Abstract
Bilirubin neurotoxicity has been studied for decades and has been shown to affect various mechanisms via significant modulation of gene expression. This suggests that vital regulatory mechanisms of gene expression, such as epigenetic mechanisms, could play a role in bilirubin neurotoxicity. Histone acetylation has recently received attention in the CNS due to its role in gene modulation for numerous biological processes, such as synaptic plasticity, learning, memory, development and differentiation. Aberrant epigenetic regulation of gene expression in psychiatric and neurodegenerative disorders has also been described. In this work, we followed the levels of histone 3 lysine 14 acetylation (H3K14Ac) in the cerebellum (Cll) of the developing (2, 9, 17 days after the birth) and adult Gunn rat, the natural model for neonatal hyperbilirubinemia and kernicterus. We observed an age-specific alteration of the H3K14Ac in the hyperbilirubinemic animals. The GeneOntology analysis of the H3K14Ac linked chromatin revealed that almost 45% of H3K14Ac ChiP-Seq TSS-promoter genes were involved in CNS development including maturation and differentiation, morphogenesis, dendritogenesis, and migration. These data suggest that the hallmark Cll hypoplasia in the Gunn rat occurs also via epigenetically controlled mechanisms during the maturation of this brain structure, unraveling a novel aspect of the bilirubin-induced neurotoxicity.
Introduction
Bilirubin toxicity to the CNS has been extensively studied for decades and has been shown to be linked to the activation of multiple complex signal cascades, and affects potential toxic/adaptation mechanisms in the brain through gene expression modulation. Examples include oxidative stress and the antioxidant response, excitotoxicity, inflammation, intracellular trafficking, protein degradation, apoptosis, as well as bilirubin transport and bilirubin oxidization (reviewed in1).
Epigenetic processes, such as histone acetylation and DNA methylation, regulate the expression of genes through modifications of DNA structure and accessibility. These regulatory mechanisms often contribute to the onset and progression of human neurological disorders, and are altered by toxic compounds (e.g.: cocaine, alcohol)2–8. Indeed, histone acetylation is considered an integral part of brain development and differentiation, synaptic plasticity, learning, memory, and neuron maintenance and survival9–12. Notably, it is reported that temporal changes in gene expression by acetylation/deacetylation of gene promoters induce persistent changes in the cell (e.g. cell fate), changes in the neurological behaviour8, as well induction of excitotoxicity, calcium overload, oxidative stress, inflammation and apoptosis13, with the last five described mechanisms in hyperbilirubinemic animals and humans. This suggests the possibility of a link between the hyperbilirubinemic phenotype and the epigenetic. On this basis, we decided to investigate the effect of hyperbilirubinemia on the epigenetic control of the Cll hypoplasia.
Results
Serum bilirubin and cerebellar development
To evaluate the possible correlation between serum bilirubin and the levels of H3K14Ac, we quantified total and free bilirubin in the blood, and the Cll weight in hyperbilirubinemic pups (jj) and normobilirubinemic littermates (control: ctrl) from 2 days after birth (P2) until the adult age. At every post-natal age, the total serum bilirubin (TSB, Fig. 1A) was statistically higher in jj animals compared to ctrl (Σ8.5 lifelong, one-way ANOVA: p ≤ 0.0001, followed by Tukey post-test, p ≤ 0.001). At P2, the TSB was about of 190 µM, peaking at P17 (Σ256 µM), and stabilizing in the adulthood (Σ126 µM), (ever significantly higher than in ctrl, one-way ANOVA: p ≤ 0.0001, followed by Tukey post-test, p ≤ 0.001).
Figure 1.
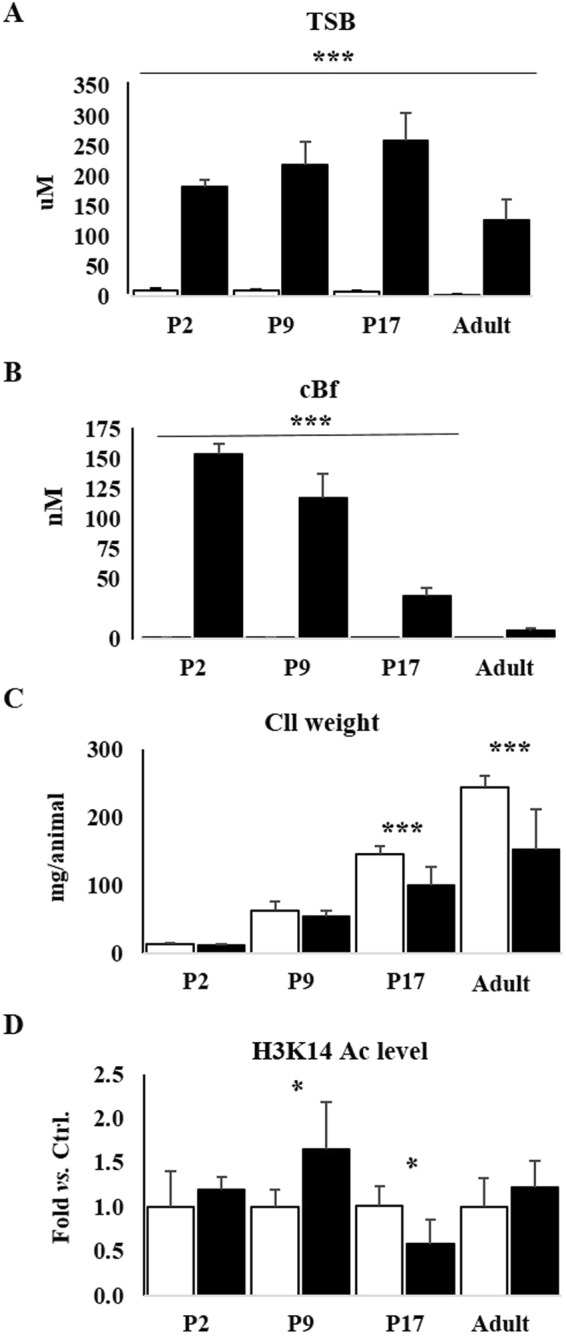
Total Serum Bilirubin (TSB), calculated free bilirubin (cBf) in the blood, cerebellar weight, and Western blot analysis of the level of histone 3 acetylation (H3K14Ac) P: post-natal age in days, Adult: more than 1-year-old. Black bars jj rats, White bars: ctrls. (A) TSB (µM); (B) cBf (nM), (C) Cll weight (mg/animal). Results are expressed as mean ± S.D. of 6–15 animals each group and post-natal age. One way ANOVA followed by Tukey post-test: ***p < 0.001. (D) H3K14Ac levels in the Cll of jj animals vs. ctrl. Results are as mean ± S.D. of 3–6 animals each group and post-natal age. Unpaired t-test with Welch correction, *p < 0.05 vs. age matched ctrl.
Free bilirubin is the moiety able to cross the blood-brain interfaces leading to neurological damage. In the absence of a reliable method for a direct quantification in the rat, free bilirubin was calculated as previously described14. Differently from TSB, the calculated Bf (cBf, Fig. 1B) level in jj pups dropped during development (P2 Σ150 nM, P9 Σ120 nM, P17 Σ35 nM, ever significantly higher than in ctrl, one-way ANOVA: p ≤ 0.0001, followed by Tukey post-test, p ≤ 0.001), felling to the levels not statistically different from those in ctrl in the adult age (adult jj Σ7 nM; One way ANOVA, followed by Tukey post-test, p > 0.05).
Cll weight (Fig. 1C) was similar in jj and ctrl littermates up to P9, becoming significantly different at P17 (Σ30% weight loss vs. age-matched ctrl, one way ANOVA followed by Tukey post-test: p < 0.001), and increasing later on (Adult: Σ40%, one way ANOVA followed by Tukey post-test: p < 0.001).
Western blot analysis of global acetylation of histone H3K14
The follow the level of H3K14Ac in the developing cerebellum of jj and controls rats by Western blot, we used the 07-353 anti-H3K13Ac antibody. At P2, no significant difference was observed in the level of H3K14Ac in the Cll of jj animals compared to age-matched ctrl (Fig. 1D) (unpaired t-test with Welch correction, p = 0.2687). The level of H3K14Ac in jj was significantly increased (1.65 ± 0.54 fold, unpaired t-test with Welch correction, p < 0.0222) at P9 and significantly decreased at P17 (0.67 ± 0.18 fold, unpaired t-test with Welch correction, p < 0.0187). In the adults there was no difference in the level of H3K14Ac between jj and ctrl (unpaired t-test with Welch correction, p = 0.4508).
