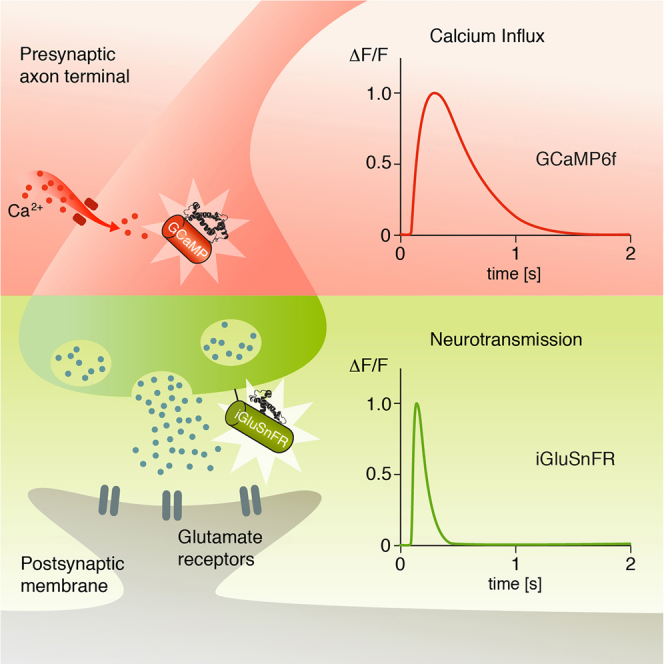
Summary
For a proper understanding of neural circuit function, it is important to know which signals neurons relay to their downstream partners. Calcium imaging with genetically encoded calcium sensors like GCaMP has become the default approach for mapping these responses. How well such measurements represent the true neurotransmitter output of any given cell, however, remains unclear. Here, we demonstrate the viability of the glutamate sensor iGluSnFR for 2-photon in vivo imaging in Drosophila melanogaster and prove its usefulness for estimating spatiotemporal receptive fields in the visual system. We compare the results obtained with iGluSnFR with the ones obtained with GCaMP6f and find that the spatial aspects of the receptive fields are preserved between indicators. In the temporal domain, however, measurements obtained with iGluSnFR reveal the underlying response properties to be much faster than those acquired with GCaMP6f. Our approach thus offers a more accurate description of glutamatergic neurons in the fruit fly.
Subject Areas: Optical Imaging, Sensory Neuroscience, Techniques in Neuroscience
Graphical Abstract
Highlights
-
•
The glutamate sensor iGluSnFR is suitable for 2-photon imaging in the fruit fly
-
•
Response properties obtained with iGluSnFR are much faster than those with GCaMP6f
-
•
Spatial aspects of receptive fields are preserved between indicators
Optical Imaging; Sensory Neuroscience; Techniques in Neuroscience
Introduction
To understand how neural circuits operate and carry out certain computations, it is essential to observe the signals that are transmitted from cell to cell. Synaptic transmission via chemical synapses proceeds in four major stages: (1) Depolarization in the presynapse opens voltage-gated calcium channels. (2) The resulting calcium influx leads to the fusion of transmitter-filled vesicles and the presynaptic membrane. (3) Transmitter molecules are released into the synaptic cleft where they diffuse and bind receptors in the postsynaptic membrane. (4) The subsequent activation of these receptors leads to opening or closing of ion channels, either directly or indirectly, with the resulting ion flux ultimately changing the postsynaptic membrane conductance and potential (reviewed in [Di Maio, 2008]). This fundamental signaling cascade, from electric potential to calcium to transmitter release to postsynaptic electric potential, orchestrates computation within any neuronal circuit.
For monitoring voltage changes, electrophysiology is the default approach. Here, direct observations of both de- and hyperpolarization in pre- or postsynaptic cells are possible. Due to the position or size of many neurons, however, direct single-cell recordings are often not feasible and have to be replaced by indirect extracellular recordings or optical imaging. Only recently genetically encoded voltage indicators (GEVIs) have emerged as powerful tools for recording neuronal activity (Cao et al., 2013, Jin et al., 2012, St-Pierre et al., 2014, Tsutsui et al., 2013, Yang et al., 2016). Experiments with optical voltage indicators such as ASAP2f that are compatible with 2-photon imaging, however, remain challenging due to weak signal-to-noise ratio (Yang et al., 2016). The fluorescence level of genetically encoded calcium indicators (GECIs) is thought to correlate with transmitter release and is therefore suitable for identifying the crucial signal to the postsynaptic cell (Zucker, 1993). Although GECIs are being improved continuously and some variants were designed to have especially fast kinetics (e.g., GCaMP6f [Chen et al., 2013]), temporal resolution is still limited due to calcium buffering (Borst and Abarbanel, 2007). This usually leads to decay constants in the order of several hundreds of milliseconds that vary depending on the system under observation (Arenz et al., 2017, Chen et al., 2013). For glutamatergic neurons, a tool to potentially overcome these limitations is the recently developed fast glutamate sensor iGluSnFR (Marvin et al., 2013).
Visual motion detection is a canonical example for computation in neural microcircuits. Prevalent models posit that, in both mammalian retina and fly visual system, local direction selectivity emerges from the nonlinear interaction between precisely tuned spatiotemporal filters (Barlow and Levick, 1965, Von Hassenstein and Reichardt, 1956). Recent work in connectomics on the visual system of Drosophila melanogaster has revealed this computation to be implemented by a circuit that consists of only a few dozen individual cells (Takemura et al., 2017). The optic lobe is the largest neuropil in the fruit fly's brain and consists of the four consecutive neuropils: lamina, medulla, lobula, and lobula plate (Figure 1). Lamina monopolar cells L1 and L2, among others, receive direct photoreceptor input and feed into two parallel pathways (Bausenwein et al., 1992, Bausenwein and Fischbach, 1992, Borst, 2014, Clark et al., 2011, Joesch et al., 2010, Rister et al., 2007, Shinomiya et al., 2014, Silies et al., 2013, Takemura et al., 2017, Tuthill et al., 2013). The ON pathway processes the motion of light increments, whereas the OFF pathway processes the motion of light decrements only (Eichner et al., 2011, Joesch et al., 2013, Joesch et al., 2010). Among the medulla interneurons that connect the lamina cells to direction-selective T4 and T5 neurons (Maisak et al., 2013, Takemura et al., 2017), we find the glutamatergic cell Mi9 that has been characterized with a receptive field responsive to OFF in the center and an antagonistic ON surround (Arenz et al., 2017, Strother et al., 2017). T4 and T5 neurons each come in four subtypes, tuned to one of the four cardinal directions, and project, according to their preferred direction, to one of the four layers in the lobula plate. Here, T4 and T5 cells make excitatory cholinergic connections onto the dendrites of large tangential cells as well as onto inhibitory lobula plate interneurons (LPis). These neurons in turn inhibit large field tangential cells in the adjacent layer during null direction motion and thus increase their flow-field selectivity (Hausen et al., 1980, Hopp et al., 2014, Schnell et al., 2010, Scott et al., 2002, Wasserman et al., 2015). To provide this inhibition, LPis release glutamate onto the glutamate receptor GluClα, which is an inhibitory glutamate receptor only found in invertebrates (Liu and Wilson, 2013, Mauss et al., 2015, Mauss et al., 2014).
