes K, Desreumaux P. Increased lymphatic vessel density and lymphangiogenesis in inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34(5):533–543. doi: 10.1111/j.1365-2036.2011.04759.x. [DOI] [PubMed] [Google Scholar]
- 149.Guo R, Zhou Q, Proulx ST, Wood R, Ji RC, Ritchlin CT, Pytowski B, Zhu Z, Wang YJ, Schwarz EM, Xing L. Inhibition of lymphangiogenesis and lymphatic drainage via vascular endothelial growth factor receptor 3 blockade increases the severity of inflammation in a mouse model of chronic inflammatory arthritis. Arthritis Rheum. 2009;60(9):2666–2676. doi: 10.1002/art.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bouta EM, Li J, Ju Y, Brown EB, Ritchlin CT, Xing L, Schwarz EM. The role of the lymphatic system in inflammatory-erosive arthritis. Semin Cell Dev Biol. 2015;38:90–97. doi: 10.1016/j.semcdb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker SA, Yla-Herttuala S, Jackson DG, Alitalo K, McDonald DM. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115(2):247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fankhauser M, Broggi MAS, Potin L, Bordry N, Jeanbart L, Lund AW, Da Costa E, Hauert S, Rincon-Restrepo M, Tremblay C, Cabello E, Homicsko K, Michielin O, Hanahan D, Speiser DE, Swartz MA. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med. 2017;9(407) doi: 10.1126/scitranslmed.aal4712. [DOI] [PubMed] [Google Scholar]
- 153.Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest. 2014;124(3):936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest. 2014;124(3):943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S, Nakamura M, Andersson P, Wang J, Sun Y, Dissing S, He X, Yang X, Cao Y. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun. 2014;5:4944. doi: 10.1038/ncomms5944. [DOI] [PubMed] [Google Scholar]
- 156.Kamath S, Buolamwini JK. Targeting EGFR and HER-2 receptor tyrosine kinases for cancer drug discovery and development. Med Res Rev. 2006;26(5):569–594. doi: 10.1002/med.20070. [DOI] [PubMed] [Google Scholar]
- 157.Saxena R, Dwivedi A. ErbB family receptor inhibitors as therapeutic agents in breast cancer: current status and future clinical perspective. Med Res Rev. 2012;32(1):166–215. doi: 10.1002/med.20209. [DOI] [PubMed] [Google Scholar]
- 158.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7(7):505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 159.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, Pinto A, Normanno N. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214(3):559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 160.Burgess AW. EGFR family: structure physiology signalling and therapeutic targets. Growth Factors. 2008;26(5):263–274. doi: 10.1080/08977190802312844. [DOI] [PubMed] [Google Scholar]
- 161.Han W, Lo HW. Landscape of EGFR signaling network in human cancers: biology and therapeutic response in relation to receptor subcellular locations. Cancer Lett. 2012;318(2):124–134. doi: 10.1016/j.canlet.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Minder P, Zajac E, Quigley JP, Deryugina EI. EGFR regulates the development and microarchitecture of intratumoral angiogenic vasculature capable of sustaining cancer cell intravasation. Neoplasia. 2015;17(8):634–649. doi: 10.1016/j.neo.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yu P, Ye L, Wang H, Du G, Zhang J, Zuo Y, Zhang J, Tian J. NSK-01105, a novel sorafenib derivative, inhibits human prostate tumor growth via suppression of VEGFR2/EGFR-mediated angiogenesis. PLoS One. 2014;9(12):e115041. doi: 10.1371/journal.pone.0115041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, Janne P, Kobayashi S, Halmos B, Tenen D, Tang XM, Engelman J, Yeap B, Folkman J, Johnson BE, Heymach JV. