Summary
Organocatalytic domino processes have become a rapidly growing area of research. N-heterocyclic carbenes (NHCs) have emerged as powerful organocatalysts for various transformations and continue to have widespread application. In the last decade, domino reactions catalyzed by NHCs have seen significant progress since the different activation modes could be successfully combined in one process. The most attractive features of these domino sequences include the readily available catalysts and substrates, the simple operational procedures, and the rapid assembly of complex molecular scaffolds with excellent levels of stereocontrol under mild reaction conditions. This review covers the advances in NHC-catalyzed domino reactions by focusing on the reaction scope, limitations, and mechanism with a close attention to the features of the reaction substrates.
Subject Areas: Organic Synthesis, Stereochemistry, Organic Chemistry Methods
Graphical Abstract

Organic Synthesis; Stereochemistry; Organic Chemistry Methods
Introduction
The synthesis of enantiomerically pure compounds continues to be an appealing and fascinating area of modern organic synthesis. There has been significant progress in the development of quite a number of methods for preparing these compounds. Organocatalytic domino reactions have attracted most attention in this respect because of their high efficiencies by decreasing the purification steps as well as the quantities of materials and solvents used. Furthermore, one of the greatest advantage of these reactions over the traditional stop-and-go synthesis is that multiple reactions are carried out in one pot under the same reaction conditions. Nature uses the same principle for the highly efficient synthesis of different chemical compounds with extraordinary selectivity via numerous parallel reactions with the aid of enzymes in a single cell. For this reason, organocatalytic domino reactions, which resemble natural intrinsic processes, are environment friendly and often proceed with excellent chemo-, regio-, and stereoselectivity (Enders et al., 2007a, Grondal et al., 2010, Albrecht et al., 2011, Pellissier, 2012, Volla et al., 2014, Hayashi, 2016, Chauhan et al., 2017).
Since about the year 2000, organocatalysis developed rapidly and revolutionized the world of synthetic organic chemistry (List et al., 2000, Bui and Barbas, 2000, Ahrendt et al., 2000). It paved the way for the new efficient asymmetric syntheses of complex chiral molecules. A significant advantage of these organocatalysts is that they are compatible with many types of reactions through different activation modes and make them ideal for application in organocatalytic one-pot reactions. Interestingly, secondary amine catalysis strongly dominated this field, and most of the organocatalytic domino reactions were governed by the well-developed iminium-enamine activation modes (Chanda and Zhao, 2018), with comparatively less applications of other organocatalysts reported in the literature. From the very beginning, N-heterocyclic carbenes (NHCs) played an important role as Lewis-base organocatalysts (Enders and Balensiefer, 2004, Enders et al., 2007b, Bode, 2013, Ryan et al., 2013, Wang and Scheidt, 2016). As time progressed, the NHCs developed, and recently they became a complete researched topic in this exciting area of asymmetric domino reactions (Flanigan et al., 2015, Reyes et al., 2017). A driving force is the ability of NHCs to promote a wide range of reactions by combining two or more activation modes.
This review illustrates the development of NHC-catalyzed domino transformations from the beginning up to the present. Special emphasis is given to the discussion of the domino reactions via different activation modes, which are the basis of our classification. Finally, we conclude with a perspective on this NHC-catalyzed asymmetric domino research field.
NHC-Catalyzed Classic Transformations via a Single Activation Mode
At present, there are six basic types of classic reactions associated with NHC catalysis. The benzoin reactions (Ukai et al., 1943, Sheehan and Hunneman, 1966) via the formation of the Breslow intermediates (Lapworth, 1903, Breslow, 1958) were the first application of NHC catalysis. This a1–d1 umpolung activation can also promote Stetter reactions (Stetter, 1976, Enders et al., 1996) with Michael acceptors or inactivated double or triple bonds (Scheme 1A) (Bugaut and Glorius, 2012).
Scheme 1.
Summary of Classic Reactions via NHC Catalysis
(A) Reactions via the Breslow intermediates.
(B) Reactions via azolium enolates.
(C) Reactions via homoenolate equivalents.
(D) Reactions via azolium dienolates.
(E) Reactions via acyl azoliums.
(F) Reactions via α,β-unsaturated acyl azolium intermediates.