ChIP-Seq analysis
To link the effect of hyperbilirubinemia on H3K14Ac with the genes controlled by this epigenetic mechanism, the 07–353 anti-H3K13Ac antibody used for Western blot analysis was also used to perform chromatin immunoprecipitation, followed by DNA sequencing (ChIP-Seq – full result available on GEO repository # GSE109145). After removal of duplicate DNA fragments and DNA fragments present in both jj and ctrl (physiological genes), 1884 unique DNA sequences were identified. Since variations in the level of histone acetylation in the promoter region positively correlate with gene transcription9,15, we focused on peaks identified by ChIP-Seq on the promoter regions (Table 1: 255 genes). As shown in Fig. 2, the functional annotation analysis of the corresponding genes16–18 revealed an enrichment for genes involved in CNS development (Σ45%), metabolism & homeostasis (Σ31%), signalling (Σ13%), response to stimuli & communication (Σ5%), transport (Σ5%), and binding (Σ2%).
Table 1.
Full list of ChIP-Seq TSS-Promoter genes.
| Gene Name | Gene Description | Nearest Refseq | Gene Type |
|---|---|---|---|
| Abcc10 | ATP-binding cassette, subfamily C (CFTR/MRP), member 10 | NM_001108201 | protein-coding |
| Acot13 | acyl-CoA thioesterase 13 | NM_001106111 | protein-coding |
| Acp1 | acid phosphatase 1, soluble | NM_021262 | protein-coding |
| Acpt | acid phosphatase, testicular | NM_001107510 | protein-coding |
| Actc1 | actin, alpha, cardiac muscle 1 | NM_019183 | protein-coding |
| Adra2b | adrenoceptor alpha 2B | NM_138505 | protein-coding |
| Agrn | agrin | NM_175754 | protein-coding |
| Ahrr | aryl-hydrocarbon receptor repressor | NM_001024285 | protein-coding |
| Aldh3a2 | aldehyde dehydrogenase 3 family, member A2 | NM_031731 | protein-coding |
| Alg11 | ALG11, alpha-1,2-mannosyltransferase | NM_001108401 | protein-coding |
| Alg8 | ALG8, alpha-1,3-glucosyltransferase | NM_001034127 | protein-coding |
| Amdhd1 | amidohydrolase domain containing 1 | NM_001191781 | protein-coding |
| Anxa2 | annexin A2 | NM_019905 | protein-coding |
| Arfgap2 | ADP-ribosylation factor GTPase activating protein 2 | NM_001033707 | protein-coding |
| Arhgap4 | Rho GTPase activating protein 4 | NM_144740 | protein-coding |
| Asl | argininosuccinate lyase | NM_021577 | protein-coding |
| Atp6v0e1 | ATPase, H+ transporting, lysosomal, V0 subunit e1 | NM_053578 | protein-coding |
| Atraid | all-trans retinoic acid-induced differentiation factor | NM_001127526 | protein-coding |
| B3galt4 | UDP-Gal:betaGlcNAc beta 1,3-galactosyltransferase, polypeptide 4 | NM_133553 | protein-coding |
| Bbs2 | Bardet-Biedl syndrome 2 | NM_053618 | protein-coding |
| Bbs5 | Bardet-Biedl syndrome 5 | NM_001108583 | protein-coding |
| Bin2 | bridging integrator 2 | NM_001012223 | protein-coding |
| Bphl | biphenyl hydrolase-like (serine hydrolase) | NM_001037206 | protein-coding |
| Brd9 | bromodomain containing 9 | NM_001107453 | protein-coding |
| Cacng8 | calcium channel, voltage-dependent, gamma subunit 8 | NM_080696 | protein-coding |
| Cap1 | CAP, adenylate cyclase-associated protein 1 (yeast) | NM_022383 | protein-coding |
| Casp6 | caspase 6 | NM_031775 | protein-coding |
| Cblc | Cbl proto-oncogene C, E3 ubiquitin protein ligase | NM_001034920 | protein-coding |
| Cct6a | chaperonin containing Tcp1, subunit 6 A (zeta 1) | NM_001033684 | protein-coding |
| Cdc20 | cell division cycle 20 | NM_171993 | protein-coding |
| Cers1 | ceramide synthase 1 | NM_001044230 | protein-coding |
| Chad | chondroadherin | NM_019164 | protein-coding |
| Chmp1a | charged multivesicular body protein 1 A | NM_001083313 | protein-coding |
| Chrnb1 | cholinergic receptor, nicotinic, beta 1 (muscle) | NM_012528 | protein-coding |
| Ciapin1 | cytokine induced apoptosis inhibitor 1 | NM_001007689 | protein-coding |
| Cidea | cell death-inducing DFFA-like effector a | NM_001170467 | protein-coding |
| Clpsl2 | colipase-like 2 | NM_001135002 | protein-coding |
| Cnksr1 | connector enhancer of kinase suppressor of Ras 1 | NM_001039011 | protein-coding |
| Col4a3 | collagen, type IV, alpha 3 | NM_001135759 | protein-coding |
| Col7a1 | collagen, type VII, alpha 1 | NM_001106858 | protein-coding |
| Cpne6 | copine VI (neuronal) | NM_001191113 | protein-coding |
| Cpsf3l | cleavage and polyadenylation specific factor 3-like | NM_001033892 | protein-coding |
| Cpsf4 | cleavage and polyadenylation specific factor 4 | NM_001012351 | protein-coding |
| Crcp | CGRP receptor component | NM_053670 | protein-coding |
| Cth | cystathionine gamma-lyase | NM_017074 | protein-coding |
| Ctr9 | CTR9 homolog, Paf1/RNA polymerase II complex component | NM_001100661 | protein-coding |
| Cyb5r1 | cytochrome b5 reductase 1 | NM_001013126 | protein-coding |
| Cyba | cytochrome b-245, alpha polypeptide | NM_024160 | protein-coding |
| Ddb1 | damage-specific DNA binding protein 1, 127 kDa | NM_171995 | protein-coding |
| Ddb2 | damage specific DNA binding protein 2 | NM_001271346 | protein-coding |
| Ddias | DNA damage-induced apoptosis suppressor | NM_001126294 | protein-coding |
| Ddit4l2 | DNA-damage-inducible transcript 4-like 2 | NM_080399 | protein-coding |
| Ddx55 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 55 | NM_001271326 | protein-coding |
| Ddx56 | DEAD (Asp-Glu-Ala-Asp) box helicase 56 | NM_001004211 | protein-coding |
| Dhdds | dehydrodolichyl diphosphate synthase subunit | NM_001011978 | protein-coding |
| Dmrtc2 | DMRT-like family C2 | NM_001109140 | protein-coding |
| Dnaja1 | DnaJ (Hsp40) homolog, subfamily A, member 1 | NM_022934 | protein-coding |
| Eif3e | eukaryotic translation initiation factor 3, subunit E | NM_001011990 | protein-coding |
| Emc3 | ER membrane protein complex subunit 3 | NM_001008355 | protein-coding |
| Emd | emerin | NM_012948 | protein-coding |
| Entpd6 | ectonucleoside triphosphate diphosphohydrolase 6 | NM_053498 | protein-coding |
| Eny2 | enhancer of yellow 2 homolog (Drosophila) | NM_001130580 | protein-coding |
| Ephx2 | epoxide hydrolase 2, cytoplasmic | NM_022936 | protein-coding |
| Fam151a | family with sequence similarity 151, member A | NM_001005558 | protein-coding |
| Fam178b | family with sequence similarity 178, member B | NM_001122653 | protein-coding |
| Fam192a | family with sequence similarity 192, member A | NM_001014014 | protein-coding |
| Fanca | Fanconi anemia, complementation group A | NM_001108455 | protein-coding |
| Fbxo44 | F-box protein 44 | NM_001191576 | protein-coding |
| Fdxr | ferredoxin reductase | NM_024153 | protein-coding |
| Fgfr1op2 | FGFR1 oncogene partner 2 | NM_201421 | protein-coding |
| Fkbp6 | FK506 binding protein 6 | NM_001105922 | protein-coding |
| Foxm1 | forkhead box M1 | NM_031633 | protein-coding |
| Fyco1 | FYVE and coiled-coil domain containing 1 | NM_001106870 | protein-coding |
| Gamt | guanidinoacetate N-methyltransferase | NM_012793 | protein-coding |
| Gdf1 | growth differentiation factor 1 | NM_001044240 | protein-coding |
| Gja4 | gap junction protein, alpha 4 | NM_021654 | protein-coding |
| Gjd4 | gap junction protein, delta 4 | NM_001100487 | protein-coding |
| Gna15 | guanine nucleotide binding protein, alpha 15 | NM_053542 | protein-coding |