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15(10):3484–3494. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li H, Takayama K, Wang S, Shiraishi Y, Gotanda K, Harada T, Furuyama K, Iwama E, Ieiri I, Okamoto I, Nakanishi Y. Addition of bevacizumab enhances antitumor activity of erlotinib against non-small cell lung cancer xenografts depending on VEGF expression. Cancer Chemother Pharmacol. 2014;74(6):1297–1305. doi: 10.1007/s00280-014-2610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Baker CH, Kedar D, McCarty MF, Tsan R, Weber KL, Bucana CD, Fidler IJ. Blockade of epidermal growth factor receptor signaling on tumor cells and tumor-associated endothelial cells for therapy of human carcinomas. Am J Pathol. 2002;161(3):929–938. doi: 10.1016/S0002-9440(10)64253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66(4):2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 168.Kuwai T, Nakamura T, Sasaki T, Kim SJ, Fan D, Villares GJ, Zigler M, Wang H, Bar-Eli M, Kerbel RS, Fidler IJ. Phosphorylated epidermal growth factor receptor on tumor-associated endothelial cells is a primary target for therapy with tyrosine kinase inhibitors. Neoplasia. 2008;10(5):489–500. doi: 10.1593/neo.08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci U S A. 2009;106(10):3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kawamoto T, Ohga N, Akiyama K, Hirata N, Kitahara S, Maishi N, Osawa T, Yamamoto K, Kondoh M, Shindoh M, Hida Y, Hida K. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One. 2012;7(3):e34045. doi: 10.1371/journal.pone.0034045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabarbara P, Seymour L, National Cancer Institute of Canada Clinical Trials G Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 172.Cripps C, Winquist E, Devries MC, Stys-Norman D, Gilbert R, Head, Neck Cancer Disease Site G Epidermal growth factor receptor targeted therapy in stages III and IV head and neck cancer. Curr Oncol. 2010;17(3):37–48. doi: 10.3747/co.v17i3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;10(11):760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baba Y, Fujii M, Tokumaru Y, Kato Y. Present and Future of EGFR Inhibitors for Head and Neck Squamous Cell Cancer. J Oncol. 2012;2012:986725. doi: 10.1155/2012/986725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chong CR, Janne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19(11):1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Marino D, Angehrn Y, Klein S, Riccardi S, Baenziger-Tobler N, Otto VI, Pittelkow M, Detmar M. Activation of the epidermal growth factor receptor promotes lymphangiogenesis in the skin. J Dermatol Sci. 2013;71(3):184–194. doi: 10.1016/j.jdermsci.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mezyk-Kopec R, Wyroba B, Stalinska K, Prochnicki T, Wiatrowska K, Kilarski WW, Swartz MA, Bereta J. ADAM17 Promotes Motility, Invasion, and Sprouting of Lymphatic Endothelial Cells. PLoS One. 2015;10(7):e0132661. doi: 10.1371/journal.pone.0132661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sarkis SA, Abdullah BH, Abdul Majeed BA, Talabani NG. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in oral squamous cell carcinoma in relation to proliferation, apoptosis, angiogenesis and lymphangiogenesis. Head Neck Oncol. 2010;2:13. doi: 10.1186/1758-3284-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rebhun RB, Langley RR, Yokoi K, Fan D, Gershenwald JE, Fidler IJ. Targeting receptor tyrosine kinase on lymphatic endothelial cells for the therapy of colon cancer lymph node metastasis. Neoplasia. 