The research groups of Rovis (Reynolds et al., 2004), Bode (He et al., 2006), Ye (Zhang et al., 2008), and Smith (Duguet et al., 2008) showed the ability of NHCs to generate azolium enolates (Scheme 1B). In particular, a series of cycloaddition reactions of ketenes via azolium enolate has been developed by Ye and co-workers (Chen and Ye, 2013) for the synthesis of a number of heterocyclic compounds. α-Halo aldehydes, esters, and acids have also been successfully employed as precursors for the generation of azolium enolates (Douglas et al., 2012).
The [3 + 2] cycloadditions via a3–d3 umpolung were independently reported by Glorius and co-workers (Burstein and Glorius, 2004) and Bode and co-workers (Scheme 1C) (Sohn et al., 2004). The corresponding intermediate shows nucleophilic reactivity at the β-position of the enal, and it is regarded as a homoenolate equivalent. This new type of homoenolate equivalent allowed the development of various novel carbon–carbon and carbon–heteroatom bond formations (Nair et al., 2011, Menon et al., 2015).
The generation of azolium dienolate using NHC-mediated catalysis is an efficient route to a range of important heterocyclic scaffolds via [4 + n] cycloaddition reactions (Scheme 1D). These protocols provide an alternate pathway for the remote functionalizations of carbonyl compounds (Chen et al., 2018). In this context, addition of an NHC to a carbonyl compound bearing an acidic γ-hydrogen atom is the main mode of azolium enolate generation.
The NHC-based intermediates can also be used as electrophiles (Vora et al., 2012, Mahatthananchai and Bode, 2014, Levens and Lupton, 2017). An early example of NHC catalysis via acyl azoliums was reported by Castells and co-workers for the conversion of furfural to the methyl ester (Scheme 1E) (Castells et al., 1977). Recently, the application of α,β-unsaturated acyl azolium intermediate has been developed into a highly active subfield of NHC catalysis (Scheme 1F) (De Sarkar et al., 2013, Zhang et al., 2017a). There have been great achievements by using α,β-unsaturated acyl azoliums in the reactions with bis-nucleophiles (Ryan et al., 2009, Kaeobamrung et al., 2010, DeSarkar and Studer, 2010, Sun et al., 2011, Cheng et al., 2013, Chen et al., 2014) and in kinetic resolution reactions (Lu et al., 2013, Lu et al., 2014).
Over the past two decades, many transformations have been developed by using these intermediates. According to the definition of Tietze (Tietze and Beifuss, 1993), most of these reactions can be considered as domino processes. Thus we will limit the discussion to NHC-catalyzed domino reactions via two or more activation modes.
NHC-Catalyzed Domino Reactions via Two or More Activation Modes
From the set of the six classic reaction types classified by the intermediates, the combination of other types of activation modes and the enolate intermediate is most common in NHC-catalyzed domino reactions. Thus a rational way to classify these domino reactions is based on the combination of the activation modes with the enolate intermediate. For instance, a common method is the use of the Breslow intermediate and the enolate approach, which was well developed in the last decade. The possible pathway of this transformation is depicted in Scheme 2. The addition of the NHC to the aldehyde gives the corresponding Breslow intermediate, which affords the Benzoin/Stetter reaction products. The following aldol/Michael reactions via an enolate give the final product. These domino reactions are included in our early minireview (Grossmann and Enders, 2012) and are not discussed here.
Scheme 2.
Domino Reactions via Breslow Intermediate and Enolate Activation Modes
In another approach, domino reactions via homoenolate-enolate activation modes are initiated by the reaction of the homoenolate equivalent, followed by the generation of NHC-bound azolium enolate (Scheme 3A). Instead, for domino reactions via α,β-unsaturated acyl azolium enolate activation modes, the first step is the Michael addition to the α,β-unsaturated acyl azolium, leading to the azolium enolate, which can then undergo a second reaction with an electrophile to afford the products (Scheme 3B). The domino reactions via dienolate-enolate activation modes are also possible if the γ-CH deprotonation of the generated acyl azolium adduct can occur to give the azolium enolate (Scheme 3C).
Scheme 3.
Classification of NHC-Catalyzed Domino Reactions via Two or More Activation Modes
(A) Domino reactions via homoenolate-enolate activation modes.
