Abstract
Through unbiased transcriptomics and multiple molecular tools, transient downregulation of the Orthodenticle homeobox 2 (OTX2) gene was recently causatively associated with the development of depressive-like behaviors in a mouse model of early life stress. The analyses presented in this manuscript test the translational applicability of these findings by examining peripheral markers of methylation of OTX2 and OTX2-regulated genes in relation to measures of depression and resting-state functional connectivity data collected as part of a larger study examining risk and resilience in maltreated children. The sample included 157 children between the ages of 8 and 15 years (χ = 11.4, SD = 1.9). DNA specimens were derived from saliva samples and processed using the Illumina 450 K beadchip. A subset of children (N = 47) with DNA specimens also had resting-state functional MRI data. After controlling for demographic factors, cell heterogeneity, and three principal components, maltreatment history and methylation in OTX2 significantly predicted depression in the children. In terms of the imaging data, increased OTX2 methylation was found to be associated with increased functional connectivity between the right vmPFC and bilateral regions of the medial frontal cortex and the cingulate, including the subcallosal gyrus, frontal pole, and paracingulate gyrus—key structures implicated in depression. Mouse models of early stress hold significant promise in helping to unravel the mechanisms by which child adversity confers risk for psychopathology, with data presented in this manuscript supporting a potential role for OTX2 and OTX2-related (e.g., WNT1, PAX6) genes in the pathophysiology of stress-related depressive disorders in children.
Introduction
Using unbiased transcriptomics, transient downregulation of the Orthodenticle homeobox 2 (Otx2) gene in the ventral tegmental area (VTA), a key reward circuit brain region, was identified as a key molecule in the development of depressive-like behaviors in a mouse model of early life stress [1]. The transient downregulation of Otx2 following early life stress was also associated with persistent downregulation of several target genes, many of which are involved in brain development. In this model, animals subjected to early life stress were found to exhibit exaggerated “depressive-like” behaviors after exposure to a second hit of stress in adulthood. The putative role of the transient downregulation of Otx2 following early life stress in promoting enhanced sensitivity to subsequent stress was demonstrated by utilizing a Herpes simplex virus vector to transiently rescue Otx2 levels immediately after early life stress. This prevented later susceptibility to stress, as did Otx2 overexpression in adult animals. Conversely, a transient, local knockdown of Otx2 in VTA in early life was found to mimic the effects of early life stress [1].
Otx2 is a highly conserved molecule. The two protein isoforms Otx2 encodes are identical in both mice and humans, and functionally indistinguishable in vitro [2]. Otx2 has multiple known roles in embryonic and experience-dependent brain development. Specifically, Otx2 regulates genetic networks directing the specification of dopaminergic and serotonergic neurons during embryonic development [3], and in the adult brain, Otx2 regulates neurogenesis of dopamine neurons in the VTA [4–6]. Otx2 is also a key protein in mediating experience-dependent plasticity and defining periods of sensitive development in the visual and auditory cortices [7–9]. Although the OTX2 protein is found in the VTA, medial prefrontal cortex (mPFC), and multiple additional cortical regions [10], a similar essential role for Otx2 in defining sensitive windows of development of other neural systems has not yet been established [8]. Given the role of OTX2 in defining sensitive windows of development, OTX2 has been proposed to be a potential “master-regulator” of plasticity, with likely important targets for treating stress-related psychiatric disorders [2].
To test the translational applicability of the mouse model findings and the potential utility of OTX2 as a biomarker of stress-related psychopathology, in this paper we examine peripheral markers of OTX2 gene methylation to determine if OTX2 gene methylation values are associated with individual differences on measures of depression in children, and individual differences in connectivity in reward circuit-related regions where OTX2 is expressed (e.g., VTA, mPFC). The relationship between OTX2 gene methylation, measures of depression, and methylation in genes downregulated by OTX2 are also explored.
Methods and materials
Subjects
The children in this study were participants in a larger study examining risk and resilience in maltreated children. The sample included 157 children from 117 families. In the year prior to study enrolment, 34% of children had an out-of-home placement due to recent reports of abuse or neglect. Another 18% of children had prior allegations of maltreatment, but were not removed, as it was determined that they could be safely maintained at home, and 48% of children were never referred to protective services. The children were between the ages of 8 and 15 years of age (χ = 11.4, SD = 1.9), with the clinical and demographic characteristics of the sample depicted in Table 1.
Table 1.
