Abstract
Sphingosine‐1‐phosphate (S1P) is an essential, bioactive lysophospholipid mediator that regulates various physiological functions such as lymphocyte trafficking, inflammation and behavioural characteristics of the vascular system. S1P signalling is mediated via a family of five GPCRs, which are expressed in various cell types and tissues. S1P concentration is maintained in a gradient through the activity of S1P degrading enzymes, and this gradient is critical for lymphocyte egress. To exert its extracellular signalling roles, S1P must be secreted out of the cells by protein transporters. The recent discovery of S1P transporters has shed light on the sources of S1P. However, these transporters still need to be clarified as they are important in defining the S1P gradient for lymphocyte recirculation and the source of S1P for maintenance of blood vessels. Here, we review the current understanding of S1P sources, highlighting the roles of S1P transporters with an emphasis on haematopoietic cells as a major source of circulatory S1P.
Abbreviations
- ABC
ATP binding cassette
- ApoM
alipoprotein M
- CLEC‐2
C‐type lectin domain family 2
- HEV
high endothelial venules
- MFS
major facilitator superfamily
- RBC
red blood cells
- S1P
sphingosine 1‐phosphate
- SGPP
sphingosine 1‐phosphate phosphatase
- SPHK
sphingosine phosphate kinase
- Spns2
protein spinster homologue 2
Introduction
During mammalian development, blood cells and blood vessels develop concurrently. Multipotent haematopoietic cells that emerge from haemogenic endothelium form blood cells. Overlapping genes in blood cells and endothelium induce them to act synergistically (Zovein et al., 2008). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=911 is a lipid molecule generated by both blood cells and endothelial cells, which has important signalling roles. In the circulation system, blood cells release this lipid mediator to maintain blood vessel integrity (Camerer et al., 2009). Furthermore, lymphatic endothelia release this molecule to guide T and B lymphocyte recirculation (Fukuhara et al., 2012). Recent findings on the role of platelet‐derived S1P in thrombus formation (Urtz et al., 2015) and platelet biogenesis (Kaushansky, 2005) further emphasize the potential of this mediator. In this review, we present some of the major signalling roles of S1P derived from the haematopoietic system. We also investigate the various animal models deficient in S1P used to explore its physiological effects. Additionally, we highlight the S1P transporters involved in regulating plasma S1P levels, focussing on their potential as pharmacological targets for various diseases.
S1P signalling roles
S1P metabolism is closely regulated by two http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=787 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2204 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2205) (Maceyka et al., 2013), which are involved in its production, and two http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=778 (Bourquin et al., 2010), an http://guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=777 and three lysophospholipid hydrolases implicated in its degradation (Serra and Saba, 2010). S1P exerts its extracellular effects through high‐affinity binding GPCRs, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=135 (Van Brocklyn et al., 1998; Van Brocklyn et al., 2000). The binding of S1P to each of these receptors induces various signalling effects depending on the heterotrimeric G proteins coupled to each of them. The different receptors have been suggested to have a role in diverse developmental and disease‐related processes. Two major functions of S1P signalling are the regulation of lymphocyte trafficking and blood vessel integrity (Proia and Hla, 2015). For instance, deletion of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=275 (Liu et al., 2000) or SPHK1/2 (Mizugishi et al., 2005) results in embryonic lethality with severe haemorrhages at early developmental stages, highlighting the critical roles of S1P signalling for blood vessel functions. In addition, S1P signalling is also vital for cell motility, and has been regarded as the central mediator for lymphocyte egress (Matloubian et al., 2004). The activation of S1P1 signalling and the maintenance of S1P gradient between tissues and the systemic circulation are vital for the egress of newly formed T cells from the thymus and mature T and B cells from secondary lymphoid organs (Nijnik et al., 2012). A disruption of the S1P gradient can be induced by inhibiting S1P degrading enzymes or S1P transporters (Fukuhara et al., 2012). Targeting S1P signalling by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2407 has been successfully used to treat multiple sclerosis. This prodrug suppresses immune responses and causes peripheral lymphopenia by blocking lymphocyte egress from the thymus and lymph nodes (Chun and Hartung, 2010; Nijnik et al., 2012). Pharmacological studies on FTY720 showed that it inhibits the expression of S1P1 receptors on lymphocytes in its phosphorylated form (Brinkmann et al., 2002; Pham et al., 2010). FTY720 and other S1P receptor modifying compounds have confirmed the importance of S1P signalling in the recruitment of inflammatory cells. Nevertheless, FTY720 causes side effects such as bradycardia (Budde et al., 2003). The S1P‐S1P receptor signalling axis is important for regulating innate and adaptive immune responses. S1P signalling through http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=278 promotes neutrophil trafficking (Allende et al., 2010) whereas signalling through http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=279 is necessary for natural killer T cell egress from lymph nodes and bone marrow (Jenne et al., 2009). Recently, S1P‐dependent signalling was detected in innate lymphoid cells in response to infection (Huang et al., 2018), emphasizing the role S1P plays in cell motility.
