Abstract
Background and Purpose
The aim of the present study was to assess the interaction of nitrergic neurotransmission within the bed nucleus of the stria terminalis (BNST) with local glutamatergic and noradrenergic neurotransmission in the control of cardiovascular responses to acute restraint stress in rats.
Experimental Approach
Interaction with local noradrenergic neurotransmission was evaluated using local pretreatment with the selective α1‐adrenoceptor antagonist WB4101 before microinjection of the NO donor NOC‐9 into the BNST. Interaction with glutamatergic neurotransmission was assessed by pretreating the BNST with a selective inhibitor of neuronal NOS (nNOS), Nω‐propyl‐L‐arginine (NPLA) before local microinjection of NMDA. The effect of intra‐BNST NPLA microinjection in animals locally pretreated with WB4101 was also evaluated.
Key Results
NOC‐9 reduced the heart rate (HR) and blood pressure increases evoked by restraint stress. These effects of NOC‐9 on HR, but not in blood pressure, was inhibited by pretreatment of BNST with WB4101. NMDA enhanced the restraint‐evoked HR increase, and this effect was abolished following BNST pretreatment with NPLA. Administration of NPLA to the BNST of animals pretreated locally with WB4101 decreased the HR and blood pressure increases induced by restraint.
Conclusion and Implications
These results indicate that inhibitory control of stress‐evoked cardiovascular responses by nitrergic signalling in the BNST is mediated by a facilitation of local noradrenergic neurotransmission. The present data also provide evidence of an involvement of local nNOS in facilitatory control of tachycardia during stress by NMDA receptors within the BNST.
Abbreviations
- BNST
bed nucleus of the stria terminalis
- HR
heart rate
- MAP
mean arterial pressure
- nNOS
neuronal isoform of the NOS enzyme
- NPLA
Nωpropyl‐L‐arginine (N5‐[imino(propylamino)methyl]‐L‐ornithine hydrochloride)
- NOC‐9
6‐(2‐hydroxy‐1‐methyl‐2‐nitrosohydrazino)‐N‐methyl‐1‐hexanamine
- sGC
soluble guanylate cyclase
- WB4101
2‐[(2,6‐dimethoxyphenoxyethyl)aminomethyl]‐1,4‐benzodioxane hydrochloride
Introduction
The physiological and behavioural responses evoked by aversive situations are essential for species survival and adaptation (Crestani, 2016; Sterling, 2012; Ulrich‐Lai and Herman, 2009). The control of such responses is performed by overlapping circuits in the CNS (Dampney, 2015; Myers, 2017). The bed nucleus of the stria terminalis (BNST) is a structure situated in the rostral prosencephalon, which has been linked to the central network that regulates stress responses (Crestani et al., 2013; Myers, 2017; Ulrich‐Lai and Herman, 2009). In particular, control of the cardiovascular responses induced by stress involves the BNST (Crestani et al., 2009; Resstel et al., 2008). Recent studies have indicated that nitrergic neurotransmission acting via activation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1251) is a prominent local neurochemical mechanism involved in the control of stress‐evoked cardiovascular responses exerted by the BNST (Barretto‐de‐Souza et al., 2018; Hott et al., 2017). For instance, blockade of nNOS, as well as of signalling mechanisms related to NO effects such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=939) and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=287, within the BNST, enhanced tachycardia and decreased the drop in tail skin temperature evoked by acute restraint stress (Barretto‐de‐Souza et al., 2018). Nevertheless, the local mechanisms related to the control of cardiovascular responses to stress by nitrergic neurotransmission in the BNST remain unknown.
Presynaptic actions that stimulate the release of several neurotransmitters are a prominent mechanism mediating the effects of NO in the CNS (Ohkuma and Katsura, 2001; Philippu, 2016; Prast and Philippu, 2001). Indeed, earlier studies have shown that NO stimulated the release of noradrenaline (Ohkuma and Katsura, 2001; Philippu, 2016; Prast and Philippu, 2001). In this sense, antagonism of http://bph_14447.docx within the BNST, but not of local α2‐ and β‐adrenoceptors, enhanced the increase in HR evoked by restraint, without affecting the increase in blood pressure (Crestani et al., 2009). As with local nitrergic signalling (Barretto‐de‐Souza et al., 2018), these results provided evidence of an inhibitory influence of α1‐adrenoceptors in the BNST in the control of restraint‐evoked cardiovascular responses. Therefore, control of restraint‐evoked cardiovascular responses by nitrergic neurotransmission in the BNST may be mediated by a facilitation of noradrenaline release within the BNST. However, a possible interaction between noradrenergic and nitrergic neurotransmission in the BNST has yet to be evaluated.
Influx of Ca2+ following activation of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=75) glutamatergic receptor is involved in NO synthesis by nNOS in the CNS (Garthwaite, 2008; Garthwaite, 2016; Prast and Philippu, 2001). We recently reported that microinjection of a selective NMDA receptor antagonist into the BNST decreased the tachycardic response to restraint stress (Adami et al., 2017). Although opposing roles of glutamatergic (facilitatory) and nitrergic (inhibitory) transmission in restraint‐evoked tachycardia are implied, NO released from nNOS is described as a prominent signalling mechanism involved in the control of cardiovascular function by NMDA receptors in the brain. For instance, blockade of nNOS completely inhibited cardiovascular changes evoked by NMDA receptor activation in several structures in the CNS (Busnardo et al., 2010; Martins‐Pinge et al., 2007; Resstel and Correa, 2006; Santini et al., 2013; Tavares et al., 2007). Nevertheless, an involvement of local nNOS in the control of cardiovascular function by NMDA receptors within the BNST has yet to be evaluated.
Based on the evidence presented above, this study aimed to test the hypothesis that nitrergic neurotransmission within the BNST plays a dual role in the control of cardiovascular responses evoked by acute restraint stress in rats, through interactions with different local neurochemical mechanisms. Thus, we tested the hypotheses that (i) the inhibitory influence of NO generated by nNOS within the BNST (previously demonstrated as an enhanced HR response following blockade of nNOS within the BNST) (Barretto‐de‐Souza et al., 2018) is mediated by a facilitation of local noradrenergic neurotransmission and (ii) activation of local nNOS is involved in the facilitatory control of restraint‐evoked cardiovascular changes by NMDA receptors in the BNST.