Demographic and clinical characteristics of the sample (N = 157)
| History of maltreatment and recent placement N = 54 | Referral to protective services, no removal N = 29 | No history of abuse or neglect N = 74 | Statistic p-value | |
|---|---|---|---|---|
| Age | 11.9 ± 2.0 | 11.1 ± 1.8 | 11.2 ± 1.9 | F (2) = 2.33 p = ns |
| Sex (%female/%male) | 59%/41% | 64%/36% | 54%/46% |
χ2 = 0.9 p = ns |
| Race (EA/AA or Biracial) | 90%/10% | 96%/4% | 85%/15% |
χ2 = 2.5 p = ns |
| Y-VACS Intra-familial Adversity Score | 27.5 ± 7.9a | 21.2 ± 8.3b | 9.2 ± 7.4c | Wald χ2 = 181.5 p < .0001 |
| Y-VACS Extra-familial Adversity Score | 6.0 ± 3.6 | 5.8 ± 3.3 | 5.4 ± 3.7 | Wald χ2 = 0.87 p = ns |
| Y-VACS Total Adversity Score | 33.5 ± 8.7a | 26.9 ± 9.7b | 14.7 ± 9.2c | Wald χ2 = 140.6 p < .0001 |
| Mood and Feelings Depression Questionnaire | 15.1 ± 12.0a | 12.8 ± 9.8a, b | 8.7 ± 7.2b | Wald χ2 = 17.6 p = .001 |
| Clinical Threshold MFQ Scores | 9 (17%) | 4 (14%) | 2 (2.7%) |
χ2 = 6.57+ p < .04 |
Means with different superscripts are statistically different from one another, Student–Newman–Keuls test
EA European American, AA African American, Y-VACS Yale–Vermont Adverse Childhood Experiences Scale, MFQ Mood and Feelings Questionnaire
+Likelihood ratio used to test the significance due to number of cells with fewer than five counts
Procedure
Assessments from parents were collected during home visits, and measures from children were collected both during home visits and during a 1-week day camp program devised specifically for our research purposes, replicating a research methodology used in prior studies [11–13]. The University of Vermont, Johns Hopkins, and Yale University Institutional Review Boards (IRB) approved the conduct of this research, with the State of Vermont Department of Children and Family Services allowing for participation of children in their custody based on the University of Vermont IRB's approval. Non-maltreated children were recruited from the community through newspaper ads and included based on parent report of an absence of significant childhood adversities, which was verified via queries of state child protective services reports of maltreatment and administration of standardized trauma screening questionnaires [14, 15]. Prior to the recruitment of maltreated children, an independent child advocate reviewed each case referred through protective services to determine that research participation was in the child’s best interest. The child’s parent or legal guardian provided informed consent, and each child provided assent for study participation. Birth parent assent for children in state custody was obtained when clinically appropriate (e.g., ongoing parent–child contact).
Childhood adversity and maltreatment experiences
Extending methods previously published by our team [16], multiple informants and data sources (e.g., parents, children, and protective services case records) were used to obtain a best estimate of each child’s adverse childhood experiences [14–16], with the Yale–Vermont Adverse Childhood Experiences Scale (Y-VACS) clinician rating used to integrate the data from these various sources to create dimensional ratings of children’s adverse life experiences [17]. The Y-VACS rates 20 types of adverse experiences, both intrafamilial (e.g., multiple forms of abuse, neglect, loss, parental incarceration, etc.) and extrafamilial (e.g., natural disasters, bullying, community violence, etc.) experiences. Each adversity receives both a frequency and severity rating. Scores generated with Y-VACS have high inter-rater reliability and strong discriminant and convergent validity [17]. In terms of maltreatment experiences, among children with a referral to protective services and no history of out-of-home placement, 58% had a history of neglect, 39% had a history of physical abuse, 19% had a history of sexual abuse, and 45% witnessed domestic violence. Among children with recent removal, 65% had a history of neglect, 67% had a history of physical abuse, 50% had a history of sexual abuse, and 70% witnessed domestic violence. A significant number of children experienced multiple adversities throughout their childhoods. The Y-VACS scores of the cohort are included in Table 1. As children with and without prior protective services involvement did not differ on the extrafamilial adversity scale, children’s scores on the intrafamilial adversity scale of Y-VACS were used in subsequent analyses with methylation data to predict children’s depression scores.
Depression
The Mood and Feelings Questionnaire (MFQ) [18], which is a 33-item self-report measure with excellent psychometric properties, was used to assess children’s depressive symptomatology. Children’s scores on the MFQ ranged from 0 to 51, with 12% of children above the clinical threshold, suggesting a likely major depression diagnosis (Table 1).
DNA specimens
DNA was extracted from the saliva samples collected while the children attended the research summer day camp program using Oragene kits, with researchers wearing protective gloves to prevent specimen contamination. Genomic DNA was extracted from the saliva using the Oragene DNA Extraction Kit (DNA Genotek, Ottawa, Ontario, Canada). To prepare specimens for methylation analysis, 500 ng of genomic DNA was treated with bisulfite reagents included in the EZ-96 DNA methylation kit (Zymo Research, Orange, CA, USA), according to the manufacturer’s protocol. Bisulfite-converted DNA samples were used in the array-based genome-wide DNA methylation assay.
Array-based genome-wide DNA methylation
Illumina 450 K Methylation BeadChip interrogates > 450,000 methylation sites per sample at single-nucleotide resolution. The methylation analyses were completed at the Keck Biotechnology Resource Laboratory at Yale University. GenomeStudio software (Illumina, San Diego, CA) was used to generate β values for each CpG site, with β values ranging from 0.0 to 1.0. Raw scanned data were normalized and the average β values were recalculated using background intensity measured by negative background probes present on the array. Standard quality control tests and functional normalization were conducted using the “Preprocessfunnorm” function in the R minfi package [19]. CpG sites with detection p > .001 were removed to ensure that only high-confidence probes were included in subsequent analysis (2704 of 485,512 probes were removed, 0.56% of sites).