S1P carriers and transporters
As a lipophilic molecule, extracellular S1P is associated with carriers. Plasma S1P binds to alipoprotein M (ApoM) in HDL and LDL, and albumin (Christoffersen et al., 2011). HDL‐bound S1P represents the major S1P fraction in plasma (Blaho et al., 2015). S1P is also present in interstitial fluid in tissue and brain (Nagahashi et al., 2016). However, its carriers in these tissues are unknown. In blood, the majority of S1P is bound to ApoM in HDL (Kono et al., 2004). Mice lacking ApoM exhibited reduced S1P levels in plasma. However, S1P production is intact in ApoM deficient mice, suggesting that ApoM stabilizes S1P in blood (Christoffersen et al., 2011). In contrast, the role of albumin‐bound S1P has been little studied.
Much knowledge about S1P source was gained from studies of knockouts of S1P kinases (SPHK1/2) (Xiong et al., 2014). Deletion of both SPHK1/2 results in loss of both the intracellular and extracellular sources of S1P (Mizugishi et al., 2005). Yet it is difficult to discern the intracellular and extracellular signalling effects. One of the major challenges in the S1P signalling field is the identification of S1P transporters. ATP‐dependent transporters such as http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=779 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=153#783 were implicated as potential S1P transporters (Mitra et al., 2006; Kobayashi et al., 2009). However, none of these ATP transporters yielded a significant amount of plasma S1P, obscuring their physiological functions as S1P transporters. The discovery of protein spinster homologue 2 (Spns2) as the first bona fide S1P transporter has shed light on the source of S1P (Kawahara et al., 2009). Spns2 exports S1P from lymphatic endothelial cells and accounts for approximately 25–50% of plasma S1P (Fukuhara et al., 2012; Nagahashi et al., 2013). Notably, Spns2 is not the S1P transporter in haematopoietic cells, which are the major source of S1P (Mendoza et al., 2012).
Solute carrier proteins represent one of the most abundant proteins in mammals. Most of these transporters including a sub‐category of transporters, namely, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=859 domains transport soluble substrates. A recent finding by Vu et al. identified Mfsd2b, an orphan transporter that is expressed in erythrocytes and platelets. This transporter provides approximately 50% of the S1P in plasma as observed in Mfsd2b−/− mice (Vu et al., 2017). This corroborates well with previous determinations of S1P sources from haematopoietic cells (Gazit et al., 2016). Mfsd2b−/− mice exhibited phenotypes that are linked to abnormal S1P signalling, such as low circulating lymphocytes and sensitivity to vascular stress (Vu et al., 2017).
Roles of erythrocyte‐derived S1P
Compelling studies have indicated that erythrocytes are the major source of plasma S1P (Hanel et al., 2007; Pappu et al., 2007). Erythrocyte‐specific deletion of both SPHK1 and SPHK2 led to embryonic lethality, and these embryos have severe vascular defects. This suggests that red blood cell (RBC)‐derived S1P is crucial during embryonic development, specifically for vascular stabilization, maturation and remodelling (Xiong et al., 2013). A detailed analysis of embryonic vascular defects further emphasizes the involvement of RBC‐specific S1P in angiogenesis and also erythropoiesis. Similarly, a global deletion of both SPHK1 and 2 resulted in vascular defects, and these embryos were not viable after E13.5 (Mizugishi et al., 2005). Additionally, these mutant embryos also exhibited severe defects in neurogenesis whereby, exencephaly was observed. This further confirms that S1P is vital during embryonic development especially for neurogenesis and angiogenesis. However, it is unknown whether Mfsd2b is the S1P provider for neurogenesis that was observed in double knockouts of SPHK1/2.