Methods
Animals
All animal care and experimental procedures were carried out according to protocols approved by the Ethical Committee for Use of Animals of the School of Pharmaceutical Science/UNESP (protocol #36/2016), which complies with Brazilian and international guidelines for animal use and welfare. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Male Wistar rats with body weights ranging from 240 to 260 g (60 days old) were used. The animals were supplied by the animal breeding facility of the UNESP (Botucatu, SP, Brazil) and were housed in standard plastic cages with sawdust as bedding in a temperature‐controlled room at 24°C in the Animal Facility of the Laboratory of Pharmacology, School of Pharmaceutical Sciences, São Paulo State University‐UNESP. The animals had ad libitum access to granulated food and water and were maintained under 12h light/dark cycles (lights on between 07:00 and 19:00 h).
Surgical preparation
Five days before the trial, animals were anesthetized with tribromoethanol (250 mg·kg−1, i.p.). The scalp was anesthetized with 2% lidocaine, and the skull was exposed. Then, using a stereotaxic apparatus (Stoelting, Wood Dale, Illinois, USA), stainless‐steel cannulae (26 G, 12 mm long) were bilaterally implanted into the BNST. Stereotaxic coordinates were as follows: antero‐posterior = +8.6 mm from interaural coordinate; lateral = 4.0 mm from the medial suture; ventral = −5.8 mm from the skull; with a lateral inclination of 23° (Paxinos and Watson, 1997). Dental cement was used to fix cannulae to the skull. After surgery, the rats were treated with a poly‐antibiotic formulation containing streptomycins and penicillins to prevent infection (560 mg·mL−1·kg−1, i.m.), and the nonsteroidal anti‐inflammatory drug flunixin meglumine to provide post‐operative analgesia (0.5 mg·mL−1·kg−1, s.c.).
One day before the experiment, animals were again anesthetized with tribromoethanol (250 mg·kg−1, i.p.), and a polyethylene cannula (a 4 cm segment of PE‐10 bound to a 13 cm segment of PE‐50) (Clay Adams, Parsippany, New Jersey, USA) was implanted into the abdominal aorta via the femoral artery for cardiovascular recording. The catheter was tunnelled under the skin and exteriorized on the animal's dorsum. After surgery, the nonsteroidal anti‐inflammatory drug flunixin meglumine was administered for post‐operation analgesia (0.5 mg·mL−1·kg−1, s.c.). The animals were kept in individual cages during the post‐operative period and the trial.
Arterial pressure and heart rate recording
The catheter implanted into the femoral artery was connected to a pressure transducer (DPT100, Utah Medical Products Inc., Midvale, UT, USA). Pulsatile arterial pressure was recorded using an amplifier (Bridge Amp, ML221, ADInstruments, Australia) connected to a computerized data acquisition system (PowerLab 4/30, ML866, ADInstruments, Australia) using an appropriate program (Lab Chart PRO, ADInstruments, Australia). The values of mean arterial pressure (MAP) and heart rate (HR) were obtained from the pulsatile arterial pressure recordings.
Tail cutaneous temperature measurement
Tail skin temperature recordings were performed using a thermal camera Multi‐Purpose imager (IRI4010, InfraRed Integrates Systems Ltd, Northampton, UK). The analysis was performed using a software for thermographic analysis, and temperature was represented by colour intensity variations (Busnardo et al., 2013; Vianna and Carrive, 2005). For image analysis, the temperature values were obtained at five points along the animal's tail, and the mean was calculated for each recording.
Restraint stress
Restraint stress consisted of introducing the animals into plastic cylindrical tubes (diameter = 6.5 cm, length = 15 cm), which were ventilated by 0.5 in. holes that comprised approximately 20% of the tube. In the present study, restraint stress session lasted 30 min (Adami et al., 2017; Barretto‐de‐Souza et al., 2018; Choi et al., 2007), after which animals were returned to their home cages. Each animal underwent only one session of stress in order to avoid habituation. This model of stress in rats has been widely used for several years (Bali and Jaggi, 2015; Buynitsky and Mostofsky, 2009).
Drug microinjections into the brain
Injection needles (33G, Small Parts, USA) used for the microinjection of drugs into the BNST were 1 mm longer than the guide cannulae fixed to the skull. The needle was connected to a 2 μL syringe (7002KH, Hamilton, USA) using PE‐10 tubing.
Experimental design
Rats in all experimental protocols were brought to the experimental room in their own cages. Animals were allowed at least 60 min to adapt to the experimental room conditions, such as sound and illumination, before starting the experiments. The experimental room was temperature controlled (24°C) and acoustically isolated from the other rooms. Cardiovascular recordings began at least 20 min before the onset of the restraint and continued throughout the period of stress. The tail skin temperature was measured 10, 5 and 0 min before the restraint for baseline values and every 5 min during restraint.
In each protocol, animals were randomly distributed among the experimental groups. Only animals in which the microinjection sites reached the BNST were included in the study. Researchers were not blinded to treatment groups during experiments and data analysis once all data were obtained via direct recording of physiological parameters, so that analysis did not include any subjective evaluation.
Involvement of local noradrenergic neurotransmission in the control of cardiovascular responses to restraint stress by NO in the BNST
This protocol aimed to investigate whether the control of restraint‐evoked cardiovascular responses by nitrergic neurotransmission in the BNST is mediated by a facilitation of local noradrenergic neurotransmission. The control of cardiovascular responses to restraint stress by noradrenergic neurotransmission in the BNST is selectively mediated by activation of local α1‐adrenoceptors (Crestani et al., 2009). Therefore, independent sets of rats received intra‐BNST microinjections of either the selective α1‐adrenoceptor antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=499 (1.5 nmol/200 nL) or vehicle (saline, 200 nL), followed 5 min later by microinjection of the NO donor NOC‐9 (75 nmol/200 nL) or vehicle (Tris–HCl, 200 nL) into the BNST (Crestani et al., 2009; Faria et al., 2016). Five minutes after the second pharmacological treatment of the BNST, animals in all experimental groups were exposed to the standard 30 min session of restraint stress.
Forty‐six animals were used in this experiment. Histological analysis confirmed that microinjection sites reached the BNST in 34 animals, which were included in the present study. The individual samples of each experimental group are presented in Table 1.
Table 1.