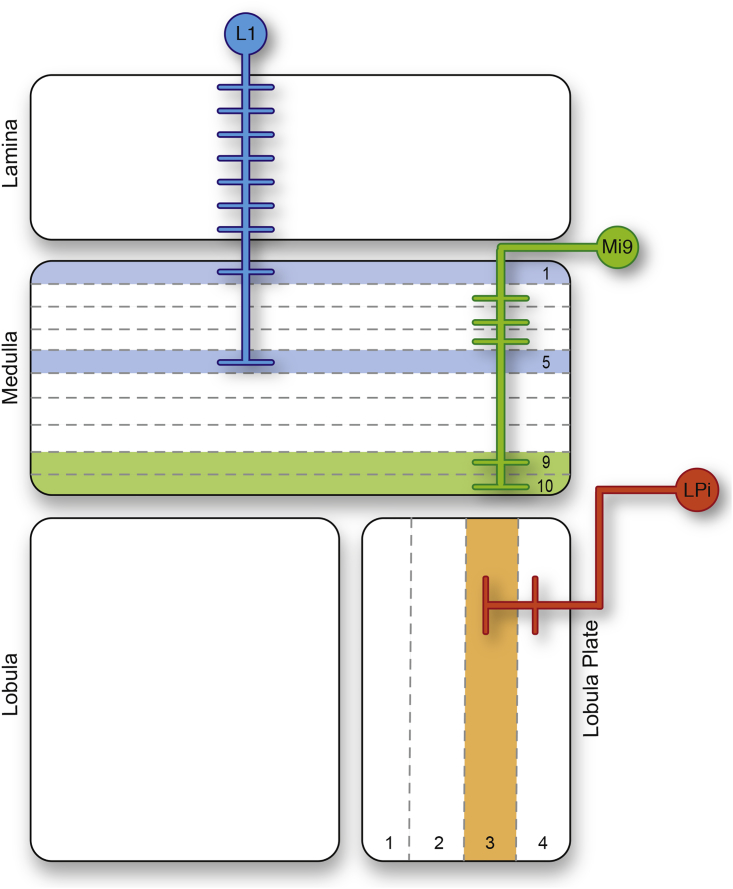
Figure 1.
Schematic of the Drosophila Optic Lobe
Schematic of the Drosophila optic lobe with glutamatergic cell types in the motion vision pathway. The three cell types are not directly connected to each other but play an import role in the circuit. For the sake of simplicity, postsynaptic partners of the glutamatergic neurons are not displayed but can be reviewed in Mauss et al. (2015) and Takemura et al., 2011, Takemura et al., 2017. Colored layers indicate area where we imaged glutamate release of the respective cell type.
The exact biophysical mechanisms by which T4 and T5 become direction selective remain unclear. To understand on a cell-by-cell level how direction selectivity is achieved, precise measurements of the signals transmitted between neurons are crucial. In this study, we focus on the final stage of the synaptic signaling cascade, i.e., transmitter release. First, we confirm the neurotransmitter phenotype of all known glutamatergic cell types (L1, Mi9, LPi) in the Drosophila motion vision pathway. Second, using the recently developed fast glutamate sensor iGluSnFR (Marvin et al., 2013), we comprehensively characterize their spatiotemporal response profiles and compare them with the ones obtained expressing the genetically encoded calcium indicator GCaMP6f (Chen et al., 2013).
Results
The Vesicular Glutamate Transporter VGlut Localizes to Axon Terminals of L1, Mi9, and LPi4-3 Neurons
VGlut or DVGLUT (CG9887) is the only vesicular glutamate transporter known in Drosophila. VGlut is located in the vesicle membrane of glutamatergic neurons where it fills the synaptic vesicles with glutamate. The protein localizes to presynaptic terminals of all known glutamatergic neuromuscular junctions (NMJs) as well as to synapses throughout the CNS neuropil in Drosophila (Daniels, 2004). Hence, VGlut is the most commonly used marker for glutamatergic neurons. Several antibodies have been raised against VGlut to identify glutamatergic neurons in the nervous system of the fruit fly (Daniels, 2004, Mahr and Aberle, 2006).
Recent studies revealed the glutamatergic phenotype of L1, Mi9, and LPi neurons—each of them a crucial element of the motion vision pathway of the fruit fly (Joesch et al., 2010, Kolodziejczyk et al., 2008, Mauss et al., 2015, Takemura et al., 2017, Takemura et al., 2011). The somata of these cell types showed positive immunoreactivity against the VGlut antibody, which was raised against a C-terminal peptide—CQMPSYDPQGYQQQ (Daniels, 2004). Interestingly, this antibody labeled mainly cell bodies of designated neurons. Since it is known that the vesicular glutamate transporter VGlut is localized to axon terminals, we investigated the glutamatergic transmitter phenotype of L1, Mi9, and LPi4-3 in more detail. We used a different anti-VGlut antibody (Mahr and Aberle, 2006), which only labels neuronal arborizations in the optic lobe neuropil and no somata. In general, the VGlut protein is highly abundant throughout all four neuropils of the optic lobe (Figure 2).