2006;8(9):747–757. doi: 10.1593/neo.06322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lesma E, Chiaramonte E, Ancona S, Orpianesi E, Di Giulio AM, Gorio A. Anti-EGFR antibody reduces lung nodules by inhibition of EGFR-pathway in a model of lymphangioleiomyomatosis. Biomed Res Int. 2015;2015:315240. doi: 10.1155/2015/315240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Davis JM, Hyjek E, Husain AN, Shen L, Jones J, Schuger LA. Lymphatic endothelial differentiation in pulmonary lymphangioleiomyomatosis cells. J Histochem Cytochem. 2013;61(8):580–590. doi: 10.1369/0022155413489311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Henske EP, McCormack FX. Lymphangioleiomyomatosis - a wolf in sheep's clothing. J Clin Invest. 2012;122(11):3807–3816. doi: 10.1172/JCI58709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bracher A, Cardona AS, Tauber S, Fink AM, Steiner A, Pehamberger H, Niederleithner H, Petzelbauer P, Groger M, Loewe R. Epidermal growth factor facilitates melanoma lymph node metastasis by influencing tumor lymphangiogenesis. J Invest Dermatol. 2013;133(1):230–238. doi: 10.1038/jid.2012.272. [DOI] [PubMed] [Google Scholar]
- 184.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;8(12):929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 185.Blobel CP. ADAMs: key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 186.Gore J, Imasuen-Williams IE, Conteh AM, Craven KE, Cheng M, Korc M. Combined targeting of TGF-beta, EGFR and HER2 suppresses lymphangiogenesis and metastasis in a pancreatic cancer model. Cancer Lett. 2016;379(1):143–153. doi: 10.1016/j.canlet.2016.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 188.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99(13):8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101(32):11658–11663. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu J, Dugas-Ford J, Chang M, Purta P, Han KY, Hong YK, Dickinson ME, Rosenblatt MI, Chang JH, Azar DT. Simultaneous in vivo imaging of blood and lymphatic vessel growth in Prox1-GFP/Flk1::myr-mCherry mice. FEBS J. 2015;282(8):1458–1467. doi: 10.1111/febs.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gurung HR, Carr MM, Bryant K, Chucair-Elliott AJ, Carr DJ. Fibroblast growth factor-2 drives and maintains progressive corneal neovascularization following HSV-1 infection. Mucosal Immunol. 2017 doi: 10.1038/mi.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cao R, Ji H, Feng N, Zhang Y, Yang X, Andersson P, Sun Y, Tritsaris K, Hansen AJ, Dissing S, Cao Y. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc Natl Acad Sci U S A. 2012;109(39):15894–15899. doi: 10.1073/pnas.1208324109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Platonova N, Miquel G, Regenfuss B, Taouji S, Cursiefen C, Chevet E, Bikfalvi A. Evidence for the interaction of fibroblast growth factor-2 with the lymphatic endothelial cell marker LYVE-1. Blood. 2013;121(7):1229–1237. doi: 10.1182/blood-2012-08-450502. [DOI] [PubMed] [Google Scholar]
- 194.Jin K, LaFevre-Bernt M, Sun Y, Chen S, Gafni J, Crippen D, Logvinova A, Ross CA, Greenberg DA, Ellerby LM. FGF-2 promotes neurogenesis and neuroprotection and prolongs survival in a transgenic mouse model of Huntington's disease. Proc Natl Acad Sci U S A. 2005;102(50):18189–18194. doi: 10.1073/pnas.0506375102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. Oncologist. 2015;20(6):660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Heldin CH, Westermark B. Platelet-derived growth factor: mechanism of action and possible in vivo function. Cell Regul. 1990;1(8):555–566. doi: 10.1091/mbc.1.8.