(B) Domino reactions via α,β-unsaturated acyl azolium-enolate activation modes.
(C) Domino reactions via dienolate-enolate activation modes.
Domino Reactions via Homoenolate-Enolate Activation Modes
Domino Reactions Initiated by Michael/Aldol Reactions
The Michael acceptors are found to be suitable multifunctional substrates for the domino processes via the homoenolate-enolate activation modes (Scheme 4). An early example of this kind of sequence was reported by Nair and co-workers in 2006 (Nair et al., 2006). The reaction proceeded smoothly using 1,3-dimesityl imidazol-2-ylidene (C1) as catalyst, with different α,β-unsaturated aldehydes 1 and chalcones 2, affording the cyclopentenes 3 in moderate to good yields. The side reactions (benzoin and Stetter reactions) were suppressed by the bulky substituents on the catalyst (Scheme 5).
Scheme 4.
The Michael Acceptors for Domino Reactions via the Homoenolate-Enolate Activation Modes
Scheme 5.
Domino a3-d3 Umpolung/Michael/Aldol/Lactonization/Decarboxylation Reaction
The authors proposed a mechanism for this a3-d3 umpolung/Michael/aldol/lactonization/decarboxylation domino sequence (Scheme 6). In the first key step, the Michael addition of the homoenolate equivalent 4 to the chalcone forms the C−C bond and generates the azolium enolate 5. The intramolecular aldol/lactonization gives the unstable β-lactone 8 and regenerates the NHC catalyst. A final decarboxylation affords the desired cyclopentene 3.
Scheme 6.
Proposed Catalytic Cycle for the Domino a3-d3 Umpolung/Michael/Aldol/Lactonization/Decarboxylation Reaction
In 2007, Bode and co-workers (Chiang et al., 2007) developed an enantioselective version of this domino process by using the aminoindanol-derived catalyst precursor C2 (Scheme 7A). Unfortunately, the enones were limited to 4-oxo-2-butenoates 9. Later, Scheidt and co-workers (Cardinal-David et al., 2010) improved this domino protocol further by using the Lewis acid and NHC co-catalysis, achieving better results on both the tolerance of substrate scopes and stereoselectivity. Interestingly, in contrast to Nair's work, in these two cases, the cis-diastereomer was obtained (Scheme 7B).
Scheme 7.
Asymmetric Domino a3-d3 Umpolung/Michael/Aldol/Lactonization/Decarboxylation Reaction
(A) Bode's work.
(B) Scheidt's work.
Very recently, Wang and co-workers (Zhang et al., 2017b) have shown the application of oxindolyl β,γ-unsaturated α-keto ester 11 in an a3-d3 umpolung/Michael/aldol/lactonization domino sequence (Scheme 8). These substrates reacted well with α,β-unsaturated aldehydes 1 in the presence of the pre-catalyst C2, affording the corresponding products 12 in moderate to good yields with a broad scope of differently substituted aromatic substrates. Furthermore, excellent diastereoselectivities and excellent enantioselectivities were achieved. In this case, the β-lactone-fused spiro[cyclopentane-oxindole] 12 was stable and the final decarboxylation did not occur.
Scheme 8.
Domino a3-d3 Umpolung/Michael/Aldol/Lactonization Reaction
Based on the previous developments, Scheidt and co-workers (Cohen et al., 2011) described an interesting a3-d3 umpolung/Michael/aldol/esterification domino reaction by employing the β,γ-unsaturated α-ketoesters 13 as the substrates (Scheme 9). This sequence, in the presence of a stoichiometric amount of Ti(OiPr)4 and a catalyst derived from C3, afforded the highly functionalized cyclopentanol 14 in generally good yields (52%–85%) and very good enantioselectivities (91%–99% ee).
Scheme 9.