| Gng5 | guanine nucleotide binding protein (G protein), gamma 5 | NM_024377 | protein-coding |
| Gnmt | glycine N-methyltransferase | NM_017084 | protein-coding |
| Gnpat | glyceronephosphate O-acyltransferase | NM_053410 | protein-coding |
| Gosr2 | golgi SNAP receptor complex member 2 | NM_031685 | protein-coding |
| Gpalpp1 | GPALPP motifs containing 1 | NM_001024875 | protein-coding |
| Gtf2e1 | general transcription factor IIE, polypeptide 1, alpha | NM_001100556 | protein-coding |
| Gtsf1 | gametocyte specific factor 1 | NM_001079707 | protein-coding |
| Gzf1 | GDNF-inducible zinc finger protein 1 | NM_001107788 | protein-coding |
| Hcfc1r1 | host cell factor C1 regulator 1 (XPO1-dependent) | NM_001100492 | protein-coding |
| Higd2a | HIG1 hypoxia inducible domain family, member 2 A | NM_001106102 | protein-coding |
| Hist3h2a | histone cluster 3, H2a | NM_021840 | protein-coding |
| Hist3h2bb | histone cluster 3, H2bb | NM_001109641 | protein-coding |
| Hoxc8 | homeobox C8 | NM_001177326 | protein-coding |
| Hoxd10 | homeo box D10 | NM_001107094 | protein-coding |
| Hrg | histidine-rich glycoprotein | NM_133428 | protein-coding |
| Icam1 | intercellular adhesion molecule 1 | NM_012967 | protein-coding |
| Idua | iduronidase, alpha-L- | NM_001172084 | protein-coding |
| Ift122 | intraflagellar transport 122 | NM_001009416 | protein-coding |
| Ikzf5 | IKAROS family zinc finger 5 | NM_001107555 | protein-coding |
| Il17rb | interleukin 17 receptor B | NM_001107290 | protein-coding |
| Itga4 | integrin, alpha 4 | NM_001107737 | protein-coding |
| Jagn1 | jagunal homolog 1 | NM_001044272 | protein-coding |
| Jtb | jumping translocation breakpoint | NM_019213 | protein-coding |
| Kb15 | type II keratin Kb15 | NM_001008825 | protein-coding |
| Kcne5 | potassium channel, voltage-gated Isk-related subfamily E regulatory beta subunit 5 | NM_001101003 | protein-coding |
| Kdelr1 | KDEL (Lys-Asp-Glu-Leu) endoplasmic reticulum protein retention receptor 1 | NM_001017385 | protein-coding |
| Kiaa0895l | hypothetical protein LOC688736 | NM_001044292 | protein-coding |
| Kif11 | kinesin family member 11 | NM_001169112 | protein-coding |
| Kif18b | kinesin family member 18B | NM_001039019 | protein-coding |
| Klrd1 | killer cell lectin-like receptor, subfamily D, member 1 | NM_012745 | protein-coding |
| Krt33b | keratin 33B | NM_001008819 | protein-coding |
| Lars2 | leucyl-tRNA synthetase 2, mitochondrial | NM_001108787 | protein-coding |
| Leng1 | leukocyte receptor cluster (LRC) member 1 | NM_001106218 | protein-coding |
| Lhx1 | LIM homeobox 1 | NM_145880 | protein-coding |
| LOC100912214 | uncharacterized LOC100912214 | NR_131101 | ncRNA |
| LOC103689982 | lysophospholipid acyltransferase 7 | NM_001313940 | protein-coding |
| LOC288913 | similar to LEYDIG CELL TUMOR 10 KD PROTEIN | NM_198728 | protein-coding |
| LOC498154 | hypothetical protein LOC498154 | NM_001025033 | protein-coding |
| LOC688925 | similar to Glutathione S-transferase alpha-4 | NM_001270386 | protein-coding |
| Lrrc14 | leucine rich repeat containing 14 | NM_001024354 | protein-coding |
| Lrrc27 | leucine rich repeat containing 27 | NM_001113789 | protein-coding |
| Lrrc36 | leucine rich repeat containing 36 | NM_001004088 | protein-coding |
| Lrrc51 | leucine rich repeat containing 51 | NM_001106284 | protein-coding |
| Lypd3 | Ly6/Plaur domain containing 3 | NM_021759 | protein-coding |
| Lzic | leucine zipper and CTNNBIP1 domain containing | NM_001013241 | protein-coding |
| Lztfl1 | leucine zipper transcription factor-like 1 | NM_001024266 | protein-coding |
| Maf1 | MAF1 homolog, negative regulator of RNA polymerase III | NM_001014085 | protein-coding |
| Mag | myelin-associated glycoprotein | NM_017190 | protein-coding |
| Mal | mal, T-cell differentiation protein | NM_012798 | protein-coding |
| Mboat7 | membrane bound O-acyltransferase domain containing 7 | NM_001134978 | protein-coding |
| Mcemp1 | mast cell-expressed membrane protein 1 | NM_001134602 | protein-coding |
| Mea1 | male-enhanced antigen 1 | NM_001044286 | protein-coding |
| Med11 | mediator complex subunit 11 | NM_001105799 | protein-coding |
| Mir137 | microRNA 137 | NR_031883 | ncRNA |
| Mir207 | microRNA 207 | NR_032107 | ncRNA |
| Mir338 | microRNA 338 | NR_031783 | ncRNA |
| Mir3562 | microRNA 3562 | NR_037344 | ncRNA |
| Misp | mitotic spindle positioning | NM_001109284 | protein-coding |
| Mrpl43 | mitochondrial ribosomal protein L43 | NM_001107598 | protein-coding |
| Mrps18b | mitochondrial ribosomal protein S18B | NM_212534 | protein-coding |
| Mrps25 | mitochondrial ribosomal protein S25 | NM_001025408 | protein-coding |
| Mt2A | metallothionein 2A | NM_001137564 | protein-coding |
| Mt3 | metallothionein 3 | NM_053968 | protein-coding |
| Mterf3 | mitochondrial transcription termination factor 3 | NM_199387 | protein-coding |
| Mtf1 | metal-regulatory transcription factor 1 | NM_001108677 | protein-coding |
| Mtf2 | metal response element binding transcription factor 2 | NM_001100898 | protein-coding |
| Myeov2 | myeloma overexpressed 2 | NM_001109044 | protein-coding |
| Naa38 | N(alpha)-acetyltransferase 38, NatC auxiliary subunit | NM_001105794 | protein-coding |
| Ncbp1 | nuclear cap binding protein subunit 1 | NM_001014785 | protein-coding |
| Ncoa4 | nuclear receptor coactivator 4 | NM_001034007 | protein-coding |
| Ndor1 | NADPH dependent diflavin oxidoreductase 1 | NM_001107818 | protein-coding |
| Ndufb8 | NADH dehydrogenase (ubiquinone) 1 beta subcomplex 8 | NM_001106360 | protein-coding |
| Ndufs5 | NADH dehydrogenase (ubiquinone) Fe-S protein 5 | NM_001030052 | protein-coding |
| Ndufv3 | NADH dehydrogenase (ubiquinone) flavoprotein 3 | NM_022607 | protein-coding |
| Nipsnap1 | nipsnap homolog 1 (C. elegans) | NM_001100730 | protein-coding |
| Nme3 | NME/NM23 nucleoside diphosphate kinase 3 | NM_053507 | protein-coding |
| Nmi | N-myc (and STAT) interactor | NM_001034148 | protein-coding |
| Nmu | neuromedin U | NM_022239 | protein-coding |
| Nob1 | NIN1/RPN12 binding protein 1 homolog | NM_199086 | protein-coding |
| Nolc1 | nucleolar and coiled-body phosphoprotein 1 | NM_022869 | protein-coding |
| Nr2c2ap | nuclear receptor 2C2-associated protein | NM_001047104 | protein-coding |
| Nsl1 | NSL1, MIS12 kinetochore complex component | NM_001109083 | protein-coding |
| Ntpcr | nucleoside-triphosphatase, cancer-related | NM_001134573 | protein-coding |
| Ntsr1 | neurotensin receptor 1 | NM_001108967 | protein-coding |
| Nubp2 | nucleotide binding protein 2 | NM_001011891 | protein-coding |
| Nudt2 | nudix (nucleoside diphosphate linked moiety X)-type motif 2 | NM_207596 | protein-coding |
| Olr437 | olfactory receptor 437 | NM_001109347 | protein-coding |
| Olr760 | olfactory receptor 760 | NM_001001069 | protein-coding |
| Ovca2 | ovarian tumor suppressor candidate 2 | NM_001109036 | protein-coding |
| Pcdha3 | protocadherin alpha 3 | NM_053941 | protein-coding |
| Pctp | phosphatidylcholine transfer protein | NM_017225 | protein-coding |
| Pex1 | peroxisomal biogenesis factor 1 | NM_001109220 | protein-coding |
| Phlda2 | pleckstrin homology-like domain, family A, member 2 | NM_001100521 | protein-coding |