OTX2 CpG sites and OTX2-regulated genes CpG sites
We examined DNA methylation of OTX2 and six genes regulated by OTX2 and early life stress in the mouse model. The Illumina 450K chip contains 31 CpG sites in the OTX2 gene. The majority are in the promotor area, eight transcription start site (TSS) affiliated, 19 5′UTR associated, and four in the gene body. The BeadChip contains 203 CpG sites in the OTX2-regulated genes; all were analyzed, except six (3%), which failed to pass the quality control tests. The sites analyzed include 16 sites in Collagen Type VIII Alpha 1 (COL8A1; all gene body affiliated), 105 sites in Paired Box 6 (PAX6; 52 in the gene body, 43 5′UTR affiliated, and 10 TSS associated), 15 sites in Retinol-Binding Protein 3 (RBP3; 7 in the first exon, 4 TSS affiliated, 3 in the gene body, and one 3′UTR associated), 20 sites in Semaphorin-3C (SEMA3C;11 in the gene body, six TSS affilated, two 5′UTR associated, and one 3′UTR associated), 18 sites in Thrombospondin-4 (THBS4; nine in the gene body, seven TSS affilated, and two 5′UTR associated), and 23 sites in WNT Family Member 1 (WNT1; 11 TSS affilated, nine in the gene body, two 3′UTR associated, and one in the first exon).
Negative control genes
For negative control comparisons, associations with depression were examined with genes that determine height [20], as maltreatment has not been associated with changes in height. Additional requirements for negative control genes included: not associated with obesity, a highly replicated outcome associated with child maltreatment [21]; not sex-linked, as both boys and girls were included in the study, not an identified risk gene for major depression, and not associated with maltreatment-related methylation differences in our prior whole epigenome study [22]. Height-related genes examined for negative control purposes included: Abhydrolase domain containing 2 (ABHD2); FLJ42289; hyaluronan binding protein 4 (HABP4); tudor domain containing 7 (TDRD7); thiosulfate sulfurtransferase (rhodanese)-like domain containing 2 (TSTD2); and tetratricopeptide repeat domain 23 (TTC23).
Targeted bisulfite sequencing
Methylation values attained with the 450 K array were validated with targeted bisulfite sequencing. Sodium bisulfite modification was performed on 500 ng of genomic DNA. Nested polymerase chain reaction (PCR) amplification was performed prior to pyrosequencing analysis using primers described in Supplementary eTable 1, which were generated to validate the methylation values of the CpG sites used in the OTX2, PAX6, and WNT1 genes used in the primary analyses, plus two other genes of interest to our group. After agarose gel electrophoresis, to ensure successful amplification and specificity, PCR amplicons were processed for pyrosequencing analysis according to the manufacturer’s standard protocol (Qiagen, Germantown, MD, USA) and run on a PyroMark 96 pyrosequencing machine. As depicted in eFigure 1, the methylation values attained with the 450 K array and the bisulfite sequencing data were highly correlated (r = 0.92, p < .001; ρ = 0.88, p < .001).
Fig. 1.
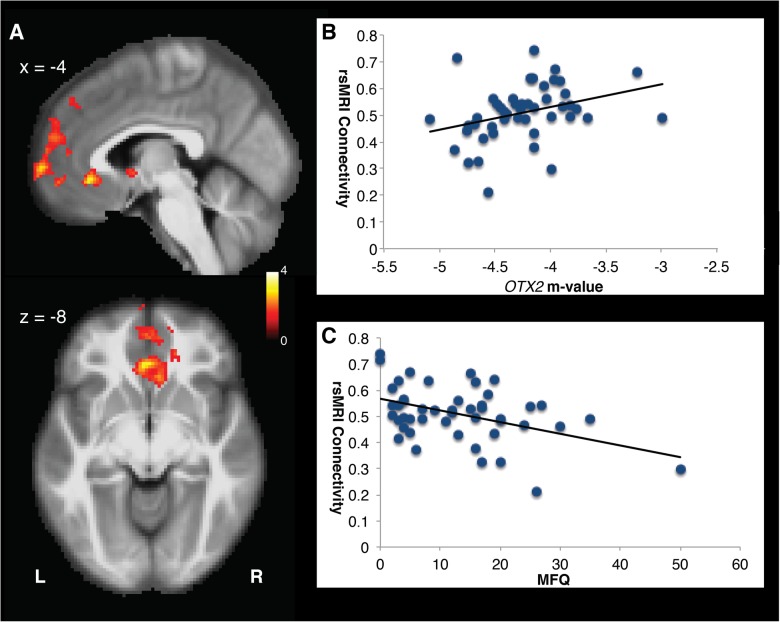
OTX2 gene methylation and functional connectivity from ventral mPFC. a Results of connectivity conjunction analysis showing functional connectivity between the right vmPFC and bilateral regions of the medial frontal cortex and the cingulate. Greater OTX2 methylation (b) and lower depression scores (c) are associated with increased connectivity between these two brain regions. Two clusters of activation reached significance: a cluster centered within the subcallosal gyrus of the cingulate and extended into the ventral frontal medial cortex; a second cluster centered within the ventral aspect of the frontal pole, extending dorsally and posterior to the paracingulate gyrus
Cell heterogeneity
Since methylation values at CpG sites can be cell-type specific [19], to control the cell heterogeneity, we estimated the relative proportion of each cell type (e.g., CD34, CD14, and buccal cells) in our heterogenous peripheral saliva samples using a modified version of Housemann et al.’s cell composition estimation analysis, as detailed in prior reports [23, 24].