Domino a3-d3 Umpolung/Michael/Aldol/Esterification Reaction
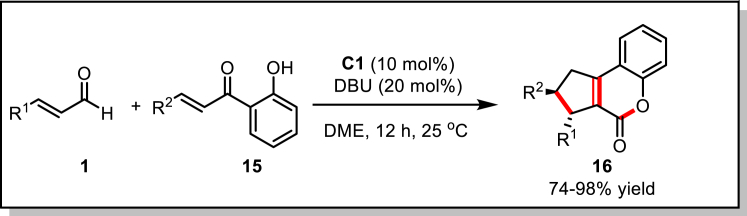
This transformation led to the further development of the domino reactions via homoenolate and enolate activation modes. A series of interesting cyclic compounds can be obtained by introducing a nucleophilic group on the Michael acceptor. This protocol was successfully extended by Biju and co-workers (Bhunia et al., 2013) via the application of o-hydroxy chalcones 15 as the substrates in combination with the α,β-unsaturated aldehydes 1, leading to a highly diastereoselective synthesis of cyclopentane-fused coumarins 16 (Scheme 10). Mechanistically, this reaction represents an a3-d3 umpolung/Michael/aldol/dehydration/lactonization sequence. The deprotonation of the pre-catalyst C1 resulted in a suitable catalyst, yielding the corresponding products 16 in moderate to good yields for a broad range of β-aryl and β-alkyl α,β-unsaturated aldehydes. Remarkably, only a single diastereomer was observed.
Scheme 10.
Domino a3-d3 Umpolung/Michael/Aldol/Dehydration/Lactonization Reaction
This concept was also used by Chi and co-workers (Fu et al., 2014), who disclosed the a3-d3 umpolung/Michael/aldol/dehydration/lactamization sequence (Scheme 11). This sequence involving the saturated ester 17 and amino enones 18, catalyzed by the catalyst C4, afforded polycyclic quinolinones in generally good yields with very good enantioselectivities. Another asymmetric domino a3-d3 umpolung/Michael/aldol/lactamization reaction was described by Hui and co-workers by employing indole-derived enones 20 and α,β-unsaturated aldehydes 1 as the substrates (Scheme 12) (Yang et al., 2017). In this case, the dehydration process did not occur, and the salient features of this transformation include the efficient synthesis of cyclopenta[3,4]pyrrolo[1,2-a]indol-4-ones 21 with four consecutive stereogenic centers in good yields with very good enantioselectivities along with a wide substrate scope and a successful scale-up reaction.
Scheme 11.
Domino a3-d3 Umpolung/Michael/Aldol/Dehydration/Lactamization Reaction
Scheme 12.
Domino a3-d3 Umpolung/Michael/Aldol/Lactamization Reaction
Domino Reactions Initiated by the Michael/Mannich Reaction
The strategy of homoenolate-enolate activation modes was then successfully expended to the reactions with α,β-unsaturated imines 22 by Bode and co-workers (Scheme 13A) (He and Bode, 2008). In this case, the a3-d3 umpolung/Michael/Mannich/lactamization domino sequence furnished the corresponding cyclopentane-fused β-lactams 23 in good yields with very good stereoselectivities. Chi and co-workers showed that the spirocyclic oxindole-annulated compounds 25 can be built when the substrates 24 are employed (Scheme 13B). Although only racemic products were obtained, the reaction proceeded with very good yields, and the formation of a quaternary stereogenic center is noteworthy (Jiang et al., 2012).
Scheme 13.
Domino a3-d3 Umpolung/Michael/Mannich/Lactamization Reaction
(A) Bode's work.
(B) Chi's work.
A related approach was developed by our group for the assembly of optically active spirocyclopentane oxindole-fused β-lactams 27 with four contiguous stereocenters, including one quaternary and one tetrasubstituted stereocenter (Scheme 14) (Wang et al., 2017). This a3-d3 umpolung/Michael/Mannich/lactamization reaction starts with the homoenolate activation of the isatin-derived enals 26 via the pre-catalyst C3. After the Michael addition of the homoenolate equivalent to the N-sulfonyl ketimines 22, the desired products 27 are obtained by the following Mannich/lactamization reaction. Despite the moderate yields, the stereoselectivities are very good and only one diastereoisomer is formed. Worth mentioning is the fact that the spirocyclopentane oxindoles 28 containing an enaminone moiety can be obtained using the same starting materials in the presence of a different pre-catalyst C6.
Scheme 14.