| Phldb3 | pleckstrin homology-like domain, family B, member 3 | NM_001191622 | protein-coding |
| Pigp | phosphatidylinositol glycan anchor biosynthesis, class P | NM_001099758 | protein-coding |
| Plcxd2 | phosphatidylinositol-specific phospholipase C, X domain containing 2 | NM_001134481 | protein-coding |
| Plp2 | proteolipid protein 2 (colonic epithelium-enriched) | NM_207601 | protein-coding |
| Pmf1 | polyamine-modulated factor 1 | NM_001191568 | protein-coding |
| Pnldc1 | poly(A)-specific ribonuclease (PARN)-like domain containing 1 | NM_001025724 | protein-coding |
| Polr3d | polymerase (RNA) III (DNA directed) polypeptide D | NM_001031653 | protein-coding |
| Pou6f1 | POU class 6 homeobox 1 | NM_001105746 | protein-coding |
| Ppp1r11 | protein phosphatase 1, regulatory (inhibitor) subunit 11 | NM_212542 | protein-coding |
| Ppt2 | palmitoyl-protein thioesterase 2 | NM_019367 | protein-coding |
| Psmg4 | proteasome (prosome, macropain) assembly chaperone 4 | NM_001109543 | protein-coding |
| Ptcd1 | pentatricopeptide repeat domain 1 | NM_001109665 | protein-coding |
| Ptk2b | protein tyrosine kinase 2 beta | NM_017318 | protein-coding |
| Qk | quaking | NM_001115021 | protein-coding |
| Rab3gap2 | RAB3 GTPase activating protein subunit 2 | NM_001008294 | protein-coding |
| Rab5c | RAB5C, member RAS oncogene family | NM_001105840 | protein-coding |
| Rad51ap1 | RAD51 associated protein 1 | NM_001079711 | protein-coding |
| Ranbp10 | RAN binding protein 10 | NM_001135875 | protein-coding |
| Rec8 | REC8 meiotic recombination protein | NM_001011916 | protein-coding |
| Rfc2 | replication factor C (activator 1) 2 | NM_053786 | protein-coding |
| RGD1307443 | similar to mKIAA0319 protein | NM_001197023 | protein-coding |
| RGD1309188 | similar to hypothetical protein BC011833 | NM_001108129 | protein-coding |
| RGD1309676 | similar to RIKEN cDNA 5730469M10 | NM_001014140 | protein-coding |
| RGD1311703 | similar to sid2057p | NM_001013898 | protein-coding |
| RGD1359334 | similar to hypothetical protein FLJ20519 | NM_001007638 | protein-coding |
| RGD1559909 | RGD1559909 | NM_001108678 | protein-coding |
| RGD1560608 | similar to novel protein | NM_001109280 | protein-coding |
| RGD1562683 | RGD1562683 | NM_001108314 | protein-coding |
| RGD1563714 | RGD1563714 | NM_001126297 | protein-coding |
| RGD1564036 | similar to RIKEN cDNA 3010026O09 | NM_001109030 | protein-coding |
| Ribc2 | RIB43 A domain with coiled-coils 2 | NM_001013949 | protein-coding |
| Rnf40 | ring finger protein 40, E3 ubiquitin protein ligase | NM_153471 | protein-coding |
| Rph3a | rabphilin 3A | NM_133518 | protein-coding |
| Rpl27 | ribosomal protein L27 | NM_022514 | protein-coding |
| Rpl27a | ribosomal protein L27a | NM_001106290 | protein-coding |
| Rspry1 | ring finger and SPRY domain containing 1 | NM_001100945 | protein-coding |
| Rxfp3 | relaxin/insulin-like family peptide receptor 3 | NM_001008310 | protein-coding |
| Sart3 | squamous cell carcinoma antigen recognized by T-cells 3 | NM_001107156 | protein-coding |
| Scly | selenocysteine lyase | NM_001007755 | protein-coding |
| Sert1 | Sertoli cell protein 1 | NR_130708 | ncRNA |
| Sfxn3 | sideroflexin 3 | NM_022948 | protein-coding |
| Skap2 | src kinase associated phosphoprotein 2 | NM_130413 | protein-coding |
| Slc19a2 | solute carrier family 19 (thiamine transporter), member 2 | NM_001030024 | protein-coding |
| Slc25a54 | solute carrier family 25, member 54 | NM_001109640 | protein-coding |
| Slc43a3 | solute carrier family 43, member 3 | NM_001107743 | protein-coding |
| Slc5a6 | solute carrier family 5 (sodium/multivitamin and iodide cotransporter), member 6 | NM_130746 | protein-coding |
| Slc6a20 | solute carrier family 6 (proline IMINO transporter), member 20 | NM_133296 | protein-coding |
| Slc6a3 | solute carrier family 6 (neurotransmitter transporter), member 3 | NM_012694 | protein-coding |
| Snrnp35 | small nuclear ribonucleoprotein 35 (U11/U12) | NM_001014127 | protein-coding |
| Snrpb2 | small nuclear ribonucleoprotein polypeptide B” | NM_001108592 | protein-coding |
| Spag7 | sperm associated antigen 7 | NM_001107016 | protein-coding |
| Spata33 | spermatogenesis associated 33 | NM_001106195 | protein-coding |
| Spata5 | spermatogenesis associated 5 | NM_001108549 | protein-coding |
| Spic | Spi-C transcription factor (Spi-1/PU.1 related) | NM_001108080 | protein-coding |
| Stam | signal transducing adaptor molecule (SH3 domain and ITAM motif) 1 | NM_001109121 | protein-coding |
| Stk19 | serine/threonine kinase 19 | NM_001013197 | protein-coding |
| Susd3 | sushi domain containing 3 | NM_001107341 | protein-coding |
| Tada3 | transcriptional adaptor 3 | NM_001025734 | protein-coding |
| Taf6l | TAF6-like RNA polymerase II, p300/CBP-associated factor (PCAF)-associated factor | NM_001107575 | protein-coding |
| Tax1bp3 | Tax1 (human T-cell leukemia virus type I) binding protein 3 | NM_001025419 | protein-coding |
| Tbc1d25 | TBC1 domain family, member 25 | NM_001106955 | protein-coding |
| Tbcb | tubulin folding cofactor B | NM_001040180 | protein-coding |
| Them4 | thioesterase superfamily member 4 | NM_001025017 | protein-coding |
| Tmem109 | transmembrane protein 109 | NM_001007736 | protein-coding |
| Tmem126a | transmembrane protein 126 A | NM_001011557 | protein-coding |
| Tnxa-ps1 | tenascin XA, pseudogene 1 | NR_024118 | pseudo |
| Trappc1 | trafficking protein particle complex 1 | NM_001039378 | protein-coding |
| Trim23 | tripartite motif-containing 23 | NM_001100637 | protein-coding |
| Trip13 | thyroid hormone receptor interactor 13 | NM_001011930 | protein-coding |
| Trip4 | thyroid hormone receptor interactor 4 | NM_001134981 | protein-coding |
| Trmt112 | tRNA methyltransferase 11-2 homolog (S. cerevisiae) | NM_001106330 | protein-coding |
| Tsc2 | tuberous sclerosis 2 | NM_012680 | protein-coding |
| Tstd2 | thiosulfate sulfurtransferase (rhodanese)-like domain containing 2 | NM_001108663 | protein-coding |
| Ttc3 | tetratricopeptide repeat domain 3 | NM_001108315 | protein-coding |
| Tuba3a | tubulin, alpha 3A | NM_001040008 | protein-coding |
| Tuba4a | tubulin, alpha 4A | NM_001007004 | protein-coding |
| Tubb2b | tubulin, beta 2B class IIb | NM_001013886 | protein-coding |
| Ufsp2 | UFM1-specific peptidase 2 | NM_001014142 | protein-coding |
| Vmp1 | vacuole membrane protein 1 | NM_138839 | protein-coding |
| Vwa7 | von Willebrand factor A domain containing 7 | NM_212499 | protein-coding |
| Zbtb26 | zinc finger and BTB domain containing 26 | NM_001107840 | protein-coding |
| Zfp142 | zinc finger protein 142 | NM_001108225 | protein-coding |
| Zfp597 | zinc finger protein 597 | NM_153732 | protein-coding |
| Zscan21 | zinc finger and SCAN domain containing 21 | NM_001012021 | protein-coding |
Figure 2.
Biological function of the identified Chip-Seq chromatin sequences (A) GeneCodis analysis (on genes with peaks found in their TSS-promoter regions) for enriched biological functions. (B) List of the 94 (45% of the total found) genes enriched for functions related to the CNS development. In red, genes confirmed by RTqPCR. Hypergeometric p-value ever <0.00005, Corrected (FDR) Hypergeometric p-value < 0.05.