Race/ethnicity effects
DNA methylation can vary by race or ethnicity [25, 26]; however, little is known about how large the effect of population stratification can be in the methylation studies. To adjust for possible population stratification within the predominantly Caucasian sample, we utilized a methylation-based principal component (PC) approach based on the sets of CpG sites within 50 kb of SNPs, using the 1000 Genomes Project variants with minor allele frequency (MAF) > 0.1 following the procedures developed by Barfield et al. [27].
Resting-state functional connectivity data
The imaging data were acquired at the University of Vermont on a Philips Achieva 3 Tesla scanner in the axial oblique plane with Anterior to Posterior phase encoding using a 2D gradient echo EPI sequence (TR = 2000 ms, TE = 35 ms, flip angle = 90°, matrix = 64 × 64, FOV = 240 × 240 mm2, slice thickness = 4 mm, slice gap of 10%). The VTA and mPFC were selected as seeds in these exploratory analyses, given their role in reward circuitry and the known expression of OTX2 in these brain regions. Resting-state functional magnetic resonance imaging (rsfMRI) was acquired on 67 children. Of this, 47 participants with (1) acceptable levels of motion during rsfMRI acquisition (17 were excluded for this reason; see online methods for detail on movement-based exclusion), (2) OTX2 methylation data, and (3) MFQ scores were included in the analyses. The subset of children with imaging data were comparable to the remainder of the sample in terms of age and sex distribution, proportion of youth with clinically significant depressive symptomatology, and range of Y-VACS scores (p = ns, all comparisons). The methods used to analyze the imaging data for these exploratory analyses are detailed in the Supplementary Information online.
Data analyses
Given the large number of CpG sites included on the 450 K beadchip in OTX2, the genes regulated by OTX2 and the negative control genes examined, to build the models for subsequent analyses zero-order correlations were first examined between children’s depression scores and the methylation values at these CpG sites. Table 2 depicts the number of CpG sites examined in OTX2 and each of the genes regulated by OTX2, the number of sites per gene that correlated significantly with children’s depression scores at nominal levels of significance (p < .05), the number that correlated after Bonferroni correction, the value of the highest correlation among the CpG sites tested in each gene, the Illumina ID associated with that CpG site, and the location of that CpG site which was then used in the analyses reported in this manuscript.
Table 2.
Descriptive information about genes and CpG sites examined
| Gene | # CpG sites in gene | # Sites correlate w depression (p < .05) | # Sites correlate w depression w bonferroni correction | Highest correlation w depression | Illumina ID of site | Site location |
|---|---|---|---|---|---|---|
| OTX2 | 31 | 12 (39%) | 2 (6%) | 0.33 | cg23706497 | 5′UTR |
| COL8A1 | 16 | 8 (50%) | 7 (44%) | −0.31 | cg18344769 | Body |
| PAX6 | 105 | 21 (20%) | 3 (3%) | 0.36 | cg04938549 | body |
| RBP3 | 15 | 5 (33%) | 1 (7%) | −0.35 | cg07304173 | 1st Exon |
| SEMA3C | 20 | 11 (55%) | 8 (40%) | −0.38 | cg08230910 | body |
| THBS4 | 18 | 4 (22%) | 3 (17%) | −0.34 | cg22636631 | body |
| WNT1 | 23 | 6 (26%) | — | −0.19 | cg27196808 | body |
The table above depicts the number of CpG sites examined in OTX2 and each of the genes regulated by OTX2, the number of sites per gene that correlated significantly with children’s depression scores at nominal levels of significance (p < .05) and after Bonferroni correction (BC), the value of the highest correlation among the CpG sites tested in each gene, the Illumina ID associated with that CpG site which was then used in the follow-up analyses, and the location of that CpG site. Bonferoni correction values were determined by dividing .05 by the number of CpG sites examined in that gene
BC Bonferroni correction, OTX2 orthodenticle homeobox 2, COL8A1 collagen type VIII alpha 1 chain, PAX6 paired box six, RBP3 retinol-binding protein 3, SEMA3C semaphorin-3C, THBS4 thrombospondin-4, WNT1 WNT family member 1
Given the heteroscedasticity of beta values, as recommended by Du et al. [28], M-values (logit transformation in the log2 scale) were used in all analyses. In order to take familial correlations into consideration while examining methylation biomarkers and childhood adversity predictors of depression, the data were analyzed using Generalized Estimating Equations (GEE). Demographic factors (e.g., age, sex, and race), cell heterogeneity measures (e.g., CD34, CD14, and buccal cell proportions), and top three PCs were also examined as covariates in the analyses, including the neuroimaging analyses. When examining the impact of OTX2-regulated genes, the effect of OTX2 gene methylation was also included in the models. Analyses were then conducted to determine if the effects of intrafamilial trauma on depression scores are mediated through methylation levels of OTX2 and related genes, with these analyses considered secondary due to the cross-sectional nature of the data set and the absence of published or publicly available databases to estimate the association of saliva and brain tissue OTX2 methylation or the role of specific CpG methylation sites on gene expression.