Basal parameters of MAP, HR and tail skin temperature (T) before and after pharmacological treatment of the BNST
| Groups | n | MAP | HR | Skin temperature | |||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||
| VEH + VEH | 9 | 106 ± 3 | 106 ± 2 | 401 ± 15 | 378 ± 14 | 29.1 ± 0.8 | 29.4 ± 0.6 |
| t = 0.6 | t = 1 | t = 0.3 | |||||
| WB + VEH | 8 | 102 ± 2 | 104 ± 2 | 365 ± 6 | 350 ± 8 | 30.4 ± 0.8 | 29.9 ± 0.7 |
| t = 0.5 | t = 1.3 | t = 0.5 | |||||
| VEH + NOC9 | 9 | 114 ± 2 | 116 ± 3 | 386 ± 15 | 370 ± 9 | 26.2 ± 0.7 | 28.0 ± 0.7 |
| t = 0.6 | t = 0.8 | t = 1.9 | |||||
| WB + NOC9 | 8 | 110 ± 2 | 109 ± 1 | 368 ± 12 | 363 ± 8 | 31.4 ± 0.7 | 30.7 ± 0.5 |
| t = 0.7 | t = 0.3 | t = 0.8 | |||||
| VEH + VEH | 8 | 105 ± 3 | 105 ± 2 | 390 ± 11 | 399 ± 15 | 27.5 ± 0.3 | 26.9 ± 0.5 |
| t = 0.03 | t = 0.4 | t = 1.1 | |||||
| NPLA+VEH | 6 | 104 ± 2 | 106 ± 2 | 389 ± 10 | 396 ± 15 | 27.4 ± 0.5 | 27.2 ± 0.9 |
| t = 0.4 | t = 0.3 | t = 0.2 | |||||
| VEH + NMDA | 6 | 111 ± 1 | 109 ± 3 | 387 ± 10 | 392 ± 12 | 27.6 ± 0.7 | 26.3 ± 0.4 |
| t = 0.2 | t = 0.2 | t = 1.7 | |||||
| NPLA+NMDA | 6 | 107 ± 1 | 106 ± 2 | 390 ± 10 | 398 ± 12 | 27.5 ± 0.6 | 27.3 ± 0.5 |
| t = 0.3 | t = 0.5 | t = 1.7 | |||||
| WB + SAL | 8 | 103 ± 2 | 105 ± 2 | 345 ± 6 | 360 ± 6 | 29.5 ± 0.6 | 29.2 ± 0.5 |
| t = 0.8 | t = 1.6 | t = 0.4 | |||||
| WB + NPLA | 11 | 98 ± 2 | 102 ± 1 | 352 ± 6 | 358 ± 8 | 30.8 ± 0.5 | 30.2 ± 0.3 |
| t = 1.8 | t = 0.5 | t = 1.5 | |||||
Data are expressed as means ± SEM. Values of t are shown as calculated for Student's t‐test. No significant differences between values before and after treatments were found
Involvement of local nNOS in the control of cardiovascular responses to restraint stress by NMDA receptors in the BNST
This protocol aimed to investigate the involvement of local NO release from nNOS in the control of cardiovascular responses to acute restraint stress by the NMDA receptors within the BNST. For this, independent sets of rats received bilateral intra‐BNST microinjections of either the selective nNOS inhibitor, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6525; 0.1 nmol/100 nL) or vehicle (saline, 100 nL), followed 5 min later by the microinjection of NMDA (0.1 nmol/100 nL) or vehicle (saline, 100 nL) into the BNST (Alves et al., 2009; Bali and Jaggi, 2015; Buynitsky and Mostofsky, 2009; Martins‐Pinge et al., 2012). Five minutes after the second pharmacological treatment of the BNST, animals in all experimental groups were subjected to the standard restraint stress.
Thirty‐six animals were used in this protocol. Histological analysis confirmed that microinjection sites reached the BNST in 26 animals, which were included in the present study. The individual samples of each experimental group are presented in Table 1.
Effect of bilateral microinjections of NPLA into the BNST on cardiovascular responses to acute restraint stress in rats pretreated with WB4101 into the BNST
To confirm the involvement of nNOS in mechanisms within the BNST related to a facilitatory control of restraint‐evoked cardiovascular responses, we investigated the effect of nNOS blockade in the absence of local neurochemical mechanisms related to the inhibitory influence of nNOS (i.e. noradrenergic neurotransmission). For this, the selective nNOS inhibitor NPLA (0.4 nmol/100 nL) or vehicle (saline, 100 nL) was microinjected bilaterally into the BNST of animals pretreated locally with the selective α1‐adrenoceptor antagonist WB4101 (10 nmol/100 nL) (Barretto‐de‐Souza et al., 2018; Crestani et al., 2009). Five minutes after the second pharmacological treatment of the BNST, animals in all experimental groups underwent the 30 min of restraint stress.
Twenty‐one animals were used in this experiment. Histological analysis confirmed that microinjection sites reached the BNST in 19 animals, which were included in the present study. The individual samples of each experimental group are presented in Table 1.
Histological determination of the microinjection sites
At the end of each experiment, rats in all experimental groups were anesthetized with urethane (250 mg·mL−1·200 g−1 body weight, i.p.) and 1% Evans blue dye at an equal volume to drug injections was microinjected into the brain as a marker of the injection sites. The brains were then removed from the cranial cavity and post‐fixed in 10% formalin solution for at least 48 h at 4°C. Then, serial 40‐μm‐thick sections of the BNST region were cut using a cryostat (CM1900, Leica, Wetzlar, Germany). The sites of injection sites were analysed using the Atlas of Paxinos and Watson (1997) as a reference.
Data and statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2018). All analysis were performed using the GraphPad Prim 7 software. Data are presented as mean ± SEM. Initially, all data were submitted to D'Agostino‐Pearson omnibus test to assess the sample variance, which indicated a homogeneity of the data in all experimental groups. Then, the basal values of MAP, HR and tail skin temperature before and after the pharmacological treatments were compared using Student's t‐test. The time‐course curves of cardiovascular changes during restraint stress were analysed using two‐way ANOVA, with treatment as main factor and time as repeated measurement, followed by Bonferroni's post hoc test. Statistical significance was set at P < 0.05.