Morphological features of the Gunn rat Cll
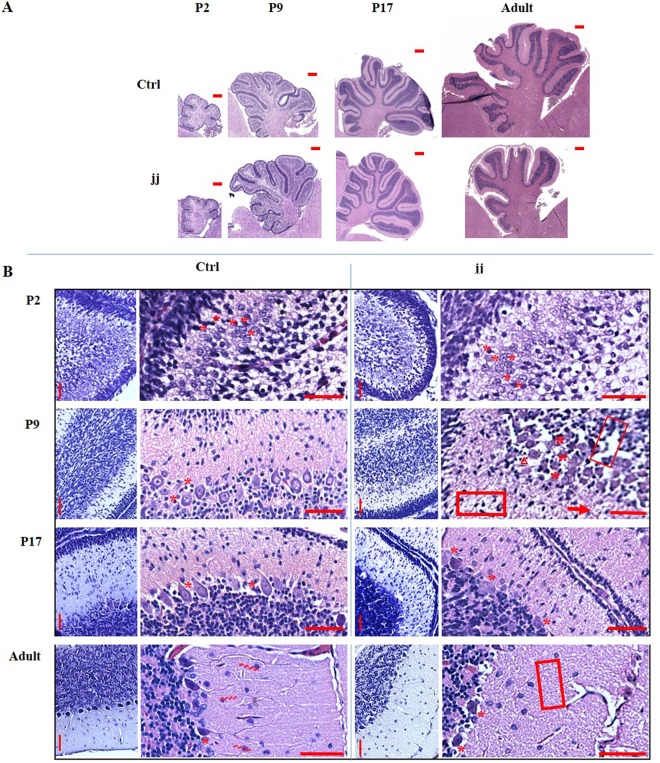
Since our results strongly suggested an impact of bilirubin on the genetic program of CNS maturation, we systematically followed the histological development of the cerebellum of jj rats in the attempt to interpret the genetic results. No morphological alterations between jj and ctrl were obvious at P2 (Fig. 3A,B). In both jj and ctrl animals, Purkinje cells were organized in 3–5 layers, with a round/oval shape and a reticulated cytoplasm (Fig. 3B). At P9, in spite of a conserved architecture, signs of cellular sufferance/death, microgliosis, extracellular matrix abnormalities and edema were evident in jj pups. PCs in ctrl displayed a clear definition of the plasma-membrane, cytoplasm, and nuclear areas, and a round/drop shape, and were organized in 3/1 layers. On the contrary, in jj pups, PCs were largely present in 4/2 layers, with an undefined, irregular shape. At P17, microgliosis and signs of cellular sufferance were still present in jj rats. PCs in ctrl were well differentiated, with a drop shape, and almost completely organized in a single layer, diffusely in 2/1 layers and still presenting the altered morphology described at P9 in jj. In the adult animal, the effect of Cll hypoplasia was well appreciable, with a less developed structure characterized by large spaces between the folia (Fig. 3A). Microgliosis was reduced but still present. No PC’s neurites were visible in jj rats, where PCs appeared atrophic and apoptotic (Fig. 3B).
Figure 3.
Histological finding (A) Full Cll images (scale bar 400 µm) showing the normal development (ctrl, upper series of pictures) and the progression of the Cll hypoplasia in jj animals (lower series of pictures). (B) Details (scale bar 100 µm) of the major histological alterations in the developing Cll of jj rat vs. age matched ctrl. P: post-natal age in days, Adult: more than 1 year old. *Purkinje cells (PCs); >PC’s neurites; ∆ microgliosis; [] extracellular matrix alteration; → oedema. 2–3 animals each genotype/age have been used. Miniatures: Nissl stain. Larger pictures: Haematoxylin & Eosin.
RTqPCR analysis of selected genes
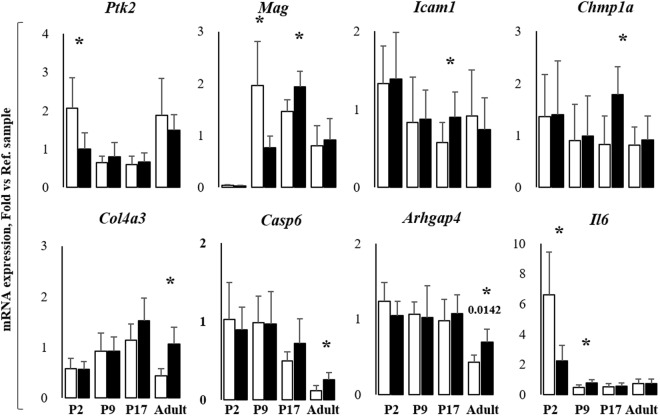
Due to the surprising percentage of enrichment for genes involved in CNS development, we decided to confirm and quantify the epigenetic control of a selected panel of genes, by assessing their expression by RTqPCR (selected genes are those in red in Fig. 2B, in which their biological functions based on the Gene Ontology analysis are indicated. RTqPCR results are in Fig. 4). Ptk2 (protein tyrosine kinase 2 beta, considered a key gene in neurite outgrowth and elongation, synapses formation, and actin reorganization19), was significantly down-regulated in P2 jj pups (Σ2 fold vs. age-matched ctrl, unpaired t-test with Welch correction, p < 0.047), normalizing thereafter. Mag (myelin-associated glycoprotein), barely detectable immediately after birth, was highly expressed in ctrl and Σ2.5 fold down-regulated in jj pups at P9 (unpaired t-test with Welch correction, p < 0.0402), reversing to a Σ1.2 fold up-regulation at P17 (unpaired t-test with Welch correction, p < 0.0306). Icam1 (intracellular adhesion molecule 1, expressed mainly by the endothelial cells forming the blood-brain barrier, involved in cell adhesion, leucocytes20 and monocytes extravasation21, and morphogenesis) was up-regulated 1.6 fold in P17 jj rats (unpaired t-test with Welch correction, p < 0.0416). Similarly, we observed a Σ2.2 fold increase (unpaired t-test with Welch correction, p < 0.0315) of Chmp1a (charged multi-vesicular body protein 1a, regulating the neural progenitor cell proliferation22). In adult jj Cll, Col4a3 (collagenase 4a3, the major structural component of the basal membrane, involved in the extracellular matrix remodeling23, providing the functional compartmentalization of the brain by clustering of growth factors, neurotransmitters/ions receptors, as well contributing to migration and differentiation24), Casp6 (caspase 6 - proliferation and morphogenesis – Fig. 2B), and Arghap4 (Rho GTPase-activating protein, inhibiting the cell motility and axon outgrowth via regulating the cytoskeleton dynamics25) were upregulated Σ2.5fold (unpaired t-test with Welch correction, p < 0.00547), Σ1.9fold (unpaired t-test with Welch correction, p < 0.0287) and Σ1.6 fold (unpaired t-test with Welch correction, p < 0.0142) respectively. No modulation of Anxa2 (annexin2), Agrn (Agrin), and Tubb2b (Tubulin2b) was detected at any post-natal age in jj rats (data not shown). Il6 (intron region segment resulting from ChIP-Seq analysis) was also investigated. In ctrl animals the Il6 level rapidly decreases from P2 to P9, stabilizing thereafter. In jj pups, a significant down-regulation of Il6 was present immediately after birth compared to ctrl animals (Σ2.9fold, unpaired t-test with Welch correction, p < 0.0315), while a 1.65 fold up-regulation was noticed at P9 (unpaired t-test with Welch correction, p < 0.0248), normalizing later on.
Figure 4.
Analysis of the expression of selected genes involved in CNS development Arghap4: Rho GTP-ase activating protein 4; Casp6: Caspase 6; Chmp1a: Charged multi-vesicular body protein 1a; Col4a3: Collagenase 4 a3; Icam1: Intracellular adhesion molecule 1; Mag: Myelin-associated glycoprotein; Ptk2: Protein tyrosine kinase 2 beta; Il6: Interleukin 6. P: post-natal age in days, Adult: more than 1-year-old. White bars: ctrl; Black bars: jj. Results are expressed as mean ± S.D. of 6 animals each genotype/age. Unpaired t-test with Welch correction, *p < 0.05; **p < 0.05; ***p < 0.005 vs. age-matched controls.
Discussion
Cll hypoplasia is a hallmark of hyperbilirubinemia in rodents26–29, and cerebellar involvement with morphological and behavioral abnormalities has also been reported in severely hyperbilirubinemic neonates30–32. Inflammation and oxidative stress are considered the major mechanisms of bilirubin neurotoxicity, whereas the impact of hyperbilirubinaemia on CNS development has been only marginally envisaged, and evaluated mostly by in vitro experiments33,34.