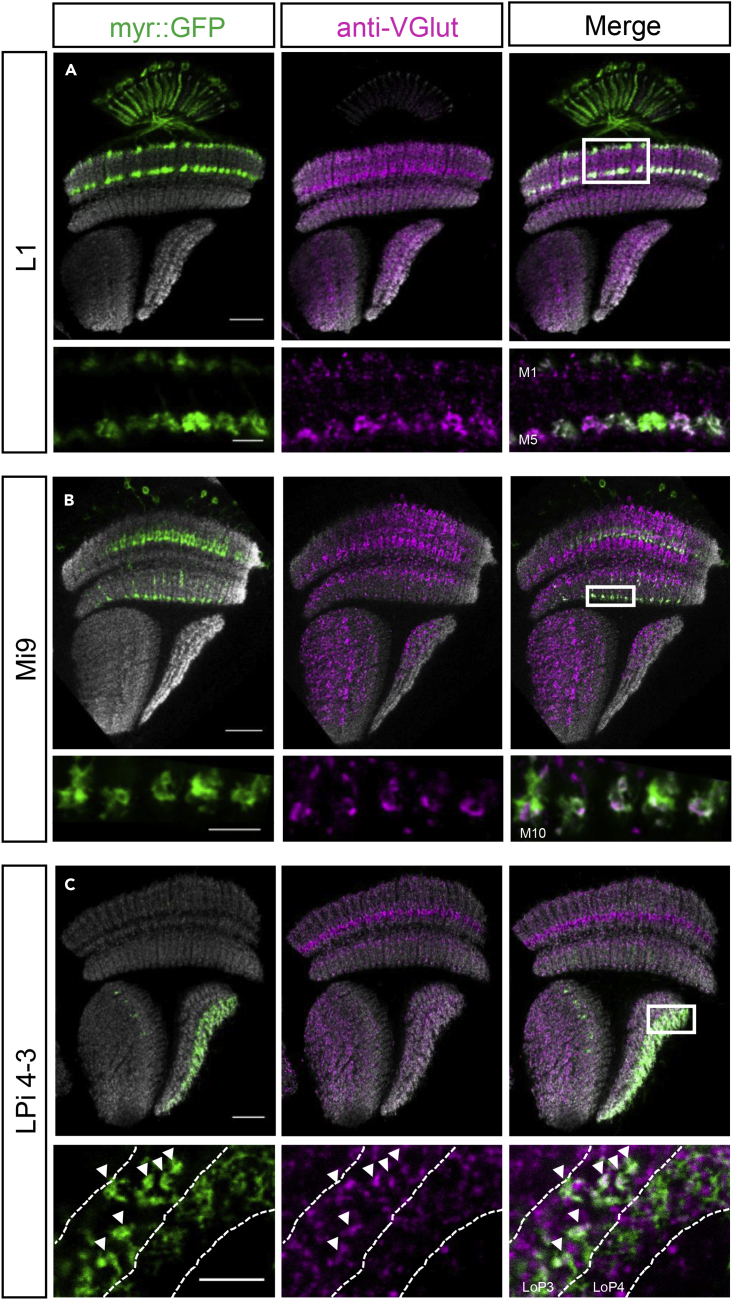
Figure 2.
Vesicular Glutamate Transporter VGlut Localizes to Axon Terminals of L1, Mi9, and LPi4-3 Neurons Indicating their Glutamatergic Phenotype
(A–C) Upper rows show overviews of optic lobes with L1 (A), Mi9 (B), and LPi4-3 (C) labeled with myr::GFP (green), background staining against bruchpilot brp (gray), and anti-VGlut staining (magenta). In the lower rows higher magnifications of axon terminals of L1, Mi9, and LPi4-3 neurons are depicted (sections marked with white boxes in overview images).
(A) L1 axon terminals in medulla layers 1 and 5 show overlapping signal with anti-VGlut staining.
(B) VGlut protein co-localizes with Mi9 axons in layer 10 of the medulla.
(C) Lobula plate intrinsic neurons LPi4-3 have their dendrites in layer 4 and project their terminals to layer 3. Labeled with arrowheads are LPi boutons in layer 3 showing overlapping signal with anti-VGlut staining. Shown here are single planes of confocal stacks. Scale bar for overview of optic lobes is 20 μm. For higher magnification close-ups the scale is 5 μm. White dashed lines in the lower panel are manually drawn and indicate layers of the lobula plate.
The axon terminals of L1 neurons show clear overlap with the anti-VGlut signal in layer M1 and M5 of the medulla (Figure 2A). The vesicular glutamate transporter VGlut resides at the presynaptic sites of L1 neurons, which indicates their glutamatergic phenotype. In layer M10 of the medulla, the same is found for Mi9 neurons: VGlut staining in this layer is co-localized with GFP-labeled Mi9 axon terminals (Figure 2B). This suggests that Mi9 neurons are glutamatergic and that they are the only source of glutamate in layer M10 of the medulla. Furthermore, we found an overlapping signal of LPi4-3 terminals in layer 3 of the lobula plate and anti-VGlut staining (Figure 2C). This confirms recent findings (Mauss et al., 2015) that described LPi neurons as glutamatergic, being presynaptic only in one of the two layers where it arborizes.
In summary, we could show that the protein VGlut localizes to axon terminals of the glutamatergic neurons L1, Mi9, and LPi4-3.
Faster Sensor Kinetics Enable More Precise Characterization of Visual Interneurons
One commonly used approach to characterize a sensory neuron is to find its preferred stimulus. This can be achieved by using a white noise input and cross-correlating the resulting output with the input (Dayan and Abbott, 2013, French, 1976, Ringach and Shapley, 2004), which yields the linear spatiotemporal receptive field as a result (e.g., Figures 3D and 3E, upper panel). The receptive field of a neuron is defined as the location of a stimulus in space and the time relative to its occurrence in which the neuron's response is modulated by the stimulus. The receptive field also describes the specific filtering properties of a system, in space as well as in time. Here, we use simple first-order low-pass, high-pass, or band-pass filters to quantify these filtering properties using the measured receptive fields. A low-pass filter only allows low frequencies to pass and attenuates high frequencies. Conversely, a high-pass filter attenuates low frequencies and allows high frequencies to pass. A band-pass filter is a combination of a high-pass and a low-pass filter in series, allowing signals within a certain frequency band to pass and attenuating all others (Cruse, 1996). In a linear system, the filters characterized this way are equivalent to the neurons' impulse responses. The temporal impulse response reveals critical aspects of the cellular response kinetics (Dayan and Abbott, 2013, Ringach and Shapley, 2004).
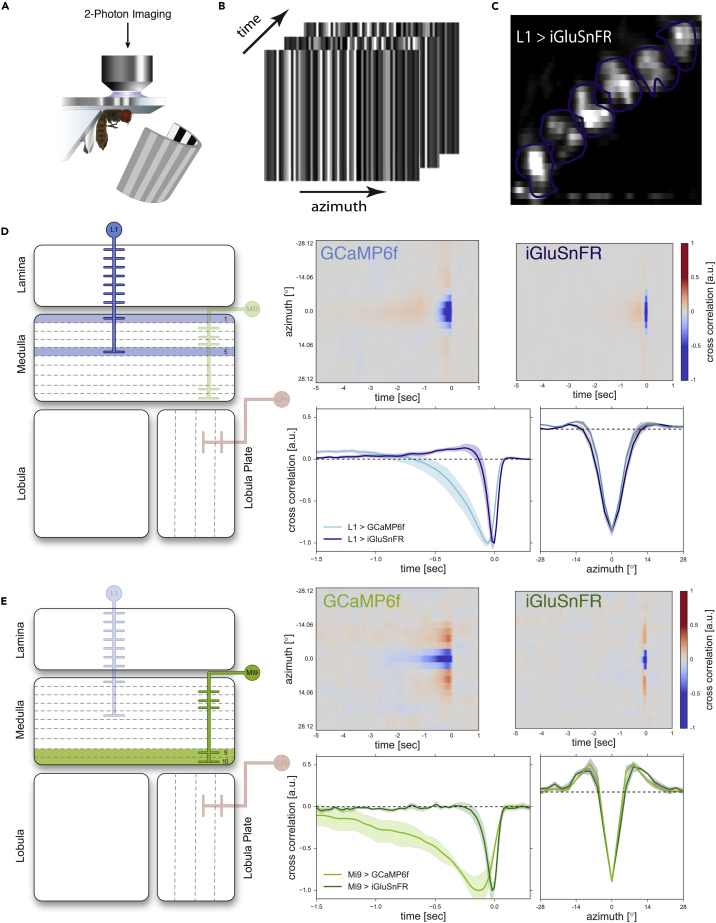
Figure 3.