Materials
Nω‐propyl‐L‐arginine (NPLA) a selective nNOS inhibitor, supplied by Tocris (Westwoods Business, Park Ellisville, MO, USA); WB4101, a selective α1‐adrenoceptor antagonist; NMDA; tribromoethanol and urethane, supplied by Sigma‐Aldrich (St Louis, MO ), were dissolved in saline (0.9% NaCl). NOC‐9 (Sigma‐Aldrich) was dissolved in 1 M Tris–HCl solution. NOC‐9 solution was prepared at pH 10 to prevent NO release before it reached the brain tissue. NOC‐9 is relatively stable at an alkaline pH (>10.0) and produces NO at physiological pH (7.4) (Keefer et al., 1996). The pH of other drugs was adjusted to 7.4. Flunixin meglumine (Banamine®; Schering‐Plough, Cotia, SP, Brazil) and the poly‐antibiotic preparation (Pentabiotico®; Fort Dodge, Campinas, SP, Brazil) were used as provided.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a, 2017b, 2017c).
Results
Involvement of local noradrenergic neurotransmission in the control of cardiovascular responses to restraint stress by NO in the BNST
Photomicrograph of a coronal brain section depicting bilateral microinjection sites in BNST of a representative animal is presented in Figure S1. Bilateral treatment of the BNST with the selective α1‐adrenoceptor antagonist WB4101 and/or the NO donor NOC‐9 did not affect baseline values of either MAP, HR or tail skin temperature (Table 1). Nevertheless, acute restraint stress evoked a sustained increase in both MAP and HR and decreased the tail skin temperature (Figure 1). Analysis also indicated a significant effect of pharmacological treatments of the BNST in MAP and HR responses to restraint stress, but without affecting the drop on tail skin temperature (Figure 1). Significant treatment × time interaction was identified for MAP and HR, but not for the skin temperature. Post hoc analysis did not reveal an effect of WB4101, when it was given alone (WB4101 + veh group) in any parameters analysed (Figure 1). However, intra‐BNST injection of NOC‐9 alone (veh + NOC‐9 group) decreased the increase of MAP and HR evoked by restraint stress (Figure 1). This effect of NOC‐9 on HR, but not on MAP, was inhibited in animals pretreated with WB4101 injected into the BNST (WB + NOC‐9 group) (Figure 1). Diagrammatic representations showing the microinjection sites into the BNST of all animals used in this protocol are presented in Figure 1.
Figure 1.
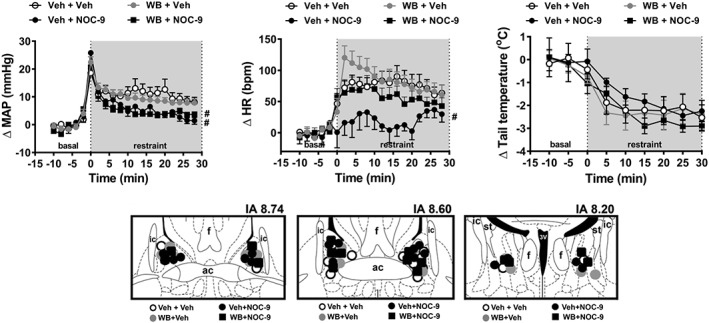
(upper Figure) Effect of BNST treatment with the selective α1‐adrenoceptor antagonist WB4101 (WB) and/or the NO donor NOC‐9 on cardiovascular responses to acute restraint stress. Time course curves of change on MAP (ΔMAP), HR (ΔHR) and tail skin temperature (Δ tail temperature) evoked by acute restraint stress in animals treated bilaterally into the BNST with either Veh + Veh (n = 9), WB + Veh (n = 8), Veh + NOC‐9 (n = 9) or WB + NOC‐9 (n = 8). Shaded area indicates the period of restraint. Data shown are means ± SEM. # P < 0.05, significantly different from the Veh + Veh group, over the whole restraint period; two‐way ANOVA followed by Bonferroni post hoc test. (lower Figure) Diagrammatic representation based on the rat brain atlas of Paxinos and Watson (1997) indicating the microinjection sites into the BNST of the groups Veh + Veh, WB + Veh, Veh + NOC‐9 and WB + NOC‐9. 3V, third ventricle; ac, anterior commissure; f, fornix; IA, interaural coordinate; ic, internal capsule; st, stria terminalis.
Involvement of local NO signalling in the control of cardiovascular responses to restraint stress by NMDA in the BNST
Bilateral microinjections into the BNST of the selective nNOS inhibitor NPLA and/or NMDA did not affect baseline values of either MAP, HR or tail skin temperature (Table 1). However, acute restraint stress evoked a sustained increase in both MAP and HR and decreased the tail skin temperature (Figure 2). Further analysis indicated a significant effect of pharmacological treatments of the BNST on restraint‐evoked tachycardia, but not in MAP and tail skin temperature responses (Figure 2). Analysis also indicated a treatment × time interaction for HR and MAP, but not for and skin temperature. Post hoc analysis did not reveal an effect of NPLA given alone (NPLA+veh group) on any of the parameters analysed (Figure 2). However, bilateral microinjection of NMDA into the BNST (veh + NMDA group) enhanced the restraint‐evoked tachycardia (Figure 2) and this effect of NMDA on HR was not observed in animals pretreated with NPLA into the BNST (NPLA+NMDA group) (Figure 2). Figure 2 presents diagrammatic representations showing microinjection sites into the BNST of all animals used in this protocol.
Figure 2.
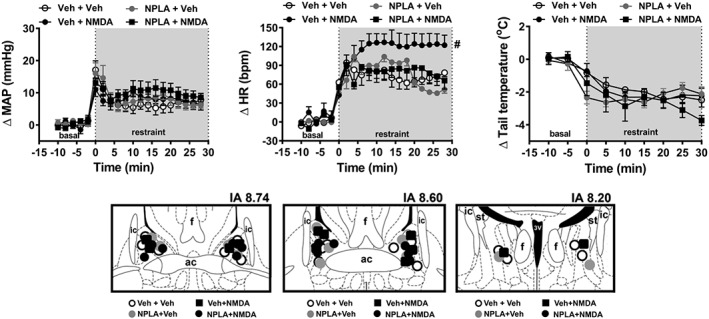
(upper Figure) Effect of BNST treatment with the selective nNOS inhibitor NPLA and/or the glutamatergic agonist NMDA on cardiovascular responses to acute restraint stress. Time course curves of change on MAP (ΔMAP), HR (ΔHR) and tail skin temperature (Δ tail temperature) evoked by acute restraint stress in animals treated bilaterally into the BNST with either Veh + Veh (n = 8), NPLA+Veh (n = 6), Veh + NMDA (n = 6) or NPLA+ NMDA (n = 6). Shaded area indicates the period of restraint. Data shown are means ± SEM. # P < 0.05, significantly different from the Veh + Veh group over the whole restraint period; two‐way ANOVA followed by Bonferroni post hoc test. (lower Figure) Diagrammatic representation based on the rat brain atlas of Paxinos and Watson (1997) indicating the microinjection sites into the BNST of the groups Veh + Veh, NPLA+Veh, Veh + NMDA and NPLA+NMDA. 3V, third ventricle; ac, anterior commissure; f, fornix; IA, interaural coordinate; ic, internal capsule; st, stria terminalis.