Unexpectedly, the known inflammatory or oxidant effectors of bilirubin neurotoxicity have been not identified in our data (ChIP-Seq, followed by Gene Ontology analysis), revealing that 45% of genes displaying a Histone 3 lysine 14 acetylation are related to CNS development. Indeed, only 3 genes among all the 255 identified TSS- Promoter sequences have been previously reported in the literature for their association with hyperbilirubinemia, namely myelin28,31,32,34, tubulin35, and Icam136.
The down-regulation of Mag has been reported in in vitro studies, in agreement with the defective myelination observed both in bilirubin neurotoxicity models28,34 and neonates32. Mag down-regulation is also a known consequence of bilirubin-induced perturbation of the oligodendrocytes maturation. A possible additional link between what has been previously described and the present results is the fact that histone acetylation is a known mechanism controlling oligodendrocyte differentiation and myelin production, both in physiological CNS development and in repair processes after demyelination6,10.
Our data are in agreement with the literature also in relation to Il6, whose intron sequence was identified by ChIP-Seq analysis. Il6 is a well-known effector of bilirubin neurotoxicity and possibly linked with the reported defective myelination. In fact, apart from the possible inflammatory activity, Il6 is involved in oligodendrogenesis37,38, a process active up to P45 in rodents and 2 years in humans39, and reactivated in pathological conditions. During reactivation, injured neurons and oligodendrocytes may reactivate myelin synthesis by overexpressing Il6 and its receptor (Il6r/CD126), restoring normal behavior in injured animals10,40.
Both Mag and Il6 present a fluctuating behavior, being significantly down-regulated in the early post-natal life, and reverting thereafter to the level of age-matched controls (Fig. 4). Notably, in our work, IL6 modulation (P9) precedes Mag increase (P17), supporting the inductor role of Il6 in myelination described in the literature10,40. The fluctuating expression of Il6 and Mag (firstly up-, then down regulated), is present also for H3K14Ac levels, increasing at P9, and reverting under the level of age-matched controls at P17, and normalizing in the adult age.
The regulation of the other genes is more difficult to be analyzed since they are very new in the bilirubin field and no data are provided by literature. While we still have to confirm the role of the various genes identified in this study through methods such as gene silencing in vitro, our work suggests that the epigenetic impairment of neurodevelopmental processes in hyperbilirubinemia may be a relevant mechanism of bilirubin neurotoxicity. It is worth mentioning that Chmp1a, Arghap4, Casp6, Ptk2, Col4a3 are genes involved in key steps of brain development as proliferation, migration, morphogenesis, neurite outgrowth and elongation, synaptogenesis, extracellular matrix formation and compartmentalization, as well the pathological axonal degeneration and apoptosis observed19,22,25,41,42 in jj rats. By adding epigenetic dysregulation to the list of the mechanisms related to bilirubin-induced neuronal damage, we can confirm and expand the concept of a widespread toxic effect of the pigment on the CNS43, improving our understanding of the cellular and molecular mechanisms of bilirubin induced damage to CNS.
Materials and Methods
Animals
Gunn rats (Hds Blue:Gunn-UDPGTj, P2, 9, 17; P ± 1 day. Adult = more than 1 year old) were obtained from the SPF animal facility of CBM S.c.a.r.l. (AREA Science Park, Basovizza). Ages were selected based on previous evidence26,44. Animals were housed in a temperature-controlled environment (22 ± 2 °C), on a 12 hours light/dark schedule, and ad-libitum access to food and water. The study was approved by the animal care and use committee of the CBM Scarl and the competent Italian Ministry. All procedures were performed according to the Italian Law (decree 87-848) and European Community directive (86-606-ECC). Maximal effort to minimize the number of the animals used and their sufferance was done.
TSB, cBf and Cerebellum weight quantification
Serum and Cll were collected as previously described26,45. In brief, blood samples were collected during the sacrifice (decapitation under urethane anaesthesia 1.0–1.2 g/kg IP) and centrifuged at 2000 rpm, 20 min RT. Total serum bilirubin (TSB) was quantified by the diazo reaction, as previously described26. Free bilirubin was calculated (cBf) by applying the formula and the albumin-bilirubin dissociation constants for Gunn pups detailed in literature14. Cerebellum was dissected immediately after the sacrifice, and the weight recorded by a precision balance.
Western blot analysis of the levels of H3K14Ac
Western blot was performed as previously described44,45. In brief, Cll were mechanically homogenized by glass-glass Dounce (in 0.25 M sucrose, 40.2 mM KH2PO4, 9.8 mM K2HPO4, 1 mM EDTA, 0.1 mM DTT, pH 7.4), and total protein concentration quantified by the Bicinchoninic Acid Protein Assay following the supplier instruction (B-9643 and C2284, Sigma, Missouri, USA). 25 μg of Cll whole extract proteins were denatured (10% of β-mercaptoethanol -Sigma Chemical, St. Louis, MO, USA, plus 5 min boiling), separated by 12% SDS-PAGE by electrophoresis in a Hoefer SE 250 System (Amersham BioSciences, UK), and electro-transferred onto immune-blot PVDF membranes (0.2 μm; Whatman Schkleicher and Schuell, Dassel, Germany) at 100 V for 60 min (Bio-Rad Laboratories, Hercules, CA, USA). Efficiency of the transfer was assessed by lack of Coomassie blue coloration of the gel after blotting, and Ponceau staining of the PVDF membrane (both chemicals: Sigma, St. Louis, MO, USA). After blocking (1.5 hrs, RT in blocking solution: 3% defatted milk in 0.2% Tween 20; 20 mM Tris-HCl pH 7.5; 500 mM NaCl), membranes were incubated O/N at 4 °C with the polyclonal anti-acetyl histone H3 (lys14) antibody (07-353, Merck Millipore, Temecula, CA, USA; final concentration 0.7 μg/mL). The day after, membranes were washed 3 × 5 min in blocking buffer, then incubated 2hrs with the secondary antibody anti-rabbit IgG peroxidase (Dako, Agilent Technologies, Santa Clara, CA, USA, final concentration 0.0625 μg/mL) in blocking solution. The signal was revealed by chemiluminescence (ECL-Plus Western blotting Detection Reagents, GE-Healthcare Bio-Science, Italy) and visualized on X-ray films (BioMax Light, Kodak Rochester, NY, USA). The results were normalized vs. the actin signal, visualized incubating the same membrane used for revealing the H3K14Ac with the anti-actin antibody A2066 (sigma- Chemical, St. Louis, MO, USA; final concentration 0.07 μg/mL, MW 42KDa). Bands intensity was quantified by the Scion Image software (GE Healthcare Europe GmbH, France).
ChIP-Seq analysis
The 07-353 anti-H3K13Ac antibody used for Western blot analysis was also used to perform chromatin immunoprecipitation, followed by DNA sequencing (ChIP-Seq – full result available on GEO repository # GSE109145). Chromatin immunoprecipitation (ChIP) was performed following the Magna ChIPTM G Tissue Kit (#17-20000, Merck Millipore, Temecula, CA, USA) procedure and applying the same Ab used in Western blot. Cll tissue (60 mg) was homogenized, DNA sheared (average size of 100–400 bp, by Sonopuls HD 3100, Bandelin, Germany, sonicator. Power 50%, 15″ × 18 cycles, 10″ pause between each cycle, on ice), cross-linked with 1% formaldehyde (5′, RT), and protein-DNA complexes immune-precipitated (5 μL, 07-353 Ab, Merck Millipore, Temecula, CA, USA) by G magnetic beads on the magnetic rack (LSKMAGS08 Pure ProteomeTM Magnetic Stand, Merck Millipore, Temecula, CA, USA). Protein-DNA crosslink was reversed (proteinase K, 62 °C, 2 h; plus 95° C × 10′), and DNA stored at −20 °C until use. As suggested by the manufacturer, the efficiency and specificity of the ChIP procedure were assessed by Western blot, and Real Time PCR (RTqPCR). Samples were quantified by Quant-iTTM PicoGreen® dsDNA Kits (Thermo Fisher Scientific, Waltham, MA, USA), according to manufacturer’s instruction.