Domino a3-d3 Umpolung/Michael/Mannich/Lactamization Reaction of Isatin-Derived Enals
Domino Reactions Initiated by the Michael/Michael Reaction
In continuation of our efforts to achieve complex structures, a different a3-d3 umpolung/Michael/Michael/esterification sequence was developed (Scheme 15) (Shu et al., 2016). Screening of the reaction conditions revealed that this reaction proceeded best in the presence of 10 mol% of the pre-catalyst C7 and 1.0 equivalent of K3PO4. Although the yield was moderate, the tetra-substituted cyclopentanes 30 were formed in high stereoselectivities. Nevertheless, the construction of four contiguous stereogenic centers demonstrates the utility of this synthetic protocol. The proposed reaction pathway for this domino reaction is outlined in Scheme 16. The first step involves the formation of the homoenolate equivalent 31, which undergoes a first Michael addition to the nitroallylic acetates 29, followed by the elimination of the acetate from the adduct 32 to generate the second Michael acceptor 33. An intramolecular Michael reaction leads to the adduct 34. Final turnover of the catalyst through esterification forms the final cyclopentanes 30.
Scheme 15.
Domino a3-d3 Umpolung/Michael/Michael/Esterification Reaction
Scheme 16.
Proposed Mechanism for Domino a3-d3 Umpolung/Michael/Michael/Esterification Reaction
In the context of the development of more complex structures, a series of new asymmetric domino reactions using benzodienones 35 with α,β-unsaturated aldehydes 1 has been developed. Chi and co-workers reported the application of the benzodienones 35 in an a3–d3 umpolung/Michael/Michael/esterification domino sequence (Scheme 17) (Fang et al., 2011). This reaction proceeded with good yields, good diastereoselectivities, and high enantioselectivities in the presence of the aminoindanol-derived catalyst precursor C2, with a broad scope of differently substituted aromatic substrates.
Scheme 17.
Domino a3-d3 Umpolung/Michael/Michael/Esterification Between Benzodienones and α,β-Unsaturated Aldehydes
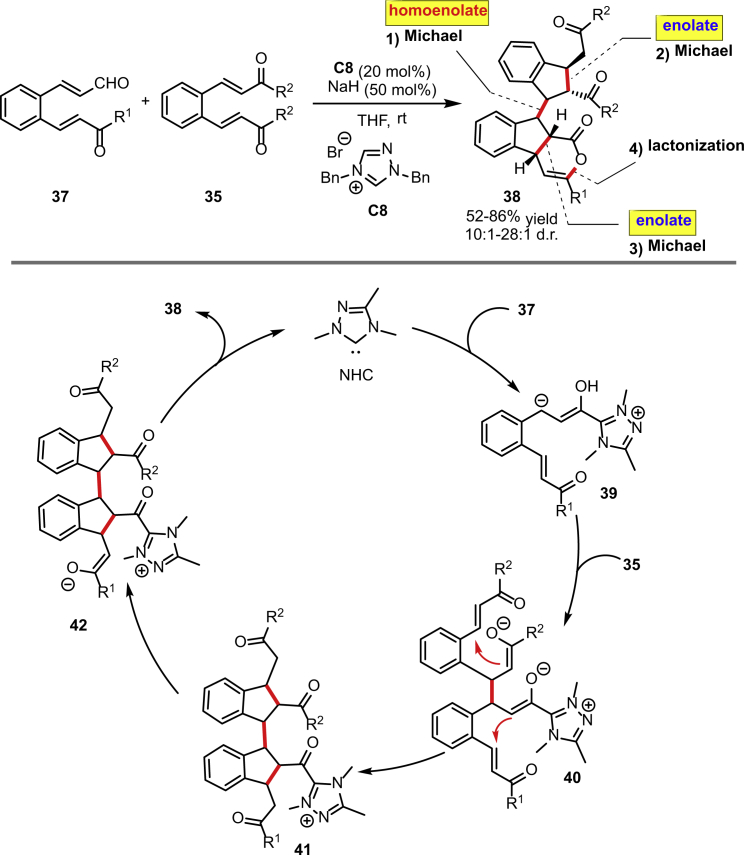
A more complex domino process initiated by Michael reaction of the homoenolate equivalent is shown in Scheme 18. Cheng and co-workers described an a3–d3 umpolung/Michael/Michael/Michael/lactonization domino reaction of 2-aroylvinylchalcones 37 and benzodienones 35 in the presence of the pre-catalyst C8 (Fan and Cheng, 2014). This protocol opens up a new entry to complex indane derivatives, which are usually found in various bioactive natural products and pharmaceuticals. The reaction sequence starts with the Michael reaction of the homoenolate equivalent 39 with one of the enone moieties of 35. The following intramolecular Michael reactions of 40 lead to the adduct 41. The final lactonization regenerates the catalyst and affords the corresponding products 38. The enantioselective version of this transformation was also attempted by the same group using chiral triazolium carbenes. Unfortunately, only very low yields of the products 38 were obtained.