Effect of bilateral microinjection of NPLA in the BNST in cardiovascular responses to acute restraint stress in rats pretreated with WB4101 in the BNST
Bilateral treatment of the BNST with the selective nNOS inhibitor NPLA in animals locally pretreated with the selective α1‐adrenoceptor antagonist WB4101 did not affect baseline values of either MAP, HR or tail skin temperature (Table 1). Nevertheless, acute restraint stress evoked a sustained increase in both MAP and HR and decreased the tail skin temperature (Figure 3). Two‐way ANOVA analysis indicated that bilateral microinjection of NPLA into the BNST of animals locally pretreated with WB4101 decreased the restraint‐evoked increase in MAP and HR, but without affecting the tail skin temperature (Figure 3). The analysis also indicated a treatment × time interaction for MAP and HR, but not for tail skin temperature. Diagrammatic representations showing microinjection sites into the BNST of all animals used in this protocol are presented in Figure 3.
Figure 3.
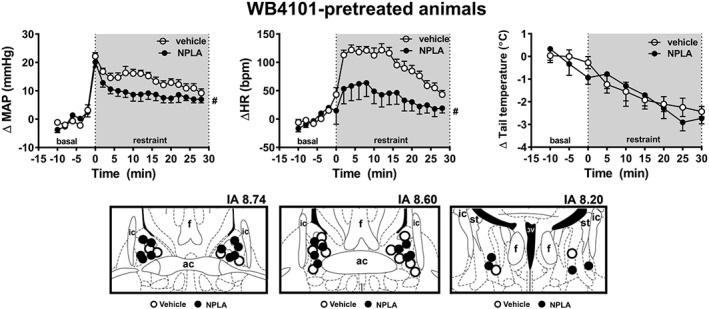
(upper Figure) Effect of BNST treatment with the selective nNOS inhibitor NPLA on cardiovascular responses to acute restraint stress in rats pretreated with the selective α1‐adrenoceptor antagonist WB4101 into the BNST. Time course curves of change on MAP (ΔMAP), HR (ΔHR) and tail skin temperature (Δ tail temperature) evoked by acute restraint stress in animals locally pretreated with WB4101 and subjected to bilateral microinjection into the BNST of either NPLA (n = 11) or vehicle (n = 8). Shaded area indicates the period of restraint. Data shown are means ± SEM. # P < 0.05, significantly different from the Veh + Veh group, over the whole restraint period; two‐way ANOVA followed by Bonferroni post hoc test. (lower Figure) Diagrammatic representation based on the rat brain atlas of Paxinos and Watson (1997) indicating the microinjection sites into the BNST of vehicle and NPLA in animals pretreated locally with WB4101. 3V, third ventricle; ac, anterior commissure; f, fornix; IA, interaural coordinate; ic, internal capsule; st, stria terminalis.
Discussion
This study shows for the first time an interaction of nitrergic neurotransmission within the BNST with local glutamatergic and noradrenergic neurotransmission. Furthermore, the present results are the first to indicate a dual role of nitrergic neurotransmission in the BNST in the control of cardiovascular responses evoked by aversive threats. Indeed, we observed that bilateral microinjection of the NO donor NOC‐9 into the BNST decreased the restraint‐evoked increase in HR and arterial pressure. The inhibitory influence of NOC‐9 in HR, but not in arterial pressure, was inhibited by local BNST pretreatment with the selective α1‐adrenoceptor antagonist WB4101. Moreover, microinjection of NMDA into the BNST enhanced the tachycardic response to restraint, and this effect was abolished by local pretreatment with the selective nNOS inhibitor NPLA. An involvement of nNOS in the BNST in local facilitatory control of cardiovascular responses to restraint stress was further reinforced by demonstration that microinjection of the nNOS inhibitor in animals pretreated with the α1‐adrenoceptor antagonist (the local neurochemical mechanism related to the inhibitory influence of nNOS) decreased the restraint‐evoked increase in HR and arterial pressure.
Recent results from our group have shown an involvement of nitrergic signalling in the BNST in the control of cardiovascular responses to restraint stress (Barretto‐de‐Souza et al., 2018), as microinjection of either a nonselective NOS inhibitor or a selective nNOS inhibitor into the BNST enhanced the restraint‐evoked HR increase. Local BNST treatment with inhibitors of signalling mechanisms related to NO effects such as sGC and PKG also enhanced HR response to restraint. Taken together, these results provided evidence of an inhibitory role of nitrergic signalling within the BNST in the cardiovascular responses to restraint stress. The decreased HR and arterial pressure responses reported in the present study in animals that received NOC‐9 administration into the BNST provides further evidence of a predominantly inhibitory role of nitrergic neurotransmission in the BNST in the cardiovascular responses evoked by restraint. However, more importantly, the present findings indicate that the inhibitory control of HR response by nitrergic signalling in the BNST is mediated by a facilitation of local noradrenergic neurotransmission acting via local α1‐adrenoceptors. These data are also compatible with previous evidence that noradrenergic neurotransmission in the BNST, acting via α1‐adrenoceptors plays an inhibitory role in restraint‐evoked tachycardia (Crestani et al., 2009). Moreover, our results are supported by reports that NO stimulated the release of noradrenaline in limbic structures such as the hippocampus and cerebral cortex (Ohkuma and Katsura, 2001; Philippu, 2016; Prast and Philippu, 2001). However, to the best of our knowledge, the present findings constitute the first evidence of an interaction between nitrergic and noradrenergic neurotransmissions within the BNST.
The decrease in arterial pressure response evoked by NOC‐9 was not affected by local α1‐adrenoceptor antagonism. This finding is in line with a previous report that BNST noradrenergic neurotransmission plays an inhibitory role in tachycardia, without affecting the arterial pressure in response to restraint (Crestani et al., 2009). Therefore, the modulation of restraint‐evoked pressor response by nitrergic neurotransmission in the BNST seems to be mediated by mechanisms other than local noradrenergic neurotransmission. One possible option is cholinergic neurotransmission, as activation of muscarinic receptors in the BNST inhibited the cardiovascular responses to restraint stress (Gouveia et al., 2016). Although there are reports of an NO‐induced release of acetylcholine in limbic structures such as the hippocampus and prefrontal cortex (Ohkuma and Katsura, 2001; Prast and Philippu, 2001), a possible interaction between nitrergic and cholinergic neurotransmissions within the BNST has never been evaluated. Therefore, further studies are needed to clarify the mechanisms related to the inhibitory influence of nitrergic neurotransmission in the BNST in the pressor response to restraint stress.