Results
OTX2 methylation-depression analyses
As depicted in Table 2, of the 31 CpG sites located in OTX2 on the 450 K chip, methylation values in 12 (39%) correlated significantly with children’s depression scores at nominal levels of significance and two (6%) correlated significantly after Bonferroni correction for multiple comparisons (p < .0016). The two sites that survived the correction for multiple comparisons were located in the 5′UTR region: Illumina ID cg19976235 (r = 0.28, p < .001) and Illumina ID cg23706497 (r = 0.33, p < .0001). Methylation values at cg23706497 correlated significantly with the methylation values at cg19976235 (r = 0.63, p < .001) and with methylation at 25 of the other 30 CpG sites (83%) included in the array. Correlations ranged from −0.64 to 0.73, with negative correlations observed between methylation values at cg23706497 and methylation values at CpG sites on the gene body, and positive correlations observed between methylation values at cg23706497 and methylation values at CpG sites on TSS and other 5′UTR-associated sites.
As depicted in Table 3, methylation in OTX2 at cg23706497 was a significant predictor of depression in children after accounting for children’s intrafamilial adversity scores and controlling for all the relevant covariates. Greater depression scores were associated with greater methylation at this site. As depicted in eFigure 2, there was modest support for a mediation model, with the effects of intrafamilial childhood adversities on children’s depression scores showing a trend in the mediation model and the main effects of trauma and OTX2, both significant predictors.
Table 3.
OTX2 methylation and intrafamilial adversity predictors of depression in children (N = 157)
| Source | Wald χ2 | df | p-value |
|---|---|---|---|
| Age | 0.49 | 1 | ns |
| Sex | 1.16 | 1 | ns |
| Race | 3.19 | 2 | ns |
| CD34 | 1.77 | 1 | ns |
| CD14 | 2.76 | 1 | ns |
| Buccal | 0.21 | 1 | ns |
| PC1 | 6.05 | 1 | .014 |
| PC2 | 5.72 | 1 | .017 |
| PC3 | 2.65 | 1 | .103 |
| Intrafamilial adversity | 5.23 | 1 | .022 |
| OTX2 | 7.37 | 1 | .007 |
Methylation in OTX2 (cg23706497) was significantly associated with depression scores in children after accounting for the effects of experiences of intrafamilial adversity and controlling for relevant covariates
PC principal components, OTX2 orthodenticle homeobox 2
Correlations between OTX2 CpG site methylation and CpG sites examined in genes regulated by OTX2
Methylation at OTX2 cg23706497 correlated significantly with methylation at the sites examined in RBP3 (r = −0.24, p < .002), SEMA3C (r = −0.28, p < .001), THBS4 (r = −.31, p < .001), WNT1 (r = .38, p < .001), and PAX2 (r = −0.39, p < .001), and showed a trend in correlating with the site examined in COL8A1 (r = −0.13, p = .10).
OTX2-regulated genes-depression analyses
The results of the analyses that examined the impact of adverse childhood experiences, the contribution of OTX2 gene methylation, and methylation in the genes regulated by OTX2 are depicted in Table 4. The genes regulated by OTX2 were each significant predictors of depression in children after accounting for the effects of intrafamilial trauma and the effect of OTX2 methylation. The effect of methylation in the OTX2-regulated genes were significant for PAX6 and WNT1 with all the covariates included in the analyses, and the effect of methylation at sites examined in the other genes were only significant after using more simplified models and removing the non-significant covariates from the analyses. As depicted in eFigure 3 and eFigure 4, in the models examining OTX2 together with PAX6 and WNTI1, there was also modest support for a mediation model with effects of intrafamilial childhood adversities on children’s depression scores showing a trend in the mediation model and the main effects of trauma, OTX2, PAX6, and WNT1 significant in the models.
Table 4.