Response Properties of the ON Pathway Columnar Elements L1 and Mi9
(A) Experimental setup: Fly tethered to a plastic holder under the 2-photon microscope looking onto the stimulus arena (see also Transparent Methods).
(B) Schematic of three frames of the white noise stimulus consisting of 64 horizontal bars.
(C) Example of 2-photon image of L1 expressing iGluSnFR. In purple are manually drawn region of interest ROIs.
(D) Left: Schematic of the Drosophila optic lobe. The cell type related to the right panel is highlighted. Right upper panel: Averaged aligned spatiotemporal receptive fields after reverse correlation of L1 expressing either the glutamate indicator iGluSnFR (5 flies and 66 cells) or GCaMP6f (5 flies and 60 cells). Cross sections along space and time axes result in receptive fields in right lower panel. Spatial receptive fields do not differ significantly for both indicators. Temporal kernels differ substantially. Impulse responses are shorter for iGluSnFR than for GCaMP6f. Shaded areas indicate a confidence interval of 95%.
(E) Same as (D) only for Mi9 (with iGluSnFR: 5 flies, 26 cells; with GCaMP6f: 5 flies, 50 cells).
For this reason, we characterized the spatial extent of the receptive fields as well as the response dynamics of all known glutamatergic cells in the motion vision circuit of Drosophila L1, Mi9, and LPi4-3. Expressing either the fast version of the genetically encoded calcium indicator GCaMP6f (Chen et al., 2013) or the fast glutamate-sensing reporter iGluSnFR (Marvin et al., 2013) with cell-type-specific Gal4 driver lines, we imaged glutamate and calcium signals in single axon terminals (Figure 3C). To precisely map the receptive fields of these cells, we used a one-dimensional white noise stimulus consisting of 2.8° wide vertical bars covering the full extent of the arena (180°, Figure 3B, see also Methods). The spatiotemporal receptive fields were then determined from the neuron's calcium or glutamate response by reverse correlation. Cross sections through the peak of the spatiotemporal receptive fields along the space axis therefore yield the one-dimensional spatial receptive fields depicted in Figures 3D and 3E. Cross sections along the time axis yield the temporal filtering properties of the neuron (Chichilnisky, 2001, Dayan and Abbott, 2013, French, 1976, Ringach, 2004).
To calculate the spatial extent of the cells' receptive field, we fitted a Mexican hat function (also called difference of Gaussians) that best resembled the center-surround structure of the estimated spatial receptive fields. Both neurons show a small confined center of ∼7° for Mi9 and 9–11° for L1. The full width at half maximum of the surround is about 40–50° for L1 and 20–30° for Mi9. Considering the uncertainty of the fitted model parameters, these values are similar and lie in the same order of magnitude when comparing results from imaging with both sensors. In addition, testing the raw data of both conditions against each other we find no significant difference (see Figures S2A and S2B, p value > 0.5, Welch's t test) of spatial receptive fields neither for L1 nor for Mi9. Both neurons show a small confined center of ∼7° for Mi9 and 9–11° for L1. The size of the surround has the same order of magnitude for both sensors, 40–50° for L1 and 20–30° for Mi9. This is within the range of uncertainty that the fit is subject to. Testing the raw data of both conditions against each other for the two cell types, however, does not yield a significant difference (see Figures S2A and S2B, right panel).
For a reliable estimation of the time constants of the temporal responses, we transferred the impulse responses of L1 and Mi9 into frequency space and fitted either a first-order low-pass or a first-order band-pass filter to the neurons' responses (see Figures S1C and S1D). For L1, we find that the data are best represented by a band-pass filter. The filter derived from the iGluSnFR signal has a low-pass time constant of 70 ms and a high-pass time constant of about 400 ms (see Figure S1A). The time constants derived from the GCaMP6f signal are significantly larger with low-pass and high-pass time constants of 350 and about 1,180 ms, respectively. For Mi9, we find that the temporal properties are best described by a low-pass filter. The estimated time constant of the Mi9 temporal kernel (Figure 3D, lower left) is 75 ms when measured with iGluSnFR compared with about 610 ms when measured with GCaMP6f (see Figure S1B).
For both cell types, the temporal kernel of the calcium response can be derived by low-pass filtering the faster glutamate signal. This is because the kinetics of the calcium sensor can be approximated by a low-pass filter when the intracellular calcium concentration is small compared to the KD value of the indicator (Borst and Abarbanel, 2007). For both cells, i.e., L1 and Mi9, we can fit the glutamatergic signal to the calcium signal by filtering it with a low-pass filter with a time constant of 360 ms (see Figures S2A and S2B, left panel). LPis, as motion-selective neurons, are not suitable for white noise analysis. To characterize the response properties of the LPi4-3 (Figure 4A), we first stimulated single ommatidia with local flicker stimuli that were placed precisely onto the lattice of the fly's eye via a custom-built telescopic device (see Transparent Methods and [Haag et al., 2017, Haag et al., 2016]). LPi4-3 cells responded to the individual pulses with different amplitudes, depending on the position of the stimulus (Figure 4C). The maximum response (Figure 4B, black center) of a recorded neuron was then set as the receptive field's center. All other responses to adjacent stimulation are normalized accordingly. Single flicker stimulations in the center of the receptive field show different time courses (Figure 4C) when using the two different indicators. The onset of the calcium response is much slower when compared with the glutamate response. In fact, whereas the glutamate signal shows a short transient peak response and then plateaus after ∼500ms, the calcium signal does not resolve any similar details in the time course of the response. The calcium signal decays back to zero in approximately 2 s after stimulus offset, whereas the glutamatergic signals are back at the baseline level in less than 200 ms. This loss-of-response features can be explained by the characteristics of the calcium indicator, which acts as a low-pass filter (Borst and Abarbanel, 2007). Low-pass filtering the glutamate response (τ = 446 ms, Figure S2C) results in a similar slope and decay as the calcium response. We also asked if the glutamatergic signal of the LPis is indeed direction selective as expected from Mauss et al. (2015). To asses this question we tested LPi4-3 cells with five light pulses of 472 ms duration positioned along the dorsoventral axis of the eye. When stimulated sequentially from dorsal to ventral (Figure 4D), the cell responded more strongly (PD, red line) than when we showed the same stimulus in the opposite direction (ND, black line, paired sample t test, p value < 0.01). We therefore conclude that the sensor is indeed also suitable for resolving glutamatergic direction-selective signals.