Libraries were prepared by using the NEBNext® UltraTM II DNA Library Prep Kit from Illumina® (E7645, New England BioLabs®Inc, MA, USA), following the manufacturer’s instructions starting from 10 ng of fragmented DNA. After end repair and adaptor ligation, adaptor-ligated DNA clean-up (without size-selection, Agencourt AMPure XP magnetic beads, Beckman Coulter Life Sciences, CA, USA), library enrichment (98°C × 30 sec; 98°C × 10 secplus 65°C × 75 min × 10 cycles; 65°C × 5 min, in a Bio-Rad thermal cycler, Bio-Rad, Richmond, CA, USA), and PCR clean up (Agencourt AMPure XP magnetic beads, Beckman Coulter Life Sciences, CA, USA), the libraries were quantified using the PicoGreen fluorescent dye, as reported above, and stored at −20 °C. Before sequencing, libraries were denatured and diluted to a final concentration of 15 pM with 10% PhiX (Illumina, New England BioLabs®Inc, MA, USA) control. Paired-end sequencing was performed using the MiSeq reagent kit v3 2 × 150 in the Illumina® MiSeq® system (Illumina, San Diego, CA, USA). A total of 4 P9 jj Cll (2 runs) and 3 P9 control Cll (1 run) were used. Reads were mapped to the Rattus norvegicus (rn4) genome using bowtie246. Duplicate reads were filtered. The quality of the sequences was evaluated using fastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Peaks were called using MACS247 and annotated using HOMER software48. Functional enrichment study was determined using GeneCodis (http://genecodis.cnb.csic.es/, hypergeometric test, FDR corrected)16–18.
Histology and morphometric analysis
Immediately after animals sacrifice, the brain was removed from the skull and fixed in 4% formalin buffered solution (4% formaldehyde 37%, 33 nM NaH2PO4, 46 mM Na2HPO4), then embedded in paraffin. Sagittal sections of the brain (3–5 μm) were obtained by a microtome (Microm-hm 340 e- BioOptica, Milan, Italy), affixed on the glass slides and dried at 60 °C for 1 hour. Hematoxylin and eosin stain (H&E) was performed by a Leica ST5020 Multistainer (Leica Microsystem, Milan, Italy). Cresyl violet (Nissl) staining was performed manually on hydrated sections (xylol 3 × 5 min; 100% ethanol 2 × 2 min; 95% ethanol 2 × 2 min; 80% ethanol 1 × 2 min; 70% ethanol 1 × 2 min; H2O 2 × 5 min) by incubating the slices for 1 hr in cresyl violet solution (0.1% cresyl violet powder, 10 drops glacial acetic acid in H2OmQ). After washing (twice H2OmQ), differentiation (75% ethanol, 95% ethanol plus 5% chloroform, 3 drops glacial acetic acid) and dehydration (100% ethanol 2 × 5 min; xylol 2 × 5 min), slices were mounted (Eukitt 03989, SIGMA Aldrich). Pictures were collected by a D-Sight plus image digital microscope & scanner (Menarini Diagnostics, Firenze, Italy). Histology was read by 3 independent pathologists, blinded to experimental design.
RTqPCR on selected genes
RTqPCR was performed as previously described26,43. Total RNA extraction (Eurogold RNA Pure reagent, Euroclone, Milan, Italy) and retro-transcription (1 μg RNA, High Capacity cDNA Reverse Transcription Kit, Applied Biosystems, Monza, Italy) were performed following the manufacturer instruction in a thermal cycler (Gene Amp PCR System 2400, Perkin-Elmer, Boston, MA, USA) at 25 °C for 5 min, 37 °C for 120 min, and 85 °C for 5 min. The final cDNA was stored at 20 °C until use. Primers were designed using the Beacon designer 8.1 software (Premier Biosoft International, Palo Alto, CA, USA) on rat sequences available in GenBank (Table 2). RtqPCR was performed in an iCycler iQ thermocycler (Bio-Rad Laboratories, Hercules, CA, USA) in presence of 25 ng of cDNA, sense and antisense gene-specific primers (250 nM each), in SSoAdvance SYBER green supermix (Bio-Rad Laboratories, Hercules, CA, USA). Amplification protocol was 95 °C × 3 min, 40 cycle of 95° C × 20 sec; 60 °C × 20 sec and 72 °C × 30 sec, followed by 72 °C × 5 min. Melting curve analysis was performed to assess product specificity. The relative quantification was made using the iCycler iQ Software, version 3.1 (Bio-Rad Laboratories, Hercules, CA, USA) by the Pfaffl modification of the ΔΔCT equation, taking into account the efficiencies of the individual genes49,50. The results were normalized to the housekeeping genes and the levels of mRNA were expressed relative to a reference sample50,51.
Table 2.
Primers specification.
| Gene | Accession number | Forward | Revers | Efficiency | Amplicon length (bp) |
|---|---|---|---|---|---|
| Agrn | NM_175754 | TACCTGTCCACTTGTATT | TTCTCATCCAATAACACATT | 98.5 | 87 |
| Arhgap4 | NM_144740 | CTTGTGAGCCATCTACTATC | GTTGAGGAAGGTGAAGAG | 88 | 75 |
| Anxa2 | NM_019905 | CTACTGTCCACGAAATCCTG | AAGTTGGTGTAGGGTTTGAC | 99.8 | 94 |
| Casp6 | NM_031775 | ACAGATGGCTTCTACAGA | AGTTCCTCTCCTCTTGTG | 102.2 | 78 |
| Chmp1a | NM_001083313 | ATCAACTTACAGGTTAGG | TACTTACGACAACATTCTA | 98.2 | 122 |
| Col4a3 | NM_001135759 | TCACCACAATGCCATTCTTA | CGACAGCCAGTATGAATAGT | 94.5 | 83 |
| Icam1 | NM_012967 | ACCTACATACATTCCTACC | ATGAGACTCCATTGTTGA | 96.3 | 91 |
| Mag | NM_017190 | ACCATCCAACCTTCTGTATC | CTGATTCCGCTCCAAGTG | 96.2 | 90 |
| Ptk2b | NM_017318 | TGTCTACACGAACCATAA | GAACTTCTCCTTGTTGTC | 93.1 | 88 |
| Tubb2b | NM_001013886 | CAGTTGGAAGAAGGAGAA | AGTGTTACATTGATGTTATCG | 107.5 | 111 |
| Il6 | NM_012589.1 | GCCCACCAGGAACGAAAGTC | TCCTCTGTGAAGTCTCCTCTCC | 107.7 | 161 |
| Hprt | NM_012583.2 | AGACTGAAGAGCTACTGTAATGAC | GGCTGTACTGCTTGACCAAG | 94.9 | 163 |
Statistics
The statistical analysis was performed by GraphPad InStat for Windows (GraphPad Software, Inc, La Jolla, CA, USA). The ANOVA test, followed by Tukey-Kramer multiple comparison tests, was used to analise TSB, cBf, and Cll weight during the development. The unpaired two-tailed Student’s t-test, based on unequal variance, was applied to evaluate the difference between jj and controls at the same age (Western blot, RTqPCR). All data are expressed as mean ± S.D. of multiple biological repetition. A p-value lower than 0.05 was considered statistically significant.
Acknowledgements
SG was supported in part by an internal grant from the Italian Liver Foundation. EV was supported in part by an internal grant from the Italian Liver Foundation, in part by the Università degli Studi di Trieste. We thanks the Alessandra Bramante and Andrea Lorenzon from the local SPF animal facility of CBM S.c.a.r.l. (AREA Science Park, Basovizza) for their support with the animal procedures, Dr. Sean M. Riordan (Mercy Children Hospital, Kansas City, MO, USA), for the final revision of the Ms. and the editing of the English, and Dr. Paola Ostano (Fondazione Edo ed Elvo Tempia Valenta, Biella) for the informatics support in loading the data on GEO.
Author Contributions
E.V. designed research, performed research, analyzed data. S.Z. performed research. T.M. performed research. F.T. analyzed data. C.B. performed research. A.D. Contributed new reagents/analytic tools. F.Z. performed research, analyzed data. C.T. wrote the paper. S.G. designed research, performed research, analyzed data, and wrote the paper. All authors read and approved the final version of the manuscript.