Methylation in OTX2-regulated genes, OTX2 methylation, and intrafamilial adversity predictors of depression in children (N = 157)
| PAX6 GEE analysis | WNT1 GEE analysis | ||||||
|---|---|---|---|---|---|---|---|
| Source | Wald χ2 | df | p-value | Source | Wald χ2 | df | p-value |
| Age | 0.60 | 1 | ns | Age | 0.44 | 1 | ns |
| Sex | 1.23 | 1 | ns | Sex | 1.19 | 1 | ns |
| Race | 3.29 | 2 | ns | Race | 1.12 | 2 | ns |
| CD34 | 2.01 | 1 | ns | CD34 | 1.19 | 1 | ns |
| CD14 | 2.26 | 1 | ns | CD14 | 3.12 | 1 | .077 |
| Buccal | 2.14 | 1 | ns | Buccal | .088 | 1 | ns |
| PC1 | 7.91 | 1 | .005 | PC1 | 12.47 | 1 | .001 |
| PC2 | 7.79 | 1 | .005 | PC2 | 8.95 | 1 | .003 |
| PC3 | 0.45 | 1 | ns | PC3 | 2.00 | 1 | ns |
| Intrafamilial adversity | 3.99 | 1 | .046 | Intrafamilial adversity | 4.38 | 1 | .036 |
| OTX2 | 7.29 | 1 | .007 | OTX2 | 6.46 | 1 | .011 |
| PAX6 | 4.07 | 1 | .044 | WNT1 | 4.84 | 1 | .028 |
| COL8A GEE analysis | RBP3 GEE analysis | ||||||
| PC1 | 6.20 | 1 | .013 | PC1 | 4.82 | 1 | .028 |
| PC2 | 5.99 | 1 | .014 | PC2 | 4.92 | 1 | .027 |
| Intrafamilial adversity | 3.56 | 1 | .06 | Intrafamilial adversity | 5.06 | 1 | .024 |
| OTX2 | 8.29 | 1 | .004 | OTX2 | 7.11 | 1 | .008 |
| COL8A1 | 4.21 | 1 | .04 | RBP3 | 6.25 | 1 | .012 |
| SEMA3C GEE analysis | THSB4 GEE analysis | ||||||
| PC1 | 5.39 | 1 | .02 | PC1 | 5.50 | 1 | .018 |
| PC2 | 5.30 | 1 | .02 | PC2 | 5.50 | 1 | .019 |
| Intrafamilial adversity | 3.65 | 1 | .056 | Intrafamilial adversity | 4.32 | 1 | .038 |
| OTX2 | 8.37 | 1 | .004 | OTX2 | 8.44 | 1 | .004 |
| SEMA3C | 4.58 | 1 | .032 | WNT1 | 4.32 | 1 | .038 |
Methylation in OTX2-related genes were significantly associated with depression scores in children after accounting for the effect of experiences of intrafamilial adversity, OTX2 gene methylation, and controlling for relevant covariates
PC principal components, OTX2 orthodenticle homeobox 2, PAX6 paired box six, WNT1 WNT family member 1, COL8A1 collagen type VIII alpha 1 chain, RBP3 retinol-binding protein 3, SEMA3C semaphorin-3C, THBS4 thrombospondin-4
Re-analyses using targeted bisulfite sequencing data
As indicated above, only the CpG sites for OTX2, PAX6, WNT1 genes used in primary analyses were validated with bisulfite sequencing. In re-running the analyses with the targeted bisulfite sequencing data for these three sites, the methylation values were first converted to M-values. After accounting for the effects of intrafamilial trauma and covariates, both PAX6 (Wald statistic = 5.72, df = 1, p < .02) and WNT1 (Wald statistic = 4.76, df = 1, p < .03) were significant predictors of depression, and the effect of OTX2 showed a trend toward significance (Wald statistic = 3.07, df = 1, p < .08). The less robust findings pertaining to OTX2 may be a function of the slightly reduced sample size in the sequencing data (N = 147); however, the pyrosequencing data and the array results are largely consistent.
Negative control gene analyses
As depicted in eTable 2 and eTable 3 online, while each of the negative control genes had some CpG sites that had significant zero-order correlations with child depression measure, none were significant predictors of depression when child maltreatment and relevant covariates were included in the GEE models, with Wald Statistic values ranging from 0.001 (p = 0.97) to 1.23 (p = 0.27). The effects of negative control genes remained non-significant even when reduced models were used that only included the significant covariates.
Resting-state functional connectivity data
Using familywise error (FEW) cluster correction with initial selection threshold set to p < 0.01, no connectivity findings from the VTA seed retained significance. As depicted in Fig. 1, after controlling for relevant covariates and FEW correction, OTX2 methylation and MFQ depression scores were predictive of functional connectivity between the right vmPFC and bilateral regions of the medial frontal cortex and the cingulate. The global conjunction illustrates that connectivity between the right vmPFC seed and target regions increased with increased methylation of OTX2 (Fig. 1b), while connectivity decreased with greater symptoms of depression (MFQ, Fig. 1c). Two clusters of activation reached significance: a cluster centered within the subcallosal gyrus of the cingulate and extended into the ventral frontal medial cortex; a second cluster centered within the ventral aspect of the frontal pole, extending dorsally and posterior to the paracingulate gyrus. No additional seeds reached significance for the targeted conjunction.