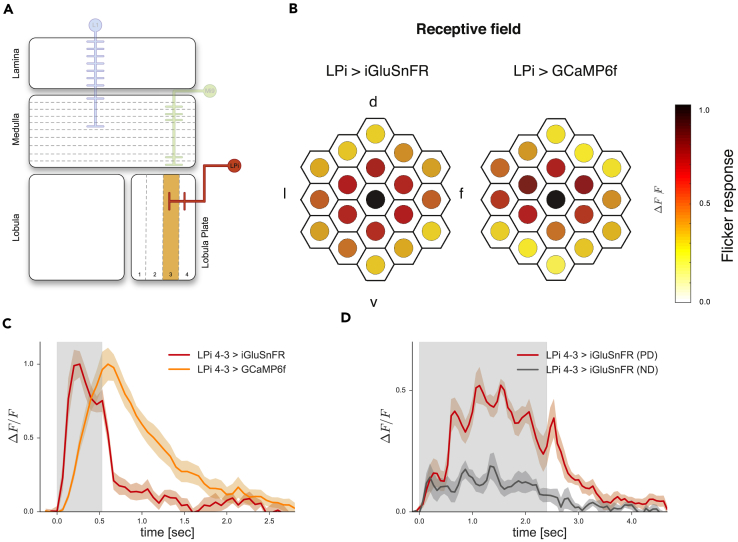
Figure 4.
Response Properties of the Direction Selective Lobula Plate Interneuron LPi4-3
(A) Schematic of the Drosophila optic lobe with LPi4-3 highlighted.
(B) Comparison of spatial receptive field size of LPi4-3 cells recorded with iGluSnFR (left, n = 24 cells from 7 flies) or GCaMP6f (right, n = 14 cells from 5 flies). The responses of individual cells to flicker stimuli presented at 19 different columnar positions were averaged after alignment to the maximum (in black) and normalization. d, Dorsal; v, ventral; l, lateral; f, frontal.
(C) Time course of LPi4-3 response upon local flicker stimulation. The decay of the signal is faster for iGluSnFR response.
(D) LPi4-3 expressing iGluSnFR show glutamatergic direction selective responses (n = 8 cells from 5 flies). Five consecutive flicker stimuli were shown along the preferred (downward) or null (upward) direction of the neuron, acting as apparent motion. Shaded areas indicate mean ± SEM.
Discussion
In this study we showed that all three investigated cell types (L1, Mi9, LPi4-3) express the vesicular transporter for glutamate, VGlut, in their axon terminals (Figure 2). To our knowledge, L1, Mi9, and LPi are the only glutamatergic cells in the Drosophila motion vision circuit. Two studies using either antibody stainings (Kolodziejczyk et al., 2008) a Flp-out analysis of the dvGlutCNSIII-Gal4 driver line (heat-shock inducible flipase excises stop-cassette upstream of mCD8-GFP to label only a few cells) (Raghu and Borst, 2011) found L2 cells to be glutamatergic. However, a recent RNA sequencing study that characterized gene expression patterns of more than 60 different cell types of the optic lobe could not confirm the expression of VGlut in L2 (Davis et al., 2018). Although they could identify other cell types like Dm cells, Lai, PB_1, Tm29, and TmY5a as glutamatergic due to their expression of VGlut, none of the other cells in the motion vision circuit (besides L1, Mi9, and LPi) seem to express VGlut. The role of Dm, Lai, PB, Tm29, and TmY5a cells in general and their potential contribution to motion vision in the fly brain are not known to date.
We also demonstrated that the spatial receptive fields measured with the glutamate sensor iGluSnFR are almost identical to the ones measured with the calcium sensor GCaMP6f (Figures 3 and 4). Both neurons possess a local OFF center receptive field with a differently strong antagonistic ON surround. Surround inhibition is a phenomenon frequently found in the early processing stages in visual systems: Bipolar and ganglion cells of the mammalian retina possess receptive fields with an antagonistic center-surround structure (reviewed in Shapley and Lennie, 1985), and first-order interneurons of the insect compound eye share this feature as well (Srinivasan et al., 1982). Functionally, a neuron with a center-surround antagonism acts as a spatial band-pass filter, enhancing the neuron's responses to edges over full field illuminations. Such band-pass filtering reduces redundancy in natural images (Srinivasan et al., 1982). We find such spatial band-pass characteristics for both cell types, L1 and Mi9. Based on their spatial receptive fields, we predict, for instance, no response of Mi9 to wide field dark flashes since the integral of the spatial receptive field is close to zero.
In the time domain, however, the glutamate signal turned out to be much faster than the calcium signal derived from the same cells. Due to their small size, many visual interneurons in the fly brain are inaccessible to electrophysiological recordings, so only a few direct recordings have been reported (Behnia et al., 2014, Gruntman et al., 2018, Juusola et al., 2016). Since data from voltage recordings from L1, Mi9, and LPi are not available so far, a direct comparison with the time constant estimated here is not possible. Simulation studies predicted time constants between 50 and 100 ms for the delayed input to the fly motion-detecting neurons (Eichner et al., 2011, Leonhardt et al., 2016). Since Mi9 is thought to provide this signal to T4 cells, the elementary motion-sensing neurons in the ON pathway, the low-pass time constant of 75 ms estimated here matches this prediction well. In addition, a previous study determined the low-pass time constant for Mi9 to be around 550 ms from calcium imaging experiments. A deconvolution of the filter with an estimated GCaMP kernel led to a resulting time constant of 63 ms (Arenz et al., 2017). This result again is in line with the time constants of the Mi9-iGluSnFR of 75 ms reported here.
In the mammalian CNS, glutamate is the most abundant and major excitatory transmitter (Meldrum, 2000, Traynelis et al., 2010). Glutamate binds to two types of receptors: metabotropic (mGluRs) and ionotropic glutamate receptors (iGluRs). iGluRs can be divided into N-methyl-D-aspartate (NMDA) and non-NMDA receptors (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid [AMPA] and kainate receptors) according to their response to agonist molecules NMDA and AMPA (Mosbacher et al., 1994). Analysis of the Drosophila genome annotated 14 iGluRs genes, which show sequence similarities with vertebrate AMPA, kainite, and NMDA receptors (Littleton and Ganetzky, 2000). However, the kainite receptor DKaiR1D and the AMPA receptor DGluR1A have different agonist/antagonist selectivity from the vertebrate's pharmacology-based classification (Li et al., 2016). Furthermore, invertebrates like Drosophila melanogaster possess a third type of iGluR, the so-called glutamate-gated chloride channel GluClα, which is inhibitory (Cully et al., 1996, Liu and Wilson, 2013). Glutamate can also act on metabotropic glutamate receptors, which signal via slower G-protein-coupled pathways. In mammals, eight mGluRs have been described (Conn and Pin, 1997). In contrast, the Drosophila genome encodes only one functional mGluR (DmGluRA), which is expressed at the glutamatergic NMJ localized in the presynaptic boutons (Bogdanik et al., 2004). Regarding the broad range of glutamate receptors in Drosophila, glutamate can act as a fast, slow, excitatory, or inhibitory transmitter (Li et al., 2016, Liu and Wilson, 2013, Mauss et al., 2015).