Scheme 18.
Domino a3-d3 Umpolung/Michael/Michael/Michael/Lactonization Reaction
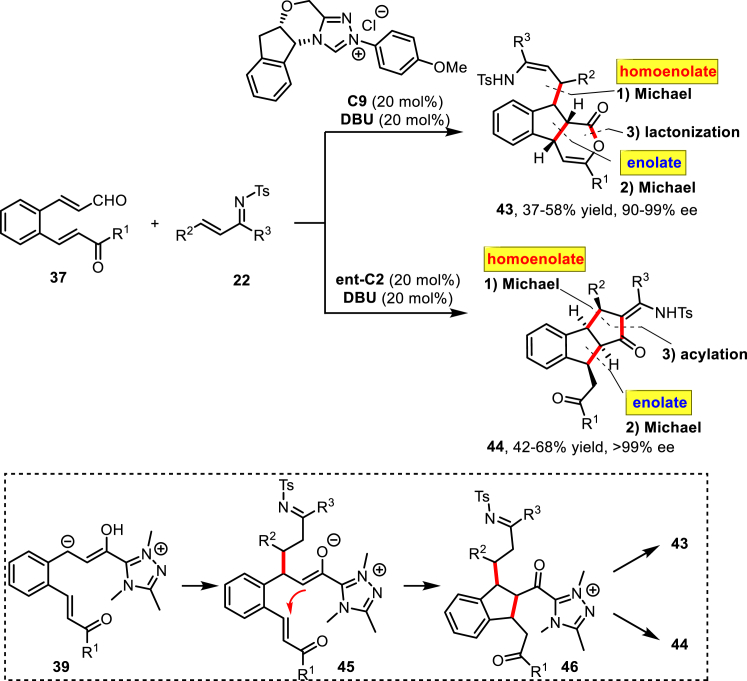
Recently, the same group developed an NHC-catalyzed asymmetric domino reaction of 2-aroylvinylcinnamaldehydes 37 and α,β-unsaturated imines 22 (Scheme 19) (Wang et al., 2015). Remarkably, the reaction pathway can be switched by changing the N-substituent on the pre-catalyst. In the presence of pre-catalyst C9, the a3–d3 umpolung/Michael/Michael/lactonization reaction leads to indeno[2,1-c]pyran-1-one derivatives 43 with ee up to 99%. Instead, by changing C9 to ent–C2 the indenocyclopentan-1-ones 44 can be formed via an a3–d3 umpolung/Michael/Michael/acylation sequence. This represents the first example of a reaction switchable only by changing the N-substituent on the NHC catalyst.
Scheme 19.
Domino a3-d3 Umpolung/Michael/Michael/Lactonization Reaction of 2-Aroylvinylcinnamaldehydes
Domino Reactions Initiated by the Homo-Aldol/Michael Reaction
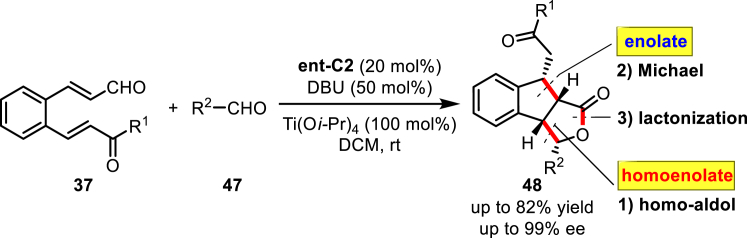
Based on this work and previous homo-addition reactions, Cheng and co-workers developed another interesting domino sequence initiated by the homo-aldol reaction (Scheme 20) (Wang et al., 2016). The domino homo-aldol/Michael/lactonization reaction between 2-aroylvinylcinnamaldehydes 37 and different aromatic aldehydes 47 in the presence of a chiral triazole carbene ent-C2 and Ti(OiPr)4 leads to the formation of the 8-(aroylmethyl)-3-arylindeno[1,2-c]furan-1-ones 48 bearing four stereogenic centers in moderate to excellent yields with excellent stereoselectivities. A drawback in the substrate scope was that an aliphatic aldehyde did not provide the desired product.