Discussion
The data presented in this manuscript suggest a potential role for OTX2 in conferring risk for depression in children following early adversity, and support the translational applicability of the recently reported mouse model of early life stress [1]. In the current investigation, measures of intrafamilial adversity (e.g., physical abuse, sexual abuse, neglect, witnessing domestic violence) and peripheral saliva-derived DNA measures of OTX2 methylation were associated with individual differences in depression in children after controlling for demographic factors, cell heterogeneity, and three principal components to prevent spurious findings associated with population stratification effects.
In the current investigation, OTX2 gene methylation in the periphery was also associated with methylation in the targeted genes downregulated by Otx2 in the mouse model of early life stress (Col8a1, Rbp3, Sema3c, Thbs4, Pax6, and Wnt1) [1]. In addition, CpG site methylation in each of the genes regulated by OTX2 were also shown to be significant predictors of depression in the children after accounting for the effects of intrafamilial trauma and the effect of OTX2 methylation, with the strongest findings seen for PAX6 and WNT1. PAX6 acts as a master control gene for neurogenesis and a positive regulator for chromatin remodeling-mediated gene expression [29]. PAX6 is also critical for differentiation of dopaminergic neurons arising from neural stem cells residing in the dorsal paraventricular zone [30]. WNT1 plays a role in the establishment of midbrain-dopaminergic neurons [31] and is a major regulator of neural plasticity in the amygdala and hippocampus following acute stress and fear conditioning [2]. Given the role of WNT1 in neuroplastic processes, it has also been proposed as a “master regulator”, which may serve as an important therapeutic target in the treatment of stress-related psychiatric disorders [2]. None of the negative control genes examined were significant predictors of depression in the GEE models.
In the subset of children with neuroimaging data, after controlling for relevant covariates and FEW correction, results of connectivity conjunction analysis found that increased OTX2 methylation was associated with increased functional connectivity between the right vmPFC and bilateral regions of the medial frontal cortex and cingulate. Two clusters of activation reached significance: a cluster centered within the subcallosal gyrus of the cingulate and extending into the ventral frontal medial cortex; and a second cluster centered within the ventral aspect of the frontal pole, extending dorsally and posterior to the paracingulate gyrus. The subcallosal cingulate is a key structure implicated in depression, with the subcallosal cingulate part of the connectome of converging white matter bundles affecting mood and behavior, including the forceps minor, uncinate fasciculus, cingulum, and fronto-striatal fibers, and also serving as a site for therapeutic deep brain stimulation [32–35]. The frontal pole and paracingulate gyrus are key structures involved in cognitive control in affective contexts, with activation in these regions decreasing after effective psychotherapeutic depression treatment in response to cognitive control stimuli presented within a sad context [36].
Limitations of the current investigation include: small size of the subsample that completed the imaging protocol, the absence of gene expression data, the cross-sectional nature of the study which limits inferences that can be made regarding the causal consequences of the methylation findings, and the absence of a cohort of depressed children without histories of intrafamilial trauma to determine if OTX2 is uniquely a biomarker for depression in children with a history of early adversity.
Mouse models of early life stress hold significant promise in helping to unravel the mechanisms by which early adversity confers risk for psychopathology. The utility of novel markers identified in model systems through unbiased genomics, transcriptomics, and proteomics can then be examined as potential biomarkers in carefully characterized clinical samples [37]. The results of the current study suggest a potential role of OTX2 and OTX2-regulated genes in the pathophysiology of stress-related disorders that warrants ongoing systematic evaluation using preclinical, postmortem, and clinical research strategies. Through iterative translational research initiatives, ongoing progress can be made to reduce the burden associated with stress-related psychiatric disorders.
Electronic supplementary material
Acknowledgements
We thank the children and families who participated in this research and the administration of the Vermont Department of Children and Families for their collaboration on this effort. This work was supported by the Zanvyl and Isabelle Krieger Fund (JK), the NIH R01MH098073 (JK, JH), R01 MD011746-01 (JK); the National Center for Posttraumatic Stress Disorder—Veterans Affairs, Connecticut (JG, JK); the VA Cooperative Study #575B, Genomics of Posttraumatic Stress Disorder in Veterans (JG, JK); and the Biological Sciences Training Program through 5T32 MH14276 (JLM-O).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41386-018-0157-y).