This gives rise to interesting speculations about the respective role of glutamate for each of the cell types investigated. In the case of the LPis, glutamate binds to the inhibitory glutamate receptor GluClα on the dendrites of large-field tangential cells, inhibiting them during null direction motion and, thus, enhancing their flow-field selectivity (Mauss et al., 2015). In the case of L1, the glutamatergic output signal seems to be key for the sign inversion of L1's OFF response in the ON pathway. This is because all Drosophila photoreceptors (R1-R8) depolarize upon illumination and release histamine onto lamina neurons, which results in the opening of chloride channels (Hardie, 1989, Hardie and Raghu, 2001). Therefore, lamina monopolar cells transiently hyperpolarize upon illumination onset and respond with a rebound excitation at illumination offset (Laughlin et al., 1987). L1 and L2 neurons respond in an identical way (Joesch et al., 2010). L1 possess an OFF receptive field center (Figure 3D) and therefore depolarizes to OFF stimuli, in contrast to its described downstream synaptic partners, which depolarize to ON stimuli (Arenz et al., 2017, Behnia et al., 2014, Strother et al., 2017, Yang et al., 2016). Hence, an inversion of the sign must occur at the synapse of L1 and its downstream partners. Since L1 is glutamatergic and GluClα is the only inhibitory receptor described in Drosophila, the glutamatergic signal is likely to be responsible for this sign inversion. Whether the downstream partners of L1 indeed express GluClα, however, is beyond the scope of this study and awaits further investigation. The hypothesis outlined above suggests that the mechanism by which a common photoreceptor input signal is split into an ON and an OFF pathway in invertebrates is different from the one in the mammalian retina where glutamatergic photoreceptors hyperpolarize in response to light. This signal is directly transmitted, i.e., without sign inversion, by ionotropic glutamate receptors expressed on the dendrites of OFF bipolar cells (Euler et al., 2014) and sign inverted by metabotropic glutamate receptors expressed on the dendrites of ON bipolar cells (Masu et al., 1995). In case of Mi9, the functional interpretation of an inhibitory glutamatergic signal is less intuitive. Mi9 directly contacts the dendrites of T4 cells, the first direction-selective neurons in the ON pathway (Takemura et al., 2017). Given the OFF response of Mi9 cells (Figure 3D), T4 cells are expected to be inhibited in darkness via the Mi9-T4 synapse. A moving ON edge would inhibit Mi9 followed by a closure of chloride channels and, thus, an increased input resistance in postsynaptic T4 cells, resulting in an amplification of a subsequently delivered excitatory input signal. Computer simulations have shown that such a two-fold signal inversion can indeed form the biophysical basis of preferred direction enhancement underlying direction selectivity in T4 cells (Borst, 2018).
Taken together our results could demonstrate the functionality of the fast glutamate reporter iGluSnFR in glutamatergic neurons of the fruit fly Drosophila melanogaster. It allowed for a more faithful description of important elements of the motion vision pathway, in particular with respect to their temporal response properties.
Limitations of the Study
Since iGluSnFR is anchored to the outer side of the plasma membrane, it senses extracellular glutamate that is present in the synaptic cleft. In addition, the iGluSnFR signal is affected by spillover and diffusion to iGluSnFR molecules outside the cleft. Thus, the iGluSnFR signal should present an upper limit to the “real” time course, i.e., the one of glutamate in the synaptic cleft as seen by the postsynaptic receptors. For the same reason, one might record an iGluSnFR signal even if the indicator is expressed on a neuron that is not glutamatergic or does not receive glutamatergic input, but ramifies within the same volume where glutamate is being released from other cells.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Aljoscha Leonhardt for careful proofreading of the manuscript. We would also like to acknowledge Hermann Aberle for sharing the VGlut antibody with us and Julia Kuhl for designing the graphical abstract. We thank Wolfgang Essbauer and Michael Sauter for fly husbandry. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 870) and the Max Planck Society. F.G.R., M.S.D., S.F., and A.B. are members of the Graduate School of Systemic Neurosciences (GSN) Munich.
Author Contributions
F.G.R., S.F., and A.B. conceived the study and designed the experiments. F.G.R. conducted and analyzed the imaging experiments for Mi9 and L1. S.F. performed and analyzed all stainings. J.H. performed and analyzed the LPi experiments. M.S.D. performed data analysis and model fitting of the receptive fields. F.G.R. wrote the manuscript with the help of all authors.
Declaration of Interests
The authors declare no competing interests.
Published: September 28, 2018
Footnotes
Supplemental Information includes Transparent Methods and two figures and can be found with this article online at https://doi.org/10.1016/j.isci.2018.08.019.
Contributor Information
Florian G. Richter, Email: frichter@neuro.mpg.de.
Alexander Borst, Email: aborst@neuro.mpg.de.
Supplemental Information
References
- Arenz A., Drews M.S., Richter F.G., Ammer G., Borst A. The temporal tuning of the Drosophila motion detectors is determined by the dynamics of their input elements. Curr. Biol. 2017;27:929–944. doi: 10.1016/j.cub.2017.01.051. [DOI] [PubMed] [Google Scholar]
- Barlow H.B., Levick W.R. The mechanism of directionally selective units in rabbit’s retina. J. Physiol. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bausenwein B., Dittrich A.P.M., Fischbach K.F. The optic lobe of Drosophila melanogaster - II. Sorting of retinotopic pathways in the medulla. Cell Tissue Res. 1992;267:17–28. doi: 10.1007/BF00318687. [DOI] [PubMed] [Google Scholar]
- Bausenwein B., Fischbach K.F. Activity labeling patterns in the medulla of Drosophila melanogaster caused by motion stimuli. Cell Tissue Res. 1992;270:25–35. doi: 10.1007/BF00381876. [DOI] [PubMed] [Google Scholar]
- Behnia R., Clark D.A., Carter A.G., Clandinin T.R., Desplan C. Processing properties of ON and OFF pathways for Drosophila motion detection. Nature. 2014;512:427–430. doi: 10.1038/nature13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanik L., Mohrmann R., Ramaekers A., Bockaert J., Grau Y., Broadie K., Parmentier M.L. The Drosophila metabotropic glutamate receptor DmGluRA regulates activity-dependent synaptic facilitation and fine synaptic morphology. J. Neurosci. 2004;24:9105–9116. doi: 10.1523/JNEUROSCI.2724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A. A biophysical mechanism for preferred direction enhancement in fly motion vision. PLoS Comput. Biol. 2018;14(6):e1006240. doi: 10.1371/journal.pcbi.1006240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A. In search of the holy grail of fly motion vision. Eur. J. Neurosci. 2014;40:3285–3293. doi: 10.1111/ejn.12731. [DOI] [PubMed] [Google Scholar]
- Borst A., Abarbanel H.D. Relating a calcium indicator signal to the unperturbed calcium concentration time-course. Theor. Biol. Med. Model. 2007;4:7. doi: 10.1186/1742-4682-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G., Platisa J., Pieribone V.A., Raccuglia D., Kunst M., Nitabach M.N. Genetically targeted optical electrophysiology in intact neural circuits. Cell. 2013;154:904–913. doi: 10.1016/j.cell.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.-W., Wardill T.J., Sun Y., Pulver S.R., Renninger S.L., Baohan A., Schreiter E.R., Kerr R.A., Orger M.B., Jayaraman V. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013;499:295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chichilnisky E.J. A simple white noise analysis of neuronal light responses. Network. 2001;12:199–213. [PubMed] [Google Scholar]
- Clark D.A., Bursztyn L., Horowitz M.A., Schnitzer M.J., Clandinin T.R. Defining the computational structure of the motion detector in Drosophila. Neuron. 2011;70:1165–1177. doi: 10.1016/j.neuron.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn P.J., Pin J.-P. Pharmacology and function of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cruse, H. (1996). Neural Networks as Cybernetics Systems.