Data Availability
ChIP-Seq – full result available on GEO repository # GSE109145.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Watchko JF, Tiribelli C. Bilirubin-Induced Neurologic Damage — Mechanisms and Management Approaches. N. Engl. J. Med. 2013;369:2021–2030. doi: 10.1056/NEJMra1308124. [DOI] [PubMed] [Google Scholar]
- 2.Konsoula Z, Barile FA. Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J. Pharmacol. Toxicol. Methods. 2012;66:215–220. doi: 10.1016/j.vascn.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Gräff J, Mansuy IM. Epigenetic dysregulation in cognitive disorders. Eur. J. Neurosci. 2009;30:1–8. doi: 10.1111/j.1460-9568.2009.06787.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun W, et al. Histone Acetylome-wide Association Study of Autism Spectrum Disorder. Cell. 2016;167:1385–1397.e11. doi: 10.1016/j.cell.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Janssen C, et al. Differential histone deacetylase mRNA expression patterns in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 2010;69:573–581. doi: 10.1097/NEN.0b013e3181ddd404. [DOI] [PubMed] [Google Scholar]
- 6.Küçükali Cİ, Kürtüncü M, Çoban A, Çebi M, Tüzün E. Epigenetics of multiple sclerosis: an updated review. Neuromolecular Med. 2015;17:83–96. doi: 10.1007/s12017-014-8298-6. [DOI] [PubMed] [Google Scholar]
- 7.Gebremedhin KG, Rademacher DJ. Histone H3 acetylation in the postmortem Parkinson’s disease primary motor cortex. Neurosci. Lett. 2016;627:121–125. doi: 10.1016/j.neulet.2016.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogge GA, Wood MA. The role of histone acetylation in cocaine-induced neural plasticity and behavior. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013;38:94–110. doi: 10.1038/npp.2012.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lilja T, Heldring N, Hermanson O. Like a rolling histone: epigenetic regulation of neural stem cells and brain development by factors controlling histone acetylation and methylation. Biochim. Biophys. Acta. 2013;1830:2354–2360. doi: 10.1016/j.bbagen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Fagiolini M, Jensen CL, Champagne FA. Epigenetic influences on brain development and plasticity. Curr. Opin. Neurobiol. 2009;19:207–212. doi: 10.1016/j.conb.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maze I, Noh K-M, Allis CD. Histone regulation in the CNS: basic principles of epigenetic plasticity. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2013;38:3–22. doi: 10.1038/npp.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gräff J, Tsai L-H. Histone acetylation: molecular mnemonics on the chromatin. Nat. Rev. Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- 13.Shein NA, Shohami E. Histone deacetylase inhibitors as therapeutic agents for acute central nervous system injuries. Mol. Med. Camb. Mass. 2011;17:448–456. doi: 10.2119/molmed.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daood MJ, Watchko JF. Calculated in vivo free bilirubin levels in the central nervous system of Gunn rat pups. Pediatr. Res. 2006;60:44–49. doi: 10.1203/01.pdr.0000219561.07550.04. [DOI] [PubMed] [Google Scholar]
- 15.Karmodiya K, Krebs AR, Oulad-Abdelghani M, Kimura H, Tora L. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics. 2012;13:424. doi: 10.1186/1471-2164-13-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona-Saez P, Chagoyen M, Tirado F, Carazo JM, Pascual-Montano A. GENECODIS: a web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 2007;8:R3. doi: 10.1186/gb-2007-8-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nogales-Cadenas R, et al. GeneCodis: interpreting gene lists through enrichment analysis and integration of diverse biological information. Nucleic Acids Res. 2009;37:W317–322. doi: 10.1093/nar/gkp416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabas-Madrid D, Nogales-Cadenas R, Pascual-Montano A. GeneCodis3: a non-redundant and modular enrichment analysis tool for functional genomics. Nucleic Acids Res. 2012;40:W478–483. doi: 10.1093/nar/gks402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Bao X, Pal R, Agbas A, Michaelis EK. Transcriptomic responses in mouse brain exposed to chronic excess of the neurotransmitter glutamate. BMC Genomics. 2010;11:360. doi: 10.1186/1471-2164-11-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietrich J-B. The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J. Neuroimmunol. 2002;128:58–68. doi: 10.1016/S0165-5728(02)00114-5. [DOI] [PubMed] [Google Scholar]
- 21.Dalmau I, Vela JM, González B, Castellano B. Expression of LFA-1α and ICAM-1 in the developing rat brain: a potential mechanism for the recruitment of microglial cell precursors. Dev. Brain Res. 1997;103:163–170. doi: 10.1016/S0165-3806(97)81792-0. [DOI] [PubMed] [Google Scholar]
- 22.Shao G, et al. Proteomic Analysis of Mouse Cortex Postsynaptic Density following Neonatal Brain Hypoxia-Ischemia. Dev. Neurosci. 2017;39:66–81. doi: 10.1159/000456030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow ML, et al. Age-dependent brain gene expression and copy number anomalies in autism suggest distinct pathological processes at young versus mature ages. PLoS Genet. 2012;8:e1002592. doi: 10.1371/journal.pgen.1002592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33:503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Vogt DL, Gray CD, Young WS, Orellana SA, Malouf AT. ARHGAP4 is a novel RhoGAP that mediates inhibition of cell motility and axon outgrowth. Mol. Cell. Neurosci. 2007;36:332–342. doi: 10.1016/j.mcn.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazzin S, et al. Bilirubin accumulation and Cyp mRNA expression in selected brain regions of jaundiced Gunn rat pups. Pediatr. Res. 2012;71:653–660. doi: 10.1038/pr.2012.23. [DOI] [PubMed] [Google Scholar]
- 27.Schutta HS, Johnson L. Bilirubin encephalopathy in the Gunn rat: a fine structure study of the cerebellar cortex. J. Neuropathol. Exp. Neurol. 1967;26:377–396. doi: 10.1097/00005072-196707000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Barateiro A, et al. Reduced Myelination and Increased Glia Reactivity Resulting from Severe Neonatal Hyperbilirubinemia. Mol. Pharmacol. 2016;89:84–93. doi: 10.1124/mol.115.098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bortolussi G, et al. Impairment of enzymatic antioxidant defenses is associated with bilirubin-induced neuronal cell death in the cerebellum of Ugt1 KO mice. Cell Death Dis. 2015;6:e1739. doi: 10.1038/cddis.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watchko JF, Painter MJ, Panigrahy A. Are the neuromotor disabilities of bilirubin-induced neurologic dysfunction disorders related to the cerebellum and its connections? Semin. Fetal. Neonatal Med. 2015;20:47–51. doi: 10.1016/j.siny.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Rose J, Vassar R. Movement disorders due to bilirubin toxicity. Semin. Fetal. Neonatal Med. 2015;20:20–25. doi: 10.1016/j.siny.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brito MA, et al. Cerebellar axon/myelin loss, angiogenic sprouting, and neuronal increase of vascular endothelial growth factor in a preterm infant with kernicterus. J. Child Neurol. 2012;27:615–624. doi: 10.1177/0883073811423975. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes A, et al. Bilirubin as a determinant for altered neurogenesis, neuritogenesis, and synaptogenesis. Dev. Neurobiol. 2009;69:568–582. doi: 10.1002/dneu.20727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barateiro A, et al. Unconjugated bilirubin restricts oligodendrocyte differentiation and axonal myelination. Mol. Neurobiol. 2013;47:632–644. doi: 10.1007/s12035-012-8364-8. [DOI] [PubMed] [Google Scholar]
- 35.Silva RFM, Rodrigues CMP, Brites D. Rat Cultured Neuronal and Glial Cells Respond Differently to Toxicity of Unconjugated Bilirubin. Pediatr. Res. 2002;51:535–541. doi: 10.1203/00006450-200204000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Mazzone GL, et al. Bilirubin inhibits the TNFα-related induction of three endothelial adhesion molecules. Biochem. Biophys. Res. Commun. 2009;386:338–344. doi: 10.1016/j.bbrc.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Mousa A, Bakhiet M. Role of cytokine signaling during nervous system development. Int. J. Mol. Sci. 2013;14:13931–13957. doi: 10.3390/ijms140713931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baune BT, et al. Interleukin-6 gene (IL-6): a possible role in brain morphology in the healthy adult brain. J. Neuroinflammation. 2012;9:125. doi: 10.1186/1742-2094-9-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108:511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jalabi W, Boehm N, Grucker D, Ghandour MS. Recovery of myelin after induction of oligodendrocyte cell death in postnatal brain. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:2885–2894. doi: 10.1523/JNEUROSCI.2748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165:1–10. doi: 10.1016/S0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- 42.Graham RK, Ehrnhoefer DE, Hayden MR. Caspase-6 and neurodegeneration. Trends Neurosci. 2011;34:646–656. doi: 10.1016/j.tins.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Dal Ben M, Bottin C, Zanconati F, Tiribelli C, Gazzin S. Evaluation of region selective bilirubin-induced brain damage as a basis for a pharmacological treatment. Sci. Rep. 2017;7:41032. doi: 10.1038/srep41032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gazzin S, et al. Modulation of Mrp1 (ABCc1) and Pgp (ABCb1) by bilirubin at the blood-CSF and blood-brain barriers in the Gunn rat. PloS One. 2011;6:e16165. doi: 10.1371/journal.pone.0016165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robert, M. C. et al. Alterations in the Cell Cycle in the Cerebellum of Hyperbilirubinemic Gunn Rat: A Possible Link with Apoptosis? PLoS ONE8 (2013). [DOI] [PMC free article] [PubMed]
- 46.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bustin SA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
ChIP-Seq – full result available on GEO repository # GSE109145.