Deletion of both S1P synthesis enzymes SPHK1/2 has demonstrated that plasma S1P is critical for several physiological functions of blood vessels. The postnatal deletion of SPHK1/2 using Mx1‐cre showed that plasma S1P is essential for protection of mice against anaphylactic shock and the vascular leakage associated with inflammation (Camerer et al., 2009). Transfusion of wild‐type RBC reversed these pathological conditions, indicative of the essential roles of RBC for S1P homeostasis. In another S1P depleted model, ApoM−/−, vascular leakage was observed followed by increased inflammatory responses to LPS‐induced sepsis (Zhu et al., 2018). Inversely, administration of an engineered ApoM‐S1P complex protects mice from cerebral and cardiac ischaemic damage and hypertension (Swendeman et al., 2017). These results indicate the dual role of S1P in blood vessels in normal and pathological conditions. Furthermore, in endothelium, S1P is thought to regulate vascular tone. Cantalupo et al. (2017) found that autocrine activation of S1P1 in endothelial cells lowered BP emphasizing its importance in vasodilatation. Nevertheless, it is unclear whether erythrocytes are responsible for this S1P pool. The identification of Mfsd2b holds great significance as its specific deletion could elucidate the role of S1P in extracellular signalling (Figure 1).
Figure 1.
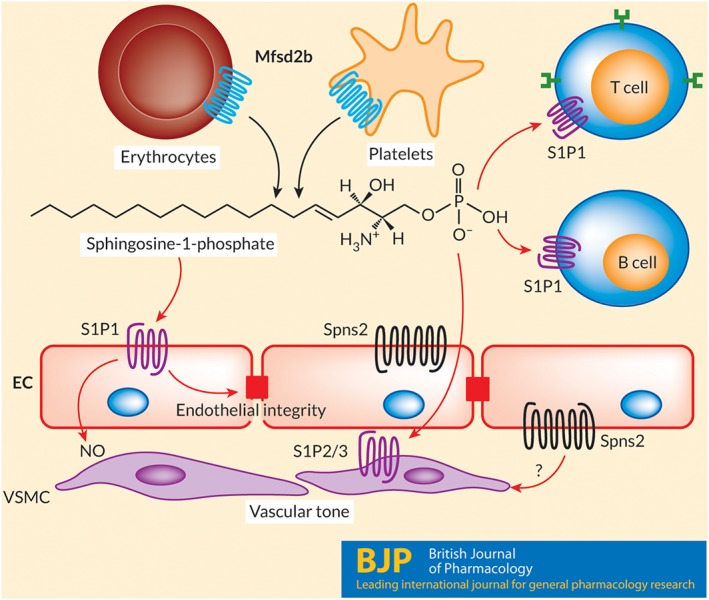
Extracellular signalling roles of S1P. Sphingosine‐1‐phosphate is transported out from haematopoietic cells by Mfsd2b and by Spns2 from endothelial cells. S1P plays a role as an extracellular signalling lipid in which it activates five different GPCRs, namely, S1P1–5. The S1P1 receptor (S1P1) is one of the most abundant receptors expressed in blood endothelial cells and T and B lymphocytes. In blood, S1P is mainly generated by erythrocytes and platelets. Mfsd2b is the transporter for S1P in these cells. In the endothelium, S1P is released via Spns2, especially in the lymphatic system. Both Mfsd2b and Spns2 contribute to blood S1P, which is critical for maintaining the functions of blood vessels via activation of S1P1 receptors on endothelial cells or S1P2/3 receptors (S1P2/3) on vascular smooth muscle cells. An active S1P signalling pathway is essential for blood vessel integrity and vascular tone. It is not known whether Spns2 releases S1P on both sides of endothelium. EC, endothelial cells; VSMC, vascular smooth muscle cells.
Release of S1P by platelets to the circulation
Platelets can produce and store large amounts of S1P, synthesis by SPHK2 being the major source of their S1P production (Urtz et al., 2015), but they lack both the de novo sphingolipid biosynthetic pathway and the S1P catabolic enzyme S1P lyase. Also, even though platelets store large amounts of S1P, they do not contribute a significant amount of S1P to the circulation at homeostasis. However, the observation that serum S1P was increased twofold to threefold during clot formation suggest that platelets do release S1P upon aggregation (Ono et al., 2013). The increase in plasma S1P concentrations by activated platelets has a critical role in the repair of endothelial vessels during injury. This mechanism is operated by receptors on vascular smooth muscle cells, which are not saturated by the plasma levels of S1P provided normally by other cell types.