- Cully D.F., Paress P.S., Liu K.K., Schaeffer J.M., Arena J.P. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J. Biol. Chem. 1996;271:20187–20191. doi: 10.1074/jbc.271.33.20187. [DOI] [PubMed] [Google Scholar]
- Daniels R.W. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J. Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F.P., Nern A., Picard S., Reiser M.B., Rubin G.M., Eddy S.R., Henry G.L. A genetic, genomic, and computational resource for exploring neural circuit function. bioRxiv. 2018 doi: 10.7554/eLife.50901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P., Abbott L.F. Theoretical neuroscience: computational and mathematical modeling of neural systems. J. Chem. Inf. Model. 2013;53:1689–1699. [Google Scholar]
- Di Maio V. Regulation of information passing by synaptic transmission: a short review. Brain Res. 2008;1225:26–38. doi: 10.1016/j.brainres.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Eichner H., Joesch M., Schnell B., Reiff D.F., Borst A. Internal structure of the fly elementary motion detector. Neuron. 2011;70:1155–1164. doi: 10.1016/j.neuron.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Euler T., Haverkamp S., Schubert T., Baden T. Retinal bipolar cells: elementary building blocks of vision. Nat. Rev. Neurosci. 2014;15:507–519. doi: 10.1038/nrn3783. [DOI] [PubMed] [Google Scholar]
- French A.S. Practical nonlinear system analysis by Wiener kernel estimation in the frequency domain. Biol. Cybern. 1976;24:111–119. [Google Scholar]
- Gruntman E., Romani S., Reiser M.B. Simple integration of fast excitation and offset, delayed inhibition computes directional selectivity in Drosophila. Nat. Neurosci. 2018;21:250–257. doi: 10.1038/s41593-017-0046-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J., Arenz A., Serbe E., Gabbiani F., Borst A. Complementary mechanisms create direction selectivity in the fly. Elife. 2016;5 doi: 10.7554/eLife.17421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag J., Mishra A., Borst A. A common directional tuning mechanism of Drosophila motion-sensing neurons in the ON and in the OFF pathway. Elife. 2017;6:1–15. doi: 10.7554/eLife.29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R.C. A histamine-activated chloride channel involved in neurotransmission at a photoreceptor synapse. Nature. 1989;339:704–706. doi: 10.1038/339704a0. [DOI] [PubMed] [Google Scholar]
- Hardie R.C., Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hausen K., Wolburg-Buchholz K., Ribi W.A. The synaptic organization of visual interneurons in the lobula complex of flies - a light and electron microscopical study using silver-intensified cobalt-impregnations. Cell Tissue Res. 1980;208:371–387. doi: 10.1007/BF00233871. [DOI] [PubMed] [Google Scholar]
- Hopp E., Borst A., Haag J. Subcellular mapping of dendritic activity in optic flow processing neurons. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2014;200:359–370. doi: 10.1007/s00359-014-0893-3. [DOI] [PubMed] [Google Scholar]
- Jin L., Han Z., Platisa J., Wooltorton J.R.A., Cohen L.B., Pieribone V.A. Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe. Neuron. 2012;75:779–785. doi: 10.1016/j.neuron.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesch M., Schnell B., Raghu S.V., Reiff D.F., Borst A. ON and OFF pathways in Drosophila motion vision. Nature. 2010;468:300–304. doi: 10.1038/nature09545. [DOI] [PubMed] [Google Scholar]
- Joesch M., Weber F., Eichner H., Borst A. Functional specialization of parallel motion detection circuits in the fly. J. Neurosci. 2013;33:902–905. doi: 10.1523/JNEUROSCI.3374-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juusola M., Dau A., Zheng L., Rien D. Electrophysiological method for recording intracellular voltage responses of Drosophila photoreceptors and interneurons to light stimuli in vivo. J. Vis. Exp. 2016;112:1–16. doi: 10.3791/54142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk A., Sun X., Meinertzhagen I.A., Nässel D.R. Glutamate, GABA and acetylcholine signaling components in the lamina of the Drosophila visual system. PLoS One. 2008;3:e2110. doi: 10.1371/journal.pone.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin S.B., Howard J., Blakeslee B. Synaptic limitations to contrast coding in the retina of the blowfly Calliphora. Proc. R. Soc. Lond. B Biol. Sci. 1987;231:437–467. doi: 10.1098/rspb.1987.0054. [DOI] [PubMed] [Google Scholar]
- Leonhardt A., Ammer G., Meier M., Serbe E., Bahl A., Borst A. Asymmetry of Drosophila ON and OFF motion detectors enhances real-world velocity estimation. Nat. Neurosci. 2016;19:706–715. doi: 10.1038/nn.4262. [DOI] [PubMed] [Google Scholar]
- Li Y., Dharkar P., Han T.H., Serpe M., Lee C.H., Mayer M.L. Novel functional properties of Drosophila CNS glutamate receptors. Neuron. 2016;92:1036–1048. doi: 10.1016/j.neuron.2016.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton J.T., Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Liu W.W., Wilson R.I. Glutamate is an inhibitory neurotransmitter in the Drosophila olfactory system. Proc. Natl. Acad. Sci. USA. 2013;110:10294–10299. doi: 10.1073/pnas.1220560110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahr A., Aberle H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns. 2006;6:299–309. doi: 10.1016/j.modgep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Maisak M.S., Haag J., Ammer G., Serbe E., Meier M., Leonhardt A., Schilling T., Bahl A., Rubin G.M., Nern A. A directional tuning map of Drosophila elementary motion detectors. Nature. 2013;500:212–216. doi: 10.1038/nature12320. [DOI] [PubMed] [Google Scholar]