Activation of nNOS in response to influx of Ca2+ following activation of the NMDA receptor is the best characterized mechanism underlying NO synthesis in the CNS (Garthwaite, 2008; Garthwaite, 2016; Prast and Philippu, 2001). Nevertheless, contrary to the inhibitory influence of nitrergic neurotransmission in restraint‐evoked cardiovascular responses, we recently reported a facilitation of the HR response to restraint by NMDA receptors in the BNST (Adami et al., 2017). The enhanced tachycardia reported in the present study following microinjection of NMDA into the BNST further reinforces the idea of a facilitatory role of local glutamatergic neurotransmission in cardiac responses to restraint stress. However, the present results provide the first evidence of a role of local nitrergic signalling in the control of stress‐evoked cardiovascular responses by NMDA receptors within the BNST. This finding is in line with previous evidence of a prominent role of nNOS activation in the control of cardiovascular function by the NMDA receptor in other CNS structures (Busnardo et al., 2010; Martins‐Pinge et al., 2007; Resstel and Correa, 2006; Santini et al., 2013; Tavares et al., 2007).
Although the present findings indicate nNOS as part of local signalling pathways related to a facilitatory influence in cardiovascular responses to stress, this is not the predominant role of nitrergic neurotransmission. Indeed, as discussed above, the microinjection of either nNOS inhibitors (Barretto‐de‐Souza et al., 2018) or NO donors (see Figure 1) provides evidence of a predominantly inhibitory role of this neurochemical mechanism within the BNST. However, we observed in the present study that the microinjection of the nNOS inhibitor, in the absence of the local neurochemical mechanism related to the inhibitory influence of nNOS (i.e. noradrenergic neurotransmission acting via α1‐adrenoceptors) unmasked the facilitatory role of nNOS, as shown by the decreased arterial pressure and HR responses to restraint (see Figure 3). This finding confirms that nNOS may act within the BNST to counteract the predominantly inhibitory influence.
The predominantly inhibitory influence of nitrergic neurotransmission in the BNST corroborates reports of protective cardiovascular effects of nitrergic neurotransmission in the CNS (Martins‐Pinge et al., 2012; Sharma and Patel, 2017; Stern et al., 2003). Central networks, providing inhinbitory control of cardiovascular responses during aversive threats, are important as they allow precise response control, fine tuning and functional state stabilization of the target organ, thus reducing the amplitude of the response (Berntson et al., 1991; Paton et al., 2005). Therefore, nNOS activation within the BNST may be an important mechanism counteracting excessive cardiac activation during stress. The nNOS involvement in the facilitatory control of restraint‐evoked cardiovascular responses, mediated by NMDA receptors, is in line with previous findings of the pro‐aversive effects of NO in the brain (Calixto et al., 2008; Guimaraes et al., 2005; Silva et al., 2012).
Corticolimbic structures such as the hippocampus, amygdala and medial prefrontal cortex have little direct anatomical connections with primary stress effector regions in hypothalamus and brainstem (Myers, 2017; Ulrich‐Lai and Herman, 2009). However, outputs from these regions converge on the BNST (Dong et al., 2001a; Myers et al., 2014) and, in turn, BNST neurons project to hypothalamic and brainstem nuclei controlling autonomic activity (Dong et al., 2001b; Dong and Swanson, 2006). Thus, the BNST has been proposed as a relay station between processing of emotional information by corticolimbic structures, and elaboration of physiological and behavioural responses to stress by hypothalamic and brainstem regions (Crestani et al., 2013; Myers, 2017; Ulrich‐Lai and Herman, 2009). Limbic inputs to the BNST are predominantly glutamatergic and GABAergic (Myers et al., 2014). In this context, the present study provides the first evidence of an involvement of nNOS activation in the control of stress responses by glutamatergic inputs in the BNST acting via NMDA receptors. However, the present study provides evidence that the nitrergic neurotransmission in the BNST acts predominantly by modulating information from noradrenergic inputs within the BNST. An interaction between noradrenergic and glutamatergic neurotransmissions within the BNST has been reported (Silberman and Winder, 2013), thus supporting a prominent role of nitrergic neurotransmission acting via facilitation of noradrenergic neurotransmission in the processing of limbic information in the BNST for elaboration of cardiovascular responses.
The opposing roles of nitrergic neurotransmission in the BNST in the control of stress‐evoked cardiovascular changes acting via modulation of local noradrenergic (inhibitory) and glutamatergic (stimulatory) are supported by earlier findings that noradrenaline evoked predominantly an inhibitory influence in activity of neurons within the BNST (Casada and Dafny, 1993). Accordingly, activation of local α1‐adrenoceptors inhibited the activity of neurons within the BNST by presynaptically facilitating local GABAergic neutransmission (Dumont and Williams, 2004). Therefore, the predominant inhibitory role of NO in cardiovascular changes to stress is possibly mediated by a facilitation of local noradrenergic neurotransmission, which in turn facilitates local GABAergic terminals via activation of α1‐adrenoceptors. A schematic representation outlining the mechanism by which NO within the BNST inhibits (via noradrenergic interaction) and facilitates (via glutamatergic interaction) the restraint‐evoked cardiovascular responses is presented in Figure 4.
Figure 4.