Platelets are prone to activation by exogenous stimuli via GPCRs (Offermanns, 2006). The release of S1P can be induced by activation of platelets with potent agonists, such as thrombin, selective PAR‐activating peptides (PARAPs) or a high concentration of collagen. This process results in the release of platelet mediators like http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1712, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2391 derivatives, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4482 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1883 from their storage granules. TXA2 and ADP are able to stimulate S1P release from platelets in vitro (Gazit et al., 2016). TXA2 activation of platelets can be largely prevented by inhibitors of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376 such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4139, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2714 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2713 (Ulrych et al., 2011). However, it is unclear whether COX‐2 inhibitors directly inhibit S1P release or platelet aggregation, which eventually leads to the release of S1P. Platelets seem to store S1P in cell membranes as well as in α‐granules but not in dense granules (Jonnalagadda et al., 2014). In addition, a series of studies from Whiteheart's laboratory has shown that a soluble N‐ethylmaleimide sensitive factor attachment protein receptor (SNARE) complex is required for S1P release in activated platelets (Joshi and Whiteheart, 2017). Selectively, Unc13d mutation causes defective exocytosis in thrombin‐induced S1P release (Ren et al., 2010) accompanied by defective aggregation and long tail bleeding times. However, the mechanisms involved in the storage of S1P by resting platelets are unknown. Several studies have reported the involvement of ABC transporters for the export of S1P in platelets. However, the biological functions of these proteins are still not completely understood. Mfsd2b is also highly expressed in platelets, and this transporter is required for S1P release from resting and activated platelets (Vu et al., 2017). In the study by Vu et al., (2017) Mfsd2b was shown to be expressed in cell membranes and intracellular membrane structures. However, the expression pattern of Mfsd2b in resting platelets is not known.
Role of platelet‐derived S1P signalling in blood vessels
Although platelets are not the major source of S1P in the circulation during normal conditions, activated platelets release significant amounts of this lipid mediator. Studies by Herzog et al., (2013) showed that S1P released by platelets is important for the maintenance of high endothelial venules (HEV). Activation of the C‐type lectin domain family 2 (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=945) receptor in platelets results in the release of S1P, which then acts in a paracrine manner to increase VE‐cadherin expression in adjacent endothelial cells, thus explaining the function of S1P released by activated platelets in overall vascular integrity. However, the specific S1P deficiency observed in platelets from SPHK2−/− does not show similar phenotypes to those with CLEC‐2 deletion. This suggests that activation of CLEC‐2 receptors in platelets might have other effects on HEV. Indeed, the specific functions of platelet‐stored S1P is still ill‐defined.
Role of platelet‐derived S1P signalling in thrombus formation
At the site of vascular injury, various mediators are released by activated platelets including S1P. Thrombus formation and stabilization require the persistent activation of recruited platelets to prevent those platelets from dissociating and detaching from the thrombus. A deficiency in SPHK2 results in defective platelet aggregation and arterial thrombosis with unaltered tail bleeding times in mice. Thus, the release of S1P by activated platelets probably activates the autocrine signalling pathway for thrombosis (Urtz et al., 2015). These findings indicate that S1P from platelets is necessary for thrombus formation in vivo (Gazit et al., 2016). This S1P signalling function should be explored further to find new inhibitors of SPHK2 that can modulate thrombus formation without interfering with the other functions of platelets. Hence, future studies will no doubt provide additional insights as to the role of platelet‐stored S1P in various diseases.
Conclusion and future perspectives
Several sources contribute to the S1P available in the circulatory system. Of significance, erythrocytes and platelets are the major source of S1P in the circulation via Mfsd2b (Vu et al., 2017). Nevertheless, endothelial cells also contribute a significant amount of S1P via Spns2 (Fukuhara et al., 2012). It is still unclear whether Mfsd2b and Spns2 are the only two exporters that contribute to the S1P in the circulation. In addition, the cellular and molecular origin and transport machinery involved in embryonic S1P have not yet been identified. However, the signalling pathways associated with S1P are being extensively explored as suitable targets for drug development for diverse inflammatory diseases and cancers. The identification of S1P transporters has provided fresh approaches for understanding the role of S1P in various pathological conditions and afforded novel molecular targets for drug development.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
This study was supported in part by NUS Young Investigator Award (YIA) grant and Ministry of Education (Moe‐Tier 1) grant (to L.N.N.).