- Marvin J.S., Borghuis B.G., Tian L., Cichon J., Harnett M.T., Akerboom J., Gordus A., Renninger S.L., Chen T.W., Bargmann C.I. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods. 2013;10:162–170. doi: 10.1038/nmeth.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu M., Iwakabe H., Tagawa Y., Miyoshi T., Yamashita M., Fukuda Y., Sasaki H., Hiroi K., Nakamura Y., Shigemoto R. Specific deficit of the ON response in visual transmission by targeted disruption of the mGIuR6 gene. Cell. 1995;80:757–765. doi: 10.1016/0092-8674(95)90354-2. [DOI] [PubMed] [Google Scholar]
- Mauss A.S., Meier M., Serbe E., Borst A. Optogenetic and pharmacologic dissection of feedforward inhibition in Drosophila motion vision. J. Neurosci. 2014;34:2254–2263. doi: 10.1523/JNEUROSCI.3938-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss A.S., Pankova K., Arenz A., Nern A., Rubin G.M., Borst A. Neural circuit to integrate opposing motions in the visual field. Cell. 2015;162:351–362. doi: 10.1016/j.cell.2015.06.035. [DOI] [PubMed] [Google Scholar]
- Meldrum B.S. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Mosbacher J., Schoepfer R., Monyer H., Burnashev N., Seeburg P., Ruppersberg J. A molecular determinant for submillisecond desensitization in glutamate receptors. Science. 1994;266:1059–1062. doi: 10.1126/science.7973663. [DOI] [PubMed] [Google Scholar]
- Raghu S.V., Borst A. Candidate glutamatergic neurons in the visual system of Drosophila. PLoS One. 2011 doi: 10.1371/journal.pone.0019472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringach D., Shapley R. Reverse correlation in neurophysiology. Cognit. Sci. 2004;28:147–166. [Google Scholar]
- Ringach D.L. Mapping receptive fields in primary visual cortex. J. Physiol. 2004;558:717–728. doi: 10.1113/jphysiol.2004.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J., Pauls D., Schnell B., Ting C.Y., Lee C.H., Sinakevitch I., Morante J., Strausfeld N.J., Ito K., Heisenberg M. Dissection of the peripheral motion channel in the visual system of Drosophila melanogaster. Neuron. 2007;56:155–170. doi: 10.1016/j.neuron.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Schnell B., Joesch M., Forstner F., Raghu S.V., Otsuna H., Ito K., Borst A., Reiff D.F. Processing of horizontal optic flow in three visual interneurons of the Drosophila brain. J. Neurophysiol. 2010;103:1646–1657. doi: 10.1152/jn.00950.2009. [DOI] [PubMed] [Google Scholar]
- Scott E.K., Raabe T., Luo L. Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J. Comp. Neurol. 2002;454:470–481. doi: 10.1002/cne.10467. [DOI] [PubMed] [Google Scholar]
- Shapley R., Lennie P. Spatial frequency analysis in the visual system. Annu. Rev. Neurosci. 1985;8:547–583. doi: 10.1146/annurev.ne.08.030185.002555. [DOI] [PubMed] [Google Scholar]
- Shinomiya K., Karuppudurai T., Lin T.Y., Lu Z., Lee C.H., Meinertzhagen I.A. Candidate neural substrates for OFF edge motion detection in Drosophila. Curr. Biol. 2014;24:1062–1070. doi: 10.1016/j.cub.2014.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silies M., Gohl D.M., Fisher Y.E., Freifeld L., Clark D.A., Clandinin T.R. Modular use of peripheral input channels tunes motion-detecting circuitry. Neuron. 2013;79:111–127. doi: 10.1016/j.neuron.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M.V., Laughlin S.B., Dubs A. Predictive coding: a fresh view of inhibition in the retina. Proc. R. Soc. Lond. B Biol. Sci. 1982;216:1471–2954. doi: 10.1098/rspb.1982.0085. [DOI] [PubMed] [Google Scholar]
- St-Pierre F., Marshall J.D., Yang Y., Gong Y., Schnitzer M.J., Lin M.Z. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat. Neurosci. 2014;17:884–889. doi: 10.1038/nn.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother J.A., Wu S.T., Wong A.M., Nern A., Rogers E.M., Le J.Q., Rubin G.M., Reiser M.B. The emergence of directional selectivity in the visual motion pathway of Drosophila. Neuron. 2017;94:168–182. doi: 10.1016/j.neuron.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Takemura S.Y., Nern A., Chklovskii D.B., Scheffer L.K., Rubin G.M., Meinertzhagen I.A. The comprehensive connectome of a neural substrate for ‘ON’ motion detection in Drosophila. Elife. 2017;6:1–16. doi: 10.7554/eLife.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura S.Y., Karuppudurai T., Ting C.Y., Lu Z., Lee C.H., Meinertzhagen I.A. Cholinergic circuits integrate neighboring visual signals in a Drosophila motion detection pathway. Curr. Biol. 2011;21:2077–2084. doi: 10.1016/j.cub.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H., Jinno Y., Tomita A., Niino Y., Yamada Y., Mikoshiba K., Miyawaki A., Okamura Y. Improved detection of electrical activity with a voltage probe based on a voltage-sensing phosphatase. J. Physiol. 2013;591:4427–4437. doi: 10.1113/jphysiol.2013.257048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill J.C., Nern A., Holtz S.L., Rubin G.M., Reiser M.B. Contributions of the 12 neuron classes in the fly lamina to motion vision. Neuron. 2013;79:128–140. doi: 10.1016/j.neuron.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hassenstein B., Reichardt W. Systemtheoretische Analyse der Zeit-, Reihenfolgen-und Vorzeichenauswertung bei der Bewegungsperzeption des Rüsselkäfers Chlorophanus. Sect. B J. Chem. Sci. 1956;11:513–524. [Google Scholar]
- Wasserman S.M., Aptekar J.W., Lu P., Nguyen J., Wang A.L., Keles M.F., Grygoruk A., Krantz D.E., Larsen C., Frye M.A. Olfactory neuromodulation of motion vision circuitry in Drosophila. Curr. Biol. 2015;25:467–472. doi: 10.1016/j.cub.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H.H., St-Pierre F., Sun X., Ding X., Lin M.Z., Clandinin T.R. Subcellular imaging of voltage and calcium signals reveals neural processing in vivo. Cell. 2016;166:245–257. doi: 10.1016/j.cell.2016.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R.S. Calcium and transmitter release. J. Physiol. Paris. 1993;87:25–36. doi: 10.1016/0928-4257(93)90021-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.