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6(4):333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 198.Nissen LJ, Cao R, Hedlund EM, Wang Z, Zhao X, Wetterskog D, Funa K, Brakenhielm E, Cao Y. Angiogenic factors FGF2 and PDGF-BB synergistically promote murine tumor neovascularization and metastasis. J Clin Invest. 2007;117(10):2766–2777. doi: 10.1172/JCI32479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hsu CW, Poche RA, Saik JE, Ali S, Wang S, Yosef N, Calderon GA, Scott L, Jr, Vadakkan TJ, Larina IV, West JL, Dickinson ME. Improved Angiogenesis in Response to Localized Delivery of Macrophage-Recruiting Molecules. PLoS One. 2015;10(7):e0131643. doi: 10.1371/journal.pone.0131643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu C, Zhang Y, Lim S, Hosaka K, Yang Y, Pavlova T, Alkasalias T, Hartman J, Jensen L, Xing X, Wang X, Lu Y, Nie G, Cao Y. A Zebrafish Model Discovers a Novel Mechanism of Stromal Fibroblast-Mediated Cancer Metastasis. Clin Cancer Res. 2017;23(16):4769–4779. doi: 10.1158/1078-0432.CCR-17-0101. [DOI] [PubMed] [Google Scholar]
- 201.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017 doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 202.Yang P, Chen N, Yang D, Crane J, Huang B, Dong R, Yi X, Guo J, Cai J, Wang Z. Cervical cancer cell-derived angiopoietins promote tumor progression. Tumour Biol. 2017;39(7):1010428317711658. doi: 10.1177/1010428317711658. [DOI] [PubMed] [Google Scholar]
- 203.Zhang L, Li G, Sessa R, Kang GJ, Shi M, Ge S, Gong AJ, Wen Y, Chintharlapalli S, Chen L. Angiopoietin-2 Blockade Promotes Survival of Corneal Transplants. Invest Ophthalmol Vis Sci. 2017;58(1):79–86. doi: 10.1167/iovs.16-20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wong BW, Wang X, Zecchin A, Thienpont B, Cornelissen I, Kalucka J, Garcia-Caballero M, Missiaen R, Huang H, Bruning U, Blacher S, Vinckier S, Goveia J, Knobloch M, Zhao H, Dierkes C, Shi C, Hagerling R, Moral-Darde V, Wyns S, Lippens M, Jessberger S, Fendt SM, Luttun A, Noel A, Kiefer F, Ghesquiere B, Moons L, Schoonjans L, Dewerchin M, Eelen G, Lambrechts D, Carmeliet P. The role of fatty acid beta-oxidation in lymphangiogenesis. Nature. 2017;542(7639):49–54. doi: 10.1038/nature21028. [DOI] [PubMed] [Google Scholar]
- 205.Garcia-Caballero M, Blacher S, Paupert J, Quesada AR, Medina MA, Noel A. Novel application assigned to toluquinol: inhibition of lymphangiogenesis by interfering with VEGF-C/VEGFR-3 signalling pathway. Br J Pharmacol. 2016;173(12):1966–1987. doi: 10.1111/bph.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hu J, Ye H, Fu A, Chen X, Wang Y, Chen X, Ye X, Xiao W, Duan X, Wei Y, Chen L. Deguelin--an inhibitor to tumor lymphangiogenesis and lymphatic metastasis by downregulation of vascular endothelial cell growth factor-D in lung tumor model. Int J Cancer. 2010;127(10):2455–2466. doi: 10.1002/ijc.25253. [DOI] [PubMed] [Google Scholar]
- 207.Dong XP, Xiao TH, Dong H, Jiang N, Zhao XG. Endostar combined with cisplatin inhibits tumor growth and lymphatic metastasis of lewis lung carcinoma xenografts in mice. Asian Pac J Cancer Prev. 2013;14(5):3079–3083. doi: 10.7314/apjcp.2013.14.5.3079. [DOI] [PubMed] [Google Scholar]
- 208.Wen J, Fu AF, Chen LJ, Xie XJ, Yang GL, Chen XC, Wang YS, Li J, Chen P, Tang MH, Shao XM, Lu Y, Zhao X, Wei YQ. Liposomal honokiol inhibits VEGF-D-induced lymphangiogenesis and metastasis in xenograft tumor model. Int J Cancer. 2009;124(11):2709–2718. doi: 10.1002/ijc.24244. [DOI] [PubMed] [Google Scholar]