Tukijan, F. , Chandrakanthan, M. , and Nguyen, L. N. (2018) The signalling roles of sphingosine‐1‐phosphate derived from red blood cells and platelets. British Journal of Pharmacology, 175: 3741–3746. 10.1111/bph.14451.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Overview. Br J Pharmacol 174: S1–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu Y‐P, Proia RL (2010). S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med 207: 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho VA, Galvani S, Engelbrecht E, Liu C, Swendeman SL, Kono M et al (2015). HDL‐bound sphingosine‐1‐phosphate restrains lymphopoiesis and neuroinflammation. Nature 523: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin F, Riezman H, Capitani G, Grütter MG (2010). Structure and function of sphingosine‐1‐phosphate lyase, a key enzyme of sphingolipid metabolism. Structure 18: 1054–1065. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R et al (2002). The immune modulator FTY720 targets sphingosine 1‐phosphate receptors. J Biol Chem 277: 21453–21457. [DOI] [PubMed] [Google Scholar]
- Budde K, Schmouder RL, Nashan B, Brunkhorst R, Lücker PW, Mayer T et al (2003). Pharmacodynamics of single doses of the novel immunosuppressant FTY720 in stable renal transplant patients. Am J Transplant 3: 846–854. [DOI] [PubMed] [Google Scholar]
- Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D et al (2009). Sphingosine‐1‐phosphate in the plasma compartment regulates basal and inflammation‐induced vascular leak in mice. J Clin Investig 119: 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T et al (2017). S1PR1 (sphingosine‐1‐phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 70: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M et al (2011). Endothelium‐protective sphingosine‐1‐phosphate provided by HDL‐associated apolipoprotein M. Proc Natl Acad Sci 108: 9613–9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hartung HP (2010). Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 33: 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T et al (2012). The sphingosine‐1‐phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Investig 122: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit SL, Mariko B, Thérond P, Decouture B, Xiong Y, Couty L et al (2016). Platelet and erythrocyte sources of S1P are redundant for vascular development and homeostasis, but both rendered essential after plasma S1P depletion in anaphylactic shock. Circ Res 119: e110–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel P, Andreani P, Graler MH (2007). Erythrocytes store and release sphingosine 1‐phosphate in blood. FASEB J 21: 1202–1209. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to immunopharmacology. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM et al (2013). Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC‐2. Nature 502: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mao K, Chen X, Sun MA, Kawabe T, Li W et al (2018). S1P‐dependent interorgan trafficking of group 2 innate lymphoid cells supports host defense. Science 359: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP et al (2009). T‐bet‐dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med 206: 2469–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonnalagadda D, Sunkara M, Morris AJ, Whiteheart SW (2014). Granule‐mediated release of sphingosine‐1‐phosphate by activated platelets. Biochimica et Biophysica Acta ‐ Molecular and Cell Biology of Lipids 1841: 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Whiteheart SW (2017). The nuts and bolts of the platelet release reaction. Platelets 28: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushansky K (2005). Review series: the molecular mechanisms that control thrombopoiesis. J Clin Investig 115: 3339–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N (2009). The sphingolipid transporter Spns2 functions in migration of zebrafish myocardial precursors. Science 323: 524–527. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Kobayashi N, Yamaguchi A, Nishi T (2009). Characterization of the ATP‐dependent sphingosine 1‐phosphate transporter in rat erythrocytes. J Biol Chem 284: 21192–21200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP et al (2004). The sphingosine‐1‐phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 279: 29367–29373. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP et al (2000). Edg‐1, the G protein‐coupled receptor for sphingosine‐1‐phosphate, is essential for vascular maturation. J Clin Investig 106: 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S (2013). Sphingosine‐1‐phosphate signaling and its role in disease. Trends Cell Biol 22: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V et al (2004). Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427: 355–360. [DOI] [PubMed] [Google Scholar]