Scheme 20.
Domino Homo-Aldol/Michael/Lactonization Reaction
Domino Reactions via Unsaturated Acyl Azolium Enolate Activation Modes
The α,β-unsaturated acyl azolium enolate activation mode is currently the protocol most employed in developing NHC-catalyzed domino reactions. In these cases, most of the examples based on this concept have one thing in common: the use of tethered, triply reactive reagents as substrates. These domino processes are initiated by an intermolecular Michael addition of a tethered, triply reactive reagent 51 to the α,β-unsaturated acyl azolium 50. The resulting azolium enolate 52 can then undergo a second reaction with the electrophilic site of 52 to afford the adduct 53. The following intramolecular acylation leads to the formation of the final product 54 and regenerates the NHC catalyst (Scheme 21). In this section, the domino reactions are classified by the types of tethered, triply reactive reagents shown in Scheme 22.
Scheme 21.
Proposed Mechanism for the Domino Reactions via α,β-Unsaturated Acyl Azolium Enolate Activation Mode
Scheme 22.
Classification of NHC-Catalyzed Domino Reactions via α,β-Unsaturated Acyl Azolium and Enolate Activation Modes
Domino Reactions Initiated by the Michael/Aldol Reaction
In 2013, Lupton and co-workers (Candish et al., 2013, Candish and Lupton, 2013) developed an NHC-catalyzed Michael/aldol/lactonization reaction via the combination of the α,β-unsaturated acyl azolium and the azolium enolate intermediate (Scheme 23). The domino process leads to the formation of cyclopentane-fused β-lactones 57 in an exceptional level of asymmetric induction via a Michael/aldol/lactonization sequence between α,β-unsaturated acid fluorides 55 and donor-acceptor cyclopropanes 56 in the presence of pre-catalyst C10. Regarding the mechanism, it is postulated that in the first step the addition of NHC to acid fluoride 55 gives the α,β-unsaturated acyl azolium 58 and enolate 59 by fluorodesilylation and retro aldol reaction. The Michael addition of enolate 59 to the α,β-unsaturated acyl azolium 58 leads to the first C–C bond and the new enolate 60. The following aldol cyclization and lactonization provides the cyclopentane fused β-lactones 57.
Scheme 23.
Domino Michael/Aldol/Lactonization Reaction
A closely related domino sequence employing the ketone malonates 62 instead of donor-acceptor cyclopropanes as substrates also worked very well to afford the corresponding cyclopentane-fused β-lactones 63 with virtually complete asymmetric induction (Scheme 24) (Bera et al., 2014). In this case, Studer and co-workers found that the lithium chloride plays an important role in enhancing the enantioselectivity of this transformation. The protocol is also suitable for the synthesis of cyclohexane-fused β-lactones in the presence of pre-catalyst C2, although the yield and enantioselectivity are diminished. The salient features of this transformation include the readily available starting materials, efficient synthesis of β-lactones with a different substitution pattern, and the first conjugate addition of tertiary prochiral C-nucleophiles to α,β-unsaturated acyl azolium. The same strategy was successfully employed by Biju and co-workers (Mondal et al., 2014) for the synthesis of cyclopentenes 66 by replacing the methyl group with a phenyl group (Scheme 25). In this case, the final decarboxylation easily occurred due to the unstability of the corresponding cyclopentane-fused β-lactones.
Scheme 24.
Domino Michael/Aldol/Lactonization Reaction between Ketone-Malonates and α,β-Unsaturated Aldehydes
Scheme 25.
Domino Michael/Aldol/Lactonization/Decarboxylation Reaction between Ketone-Malonates and α,β-Unsaturated Aldehydes
An extension of this type of the NHC-catalyzed domino reaction has recently been reported by Chi and co-workers using α-amino ketones as one of the reaction partners to produce a new series of pyrrolidine-fused β-lactones (Scheme 26) (Wu et al., 2017). Using the nitro-substituted triazolium salt C11 as the pre-catalyst, the products 68 were obtained in good yields and good stereoselectivities via an aza-Michael/aldol/lactonization sequence. The reaction worked well for differently substituted α-amino ketones 67 and a broad range of aromatic and aliphatic α,β-unsaturated aldehydes 1. It is worth mentioning that the challenging intermolecular aza-Michael addition of a nitrogen nucleophile to the α,β-unsaturated acyl azolium was realized.