- 209.Yashiro M, Shinto O, Nakamura K, Tendo M, Matsuoka T, Matsuzaki T, Kaizaki R, Ohira M, Miwa A, Hirakawa K. Effects of VEGFR-3 phosphorylation inhibitor on lymph node metastasis in an orthotopic diffuse-type gastric carcinoma model. Br J Cancer. 2009;101(7):1100–1106. doi: 10.1038/sj.bjc.6605296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M, Miyazono K. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67(21):10181–10189. doi: 10.1158/0008-5472.CAN-07-2366. [DOI] [PubMed] [Google Scholar]
- 211.Nakamura ES, Koizumi K, Kobayashi M, Saiki I. Inhibition of lymphangiogenesis-related properties of murine lymphatic endothelial cells and lymph node metastasis of lung cancer by the matrix metalloproteinase inhibitor MMI270. Cancer Sci. 2004;95(1):25–31. doi: 10.1111/j.1349-7006.2004.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Matsumoto G, Yajima N, Saito H, Nakagami H, Omi Y, Lee U, Kaneda Y. Cold shock domain protein A (CSDA) overexpression inhibits tumor growth and lymph node metastasis in a mouse model of squamous cell carcinoma. Clin Exp Metastasis. 2010;27(7):539–547. doi: 10.1007/s10585-010-9343-y. [DOI] [PubMed] [Google Scholar]
- 213.Li XP, Jing W, Sun JJ, Liu ZY, Zhang JT, Sun W, Zhu W, Fan YZ. A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2-3 "multi-points priming" mechanisms in vitro and in vivo. BMC Cancer. 2015;15:527. doi: 10.1186/s12885-015-1521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang Y, Gao Z, Ma Y, Teng H, Liu Z, Wei H, Lu Y, Cheng X, Hou L, Zou X. Fucoidan inhibits lymphangiogenesis by downregulating the expression of VEGFR3 and PROX1 in human lymphatic endothelial cells. Oncotarget. 2016;7(25):38025–38035. doi: 10.18632/oncotarget.9443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Saif MW, Knost JA, Chiorean EG, Kambhampati SR, Yu D, Pytowski B, Qin A, Kauh JS, O'Neil BH. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother Pharmacol. 2016;78(4):815–824. doi: 10.1007/s00280-016-3134-3. [DOI] [PubMed] [Google Scholar]
- 216.He XW, Liu T, Chen YX, Cheng DJ, Li XR, Xiao Y, Feng YL. Calcium carbonate nanoparticle delivering vascular endothelial growth factor-C siRNA effectively inhibits lymphangiogenesis and growth of gastric cancer in vivo. Cancer Gene Ther. 2008;15(3):193–202. doi: 10.1038/sj.cgt.7701122. [DOI] [PubMed] [Google Scholar]
- 217.Li T, Wang GD, Tan YZ, Wang HJ. Inhibition of lymphangiogenesis of endothelial progenitor cells with VEGFR-3 siRNA delivered with PEI-alginate nanoparticles. Int J Biol Sci. 2014;10(2):160–170. doi: 10.7150/ijbs.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cho YK, Uehara H, Young JR, Tyagi P, Kompella UB, Zhang X, Luo L, Singh N, Archer B, Ambati BK. Flt23k nanoparticles offer additive benefit in graft survival and anti-angiogenic effects when combined with triamcinolone. Invest Ophthalmol Vis Sci. 2012;53(4):2328–2336. doi: 10.1167/iovs.11-8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kobayashi H, Kawamoto S, Brechbiel MW, Bernardo M, Sato N, Waldmann TA, Tagaya Y, Choyke PL. Detection of lymph node involvement in hematologic malignancies using micromagnetic resonance lymphangiography with a gadolinum-labeled dendrimer nanoparticle. Neoplasia. 2005;7(11):984–991. doi: 10.1593/neo.05454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yang H, Zou LG, Zhang S, Gong MF, Zhang D, Qi YY, Zhou SW, Diao XW. Feasibility of MR imaging in evaluating breast cancer lymphangiogenesis using Polyethylene glycol-GoldMag nanoparticles. Clin Radiol. 2013;68(12):1233–1240. doi: 10.1016/j.crad.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 221.Harisinghani MG, Dixon WT, Saksena MA, Brachtel E, Blezek DJ, Dhawale PJ, Torabi M, Hahn PF. MR lymphangiography: imaging strategies to optimize the imaging of lymph nodes with ferumoxtran-10. Radiographics. 2004;24(3):867–878. doi: 10.1148/rg.243035190. [DOI] [PubMed] [Google Scholar]