- Mendoza A, Bréart B, Ramos‐Perez WD, Pitt LA, Gobert M, Sunkara M et al (2012). The transporter Spns2 is required for secretion of lymph but not plasma sphingosine‐1‐phosphate. Cell Rep 2: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S (2006). Role of ABCC1 in export of sphingosine‐1‐phosphate from mast cells. Proc Natl Acad Sci U S A 103: 16394–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizugishi K, Yamashita T, Olivera A, Miller GF, Spiegel S, Proia RL (2005). Essential role for sphingosine kinases in neural and vascular development. Mol Cell Biol 25: 11113–11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M, Kim EY, Yamada A, Ramachandran S, Allegood JC, Hait NC et al (2013). Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J 27: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahashi M, Yamada A, Miyazaki H, Allegood JC, Tsuchida J, Aoyagi T et al (2016). Interstitial fluid sphingosine‐1‐phosphate in murine mammary gland and cancer and human breast tissue and cancer determined by novel methods. J Mammary Gland Biol Neoplasia 21: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijnik A, Clare S, Hale C, Chen J, Raisen C, Mottram L et al (2012). The role of sphingosine‐1‐phosphate transporter Spns2 in immune system function. J Immunol 189: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S (2006). Activation of platelet function through G protein‐coupled receptors. Circ Res 99: 1293–1304. [DOI] [PubMed] [Google Scholar]
- Ono Y, Kurano M, Ohkawa R, Yokota H, Igarashi K, Aoki J et al (2013). Sphingosine 1‐phosphate release from platelets during clot formation: close correlation between platelet count and serum sphingosine 1‐phosphate concentration. Lipids Health Dis 12: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y et al (2007). Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine‐1‐phosphate. Science 316: 295–298. [DOI] [PubMed] [Google Scholar]
- Pham THM, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R et al (2010). Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med 207: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proia RL, Hla T (2015). Emerging biology of sphingosine‐1‐phosphate: its role in pathogenesis and therapy. J Clin Invest 125: 1379–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Q, Wimmer C, Chicka MC, Ye S, Ren Y, Hughson FM et al (2010). Munc13‐4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood 116: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Saba JD (2010). Sphingosine 1 phosphate lyase, a key regulator of sphingosine 1‐phopshate signaling and function. Adv Enzyme Regul 50: 349–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman SL, Xiong Y, Cantalupo A, Yuan H, Burg N, Hisano Y et al (2017). An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci Signal 10. pii: eaal2722. [DOI] [PMC free article] [PubMed]
- Ulrych T, Böhm A, Polzin A, Daum G, Nüsing RM, Geisslinger G et al (2011). Release of sphingosine‐1‐phosphate from human platelets is dependent on thromboxane formation. Journal of Thrombosis and Haemostasis 9: 790–798. [DOI] [PubMed] [Google Scholar]
- Urtz N, Gaertner F, Von Bruehl ML, Chandraratne S, Rahimi F, Zhang L et al (2015). Sphingosine 1‐phosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo . Circ Res 117: 376–387. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Gräler MH, Bernhardt G, Hobson JP, Lipp M, Spiegel S (2000). Sphingosine‐1‐phosphate is a ligand for the G protein‐coupled receptor EDG‐6. Blood 95: 2624–2629. [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee M, Menzeleev R, Olivera A, Edsall L, Cuvillier O et al (1998). Dual actions of sphingosine‐1‐phosphate: extracellular through the G. J Cell Biol 142: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TM, Ishizu AN, Foo JC, Toh XR, Zhang F, Whee DM et al (2017). Mfsd2b is essential for the sphingosine‐1‐phosphate export in erythrocytes and platelets. Nature 550: 524–528. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Lee HJ, Mariko B, Lu YC, Dannenberg AJ, Haka AS et al (2013). Sphingosine kinases are not required for inflammatory responses in macrophages. J Biol Chem 288: 32563–32573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Yang P, Proia RL, Hla T (2014). Erythrocyte‐derived sphingosine 1‐phosphate is essential for vascular development. J Clin Investig 124: 4823–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Luo G, Feng Y, Yu M, Zhang J, Wei J et al (2018). Apolipoprotein M protects against lipopolysaccharide‐induced acute lung injury via sphingosine‐1‐phosphate signaling. Inflammation 41: 643–653. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y et al (2008). Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 4: 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]