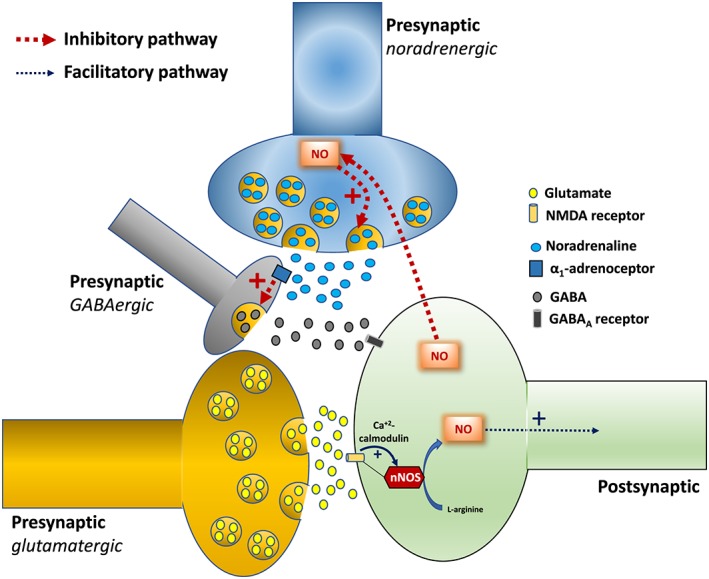
Schematic representation illustrating the local mechanisms by which NO released from nNOS into the BNST modulates the cardiovascular responses to restraint stress. (Inhibitory mechanism) The red dotted arrows indicate the pathway related to the predominant inhibitory influence of BNST nitrergic neurotransmission in the control of cardiovascular responses to restraint. The NO synthesized by nNOS stimulates the release of noradrenaline which in turn facilitates local GABA release via activation of α1‐adrenoceptors in GABAergic terminals. The GABA evokes inhibitory postsynaptic currents (IPSCs) via activation of the GABAA receptor. (Facilitatory mechanism) The blue arrows indicate the facilitatory pathway of nitrergic neurotransmission in the BNST in controlling the cardiovascular responses to restraint stress. The NO synthesized by nNOS is a prominent signalling mechanism involved in the effects of NMDA receptor activation (yellow channel). The thickness of the arrows indicates the predominance of the pathways (i.e. inhibitory and facilitatory mechanism). See text for further details.
In summary, the results of the present study provide evidence that nitrergic neurotransmission in the BNST plays a dual role in the cardiovascular responses to stress. A predominantly inhibitory influence in HR responses is mediated by a facilitation of local noradrenergic neurotransmission acting via α1‐adrenoceptors. However, inhibition of arterial pressure response seems to be mediated by mechanisms others than local noradrenergic neurotransmission. Furthermore, our data suggest that local NO release from nNOS is part of the signalling pathway related to the facilitatory control of HR response to restraint stress by NMDA receptors within the BNST.
Author contributions
L.B.S. and C.C.C. conceived and designed this research; L.B.S., M.B.A. and R.B performed the experiments and analysed the data; L.B.S., M.B.A., R.B. and C.C.C. interpreted the results of experiments; L.B.S. prepared the figures and drafted the manuscript; L.B.S. and C.C.C. edited and revised the manuscript; C.C.C. approved the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This http://onlinelibrary.wiley.com/doi/10.1111/bph.13405/abstract acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 Photomicrograph of a coronal brain section depicting bilateral microinjection sites in the bed nucleus of the stria terminalis (BNST) of a representative animal. Arrows indicate the microinjection sites. ac – anterior commissure; cc – corpus callosum; f – fornix; LV – lateral ventricle.
Acknowledgements
The authors wish to acknowledge Elisabete Lepera and Rosana Silva for technical assistance. The present research was supported by grants from FAPESP (grant # 2015/05922‐9), CNPq (grant # 456405/2014‐3) and Scientific Support and Development Program of School of Pharmaceutical Sciences (UNESP). C.C.C. is a CNPq research fellow (process # 305583/2015‐8).
Barretto‐de‐Souza, L. , Adami, M. B. , Benini, R. , and Crestani, C. C. (2018) Dual role of nitrergic neurotransmission in the bed nucleus of the stria terminalis in controlling cardiovascular responses to emotional stress in rats. British Journal of Pharmacology, 175: 3773–3783. 10.1111/bph.14447.
References
- Adami MB, Barretto‐de‐Souza L, Duarte JO, Almeida J, Crestani CC (2017). Both N‐methyl‐D‐aspartate and non‐N‐methyl‐D‐aspartate glutamate receptors in the bed nucleus of the stria terminalis modulate the cardiovascular responses to acute restraint stress in rats. J Psychopharmacol 31: 674–681. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174 (Suppl. 1): S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174 (Suppl. 1): S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion NV, Faccenda E, Harding SD et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Ligand‐gated ion channels. Br J Pharmacol 174 (Suppl. 1): S130–S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves FH, Crestani CC, Resstel LB, Correa FM (2009). Bed nucleus of the stria terminalis N‐methyl‐D‐aspartate receptors and nitric oxide modulate the baroreflex cardiac component in unanesthetized rats. J Neurosci Res 87: 1703–1711. [DOI] [PubMed] [Google Scholar]
- Bali A, Jaggi AS (2015). Preclinical experimental stress studies: protocols, assessment and comparison. Eur J Pharmacol 746: 282–292. [DOI] [PubMed] [Google Scholar]
- Barretto‐de‐Souza L, Adami MB, Oliveira LA, Gomes‐de‐Souza L, Duarte JO, Almeida J et al (2018). Nitric oxide‐cGMP‐PKG signaling in the bed nucleus of the stria terminalis modulates the cardiovascular responses to stress in male rats. Eur Neuropsychopharmacol 28: 75–84. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS (1991). Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev 98: 459–487. [DOI] [PubMed] [Google Scholar]
- Busnardo C, Alves FH, Crestani CC, Scopinho AA, Resstel LB, Correa FM (2013). Paraventricular nucleus of the hypothalamus glutamate neurotransmission modulates autonomic, neuroendocrine and behavioral responses to acute restraint stress in rats. Eur Neuropsychopharmacol 23: 1611–1622. [DOI] [PubMed] [Google Scholar]
- Busnardo C, Crestani CC, Tavares RF, Resstel LB, Correa FM (2010). Cardiovascular responses to L‐glutamate microinjection into the hypothalamic paraventricular nucleus are mediated by a local nitric oxide‐guanylate cyclase mechanism. Brain Res 1344: 87–95. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI (2009). Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev 33: 1089–1098. [DOI] [PubMed] [Google Scholar]
- Casada JH, Dafny N (1993). Responses of neurons in bed nucleus of the stria terminalis to microiontophoretically applied morphine, norepinephrine and acetylcholine. Neuropharmacology 32: 279–284. [DOI] [PubMed] [Google Scholar]