References
- 1.Pena CJ, et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science. 2017;356:1185–8. doi: 10.1126/science.aan4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maheu ME, Ressler KJ. Developmental pathway genes and neural plasticity underlying emotional learning and stress-related disorders. Learn Mem. 2017;24:492–501. doi: 10.1101/lm.044271.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jukic MM, et al. Abnormal development of monoaminergic neurons is implicated in mood fluctuations and bipolar disorder. Neuropsychopharmacology. 2015;40:839–48. doi: 10.1038/npp.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernay B, et al. Otx2 regulates subtype specification and neurogenesis in the midbrain. J Neurosci. 2005;25:4856–67. doi: 10.1523/JNEUROSCI.5158-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simeone A, Puelles E, Acampora D. The Otx family. Curr Opin Genet Dev. 2002;12:409–15. doi: 10.1016/S0959-437X(02)00318-0. [DOI] [PubMed] [Google Scholar]
- 6.Puelles E, et al. Otx2 regulates the extent, identity and fate of neuronal progenitor domains in the ventral midbrain. Development. 2004;131:2037–48. doi: 10.1242/dev.01107. [DOI] [PubMed] [Google Scholar]
- 7.Sugiyama S, et al. Experience-dependent transfer of Otx2 homeoprotein into the visual cortex activates postnatal plasticity. Cell. 2008;134:508–20. doi: 10.1016/j.cell.2008.05.054. [DOI] [PubMed] [Google Scholar]
- 8.Lee HHC, et al. Genetic Otx2 mis-localization delays critical period plasticity across brain regions. Mol Psychiatry. 2017;22:680–8. doi: 10.1038/mp.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beurdeley M, et al. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J Neurosci. 2012;32:9429–37. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spatazza J, et al. Choroid-plexus-derived Otx2 homeoprotein constrains adult cortical plasticity. Cell Rep. 2013;3:1815–23. doi: 10.1016/j.celrep.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman J. Depressive disorders in maltreated children. J Am Acad Child Adolesc Psychiatry. 1991;30:257–65. doi: 10.1097/00004583-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Cicchetti D. The effects of maltreatment on school-aged children’s socio-emotional development: assessments in a day camp setting. Dev Psychol. 1989;25:516–24. doi: 10.1037/0012-1649.25.4.516. [DOI] [Google Scholar]
- 13.Kaufman J, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci USA. 2004;101:17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein D. A new screening measure for detecting ‘Hidden’ domestic violence. Psychiatr Times. 1998;15:448–53. [Google Scholar]
- 15.Pynoos, RS, Rodriguez, N, Steinberg, A. PTSD index for DSM-5. Los Angeles, CA: University of California Los Angeles; 2013.
- 16.Kaufman J, et al. The use of multiple informants to assess children’s maltreatment experiences. J Fam Violence. 1994;9:227–48. doi: 10.1007/BF01531949. [DOI] [Google Scholar]
- 17.Holbrook, H, et al. The Yale-Vermont Adversity in Childhood Scale: A Quantitative Approach to Adversity Assessment. In: American Academy of Child and Adolescent Psychiatry’s 61st Annual Meeting. San Diego, CA; 2015.
- 18.Angold A, et al. Development of a Short Questionnaire for use in Epidemiological Studies of Depression in Children and Adolescents. Int J Methods Psychiatr Res. 1995;5:237–49. [Google Scholar]
- 19.Aryee MJ, et al. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA Methylation microarrays. Bioinformatics. 2014;30:363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood AR, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–86. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. 2014;19:544–54. doi: 10.1038/mp.2013.54. [DOI] [PubMed] [Google Scholar]
- 22.Yang BZ, et al. Child abuse and epigenetic mechanisms of disease risk. Am J Prev Med. 2013;44:101–7. doi: 10.1016/j.amepre.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houseman EA, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13:86. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith AK, et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet. 2015;168B:36–44. doi: 10.1002/ajmg.b.32278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adkins RM, et al. Racial differences in gene-specific DNA methylation levels are present at birth. Birth Defects Res A Clin Mol Teratol. 2011;91:728–36. doi: 10.1002/bdra.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyn H, et al. DNA methylation contributes to natural human variation. Genome Res. 2013;23:1363–72. doi: 10.1101/gr.154187.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barfield RT, et al. Accounting for population stratification in DNA methylation studies. Genet Epidemiol. 2014;38:231–41. doi: 10.1002/gepi.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du P, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soumiya H, Fukumitsu H, Furukawa S. Prenatal immune challenge compromises the normal course of neurogenesis during development of the mouse cerebral cortex. J Neurosci Res. 2011;89:1575–85. doi: 10.1002/jnr.22704. [DOI] [PubMed] [Google Scholar]
- 30.de Chevigny A, et al. Dynamic expression of the pro-dopaminergic transcription factors Pax6 and Dlx2 during postnatal olfactory bulb neurogenesis. Front Cell Neurosci. 2012;6:6. doi: 10.3389/fncel.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prakash N, et al. A Wnt1-regulated genetic network controls the identity and fate of midbrain-dopaminergic progenitors in vivo. Development. 2006;133:89–98. doi: 10.1242/dev.02181. [DOI] [PubMed] [Google Scholar]
- 32.Riva-Posse P, et al. A connectomic approach for subcallosal cingulate deep brain stimulation surgery: prospective targeting in treatment-resistant depression. Mol Psychiatry. 2017;11:59. doi: 10.1038/mp.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akil H, et al. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci Biobehav Rev. 2018;84:272–88. doi: 10.1016/j.neubiorev.2017.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayberg HS, et al. Cingulate function in depression: a potential predictor of treatment response [see comments] Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 35.Ducharme S, et al. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24:2941–50. doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dichter GS, Felder JN, Smoski MJ. The effects of brief behavioral activation therapy for depression on cognitive control in affective contexts: an fMRI investigation. J Affect Disord. 2010;126:236–44. doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaufman J, et al. Annual Meeting of the Society of Biological Psychiatry. New York, NY: Elsevier Press; 2018. New perspectives on the study of early life stress. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.