- Calixto AV, Duarte FS, Moraes CK, Faria MS, De Lima TC (2008). Nitric oxide involvement and neural substrates of the conditioned and innate fear as evaluated in the T‐maze test in rats. Behav Brain Res 189: 341–349. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich‐Lai YM, Herman JP (2007). Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic‐pituitary‐adrenal axis activity: implications for the integration of limbic inputs. J Neurosci 27: 2025–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC (2016). Emotional stress and cardiovascular complications in animal models: a review of the influence of stress type. Front Physiol 7: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP (2013). Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol 11: 141–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Tavares RF, Correa FM (2009). Role of the bed nucleus of the stria terminalis in the cardiovascular responses to acute restraint stress in rats. Stress 12: 268–278. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Alexander S, Cirino G, Docherty JR, George CH, Giembycz MA et al (2018). Experimental design and analysis and their reporting II: updated and simplified guidance for authors and peer reviewers. Brit J Pharmacol 175: 987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RA (2015). Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am J Physiol Regul Integr Comp Physiol 309: R429–R443. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW (2001a). Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev 38: 192–246. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW (2001b). Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol 436: 430–455. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW (2006). Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol 494: 142–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT (2004). Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24: 8198–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria MP, Miguel TT, Gomes KS, Nunes‐de‐Souza RL (2016). Anxiety‐like responses induced by nitric oxide within the BNST in mice: Role of CRF1 and NMDA receptors. Horm Behav 79: 74–83. [DOI] [PubMed] [Google Scholar]
- Garthwaite J (2008). Concepts of neural nitric oxide‐mediated transmission. Eur J Neurosci 27: 2783–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J (2016). From synaptically localized to volume transmission by nitric oxide. J Physiol 594: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia MK, Miguel TT, Busnardo C, Scopinho AA, Correa FM, Nunes‐de‐Souza RL et al (2016). Dissociation in control of physiological and behavioral responses to emotional stress by cholinergic neurotransmission in the bed nucleus of the stria terminalis in rats. Neuropharmacology 101: 379–388. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Beijamini V, Moreira FA, Aguiar DC, de Lucca AC (2005). Role of nitric oxide in brain regions related to defensive reactions. Neurosci Biobehav Rev 29: 1313–1322. [DOI] [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hott SC, Gomes FV, Uliana DL, Vale GT, Tirapelli CR, Resstel LB (2017). Bed nucleus of the stria terminalis NMDA receptors and nitric oxide modulate contextual fear conditioning in rats. Neuropharmacology 112: 135–143. [DOI] [PubMed] [Google Scholar]
- Keefer LK, Nims RW, Davies KM, Wink DA (1996). “NONOates” (1‐substituted diazen‐1‐ium‐1,2‐diolates) as nitric oxide donors: convenient nitric oxide dosage forms. Methods Enzymol 268: 281–293. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins‐Pinge MC, Garcia MR, Zoccal DB, Crestani CC, Pinge‐Filho P (2007). Differential influence of iNOS and nNOS inhibitors on rostral ventrolateral medullary mediated cardiovascular control in conscious rats. Auton Neurosci 131: 65–69. [DOI] [PubMed] [Google Scholar]
- Martins‐Pinge MC, Mueller PJ, Foley CM, Heesch CM, Hasser EM (2012). Regulation of arterial pressure by the paraventricular nucleus in conscious rats: interactions among glutamate, GABA, and nitric oxide. Front Physiol 3: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B (2017). Corticolimbic regulation of cardiovascular responses to stress. Physiol Behav 172: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP (2014). Central stress‐integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct 219: 1287–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S, Katsura M (2001). Nitric oxide and peroxynitrite as factors to stimulate neurotransmitter release in the CNS. Prog Neurobiol 64: 97–108. [DOI] [PubMed] [Google Scholar]
- Paton JF, Boscan P, Pickering AE, Nalivaiko E (2005). The yin and yang of cardiac autonomic control: vago‐sympathetic interactions revisited. Brain Res Brain Res Rev 49: 555–565. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1997). The Rat Brain in Stereotaxic Coordinates, 3rd edn. Academic Press: Sidney, Australia. [Google Scholar]
- Philippu A (2016). Nitric oxide: a universal modulator of brain function. Curr Med Chem 23: 2643–2652. [DOI] [PubMed] [Google Scholar]
- Prast H, Philippu A (2001). Nitric oxide as modulator of neuronal function. Prog Neurobiol 64: 51–68. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Alves FH, Reis DG, Crestani CC, Correa FM, Guimaraes FS (2008). Anxiolytic‐like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience 154: 869–876. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM (2006). Injection of l‐glutamate into medial prefrontal cortex induces cardiovascular responses through NMDA receptor – nitric oxide in rat. Neuropharmacology 51: 160–167. [DOI] [PubMed] [Google Scholar]
- Santini CO, Fassini A, Scopinho AA, Busnardo C, Correa FM, Resstel LB (2013). The ventral hippocampus NMDA receptor/nitric oxide/guanylate cyclase pathway modulates cardiovascular responses in rats. Auton Neurosci 177: 244–252. [DOI] [PubMed] [Google Scholar]
- Sharma NM, Patel KP (2017). Post‐translational regulation of neuronal nitric oxide synthase: implications for sympathoexcitatory states. Expert Opin Ther Targets 21: 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG (2013). Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front Psychiatry 4: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Aguiar DC, Diniz CR, Guimaraes FS, Joca SR (2012). Neuronal NOS inhibitor and conventional antidepressant drugs attenuate stress‐induced fos expression in overlapping brain regions. Cell Mol Neurobiol 32: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P (2012). Allostasis: a model of predictive regulation. Physiol Behav 106: 5–15. [DOI] [PubMed] [Google Scholar]
- Stern JE, Li Y, Zhang W (2003). Nitric oxide: a local signalling molecule controlling the activity of pre‐autonomic neurones in the paraventricular nucleus of the hypothalamus. Acta Physiol Scand 177: 37–42. [DOI] [PubMed] [Google Scholar]
- Tavares RF, Resstel LB, Correa FM (2007). Interaction between glutamatergic and nitrergic mechanisms mediating cardiovascular responses to L‐glutamate injection in the diagonal band of Broca in anesthetized rats. Life Sci 81: 855–862. [DOI] [PubMed] [Google Scholar]
- Ulrich‐Lai YM, Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianna DM, Carrive P (2005). Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci 21: 2505–2512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Photomicrograph of a coronal brain section depicting bilateral microinjection sites in the bed nucleus of the stria terminalis (BNST) of a representative animal. Arrows indicate the microinjection sites. ac – anterior commissure; cc – corpus callosum; f – fornix; LV – lateral ventricle.