Scheme 26.
Domino aza-Michael/Aldol/Lactonization Reaction
Very recently, Ye and co-workers have shown the application of ketone-tethered oxindoles 69 in a Michael/aldol/lactonization/decarboxylation domino sequence (Zhang et al., 2017c). In this case, the final decarboxylation occurred in the presence of C7 as the pre-catalyst at room temperature affording the spiroproducts 70 in moderate to good yields (35%–74%) with a broad range of differently substituted aromatic 2-bromoenals 64. Furthermore, good diastereoselectivities (up to 20:1) and good enantioselectivities (up to 92%) were achieved (Scheme 27).
Scheme 27.
Domino Michael/Aldol/Lactonization/Decarboxylation Reaction
The synthesis of six-membered rings such as cyclohexadienes initiated by a Michael/aldol reaction between α,β-unsaturated acid fluorides 55 and silyl dienol ethers 71 was performed by Lupton and co-workers (Scheme 28) (Ryan et al., 2011). Using the catalyst C12, the corresponding 1,3-cyclohexadienes 72 were isolated in good yields with excellent diastereoselectivities. In this case, the addition of imidazolylidene NHC C12 to α,β-unsaturated acid fluorides 55 provides the α,β-unsaturated acyl azolium 73 and dienolate 74 by fluorodesilylation. The Michael addition of the dienolate 74 to the α,β-unsaturated acyl azolium 73 forms the C−C bond and generates the enolate 75. Subsequent aldol lactonization affords β-lactone 77 with decarboxylation, leading to the final 1,3-cyclohexadiene 72. Later they studied the challenge to develop an enantioselective version of this reaction using t-butyl-substituted triazolylidene NHCs. Unfortunately, both the enantioselectivity and yield were poor, with the formation of undesired enol esters.
Scheme 28.
Domino Michael/Aldol/Lactonization/Decarboxylation Reaction between Silyl Dienol Ethers and α,β-Unsaturated Acid Fluorides
Thus the authors decided to employ these enol esters 78 as replacements for the dienol trimethylsilyl (TMS) ether to overcome these problems (Scheme 29) (Levens et al., 2015). Screening of the solvents and catalysts revealed that this reaction proceeds best in tetrahydrofuran (THF) in the presence of 10 mol% pre-catalyst C13 with a dimethoxyphenyl N-substitution. Good stereoselectivities combined with good yields make this a useful protocol for the preparation of these 1,3-cyclohexadienes 79.
Scheme 29.
Domino Reactions of Enol Esters via α,β-Unsaturated Acyl Azolium and Enolate Activation Modes
In contrast, the corresponding β-lactones 80 can be prepared by using dienyl esters with only one substituent at the C2 position (Candish et al., 2014). In this case, the optimal catalyst was found to be the t-butyl-substituted triazolylidene NHC C10. The scope of the reaction was very broad, as demonstrated by the application of various R′ and aryl groups with consistently good yields and high enantioselectivities. It is worth mentioning is that olefin isomerization occurred in this reaction. The isomerization is believed to occur before the aldol lactonization to form the final cyclohexyl β-lactones. The decarboxylation of 80 is less favorable. This methodology allows a quick entry to 1,3-diol and β-hydroxy ester.
Interestingly, utilizing the pre-catalyst C1 or C13, and benzene as the solvent, the benzaldehydes 81 were obtained in moderate to good yields at 80°C (Candish et al., 2015). Nuclear magnetic resonance (NMR) studies indicate that the reaction involves the formation of β-lactones 80 followed by the addition of the NHC. Subsequent proton transfers and dehydration give the acyl anion 88. Finally, the regeneration of the NHC provides the benzaldehyde 81.
A nice example for the synthesis of multi-substituted benzenes via a Michael/aldol/lactonization/decarboxylation sequence was reported independently by Ye (Zhang et al., 2016) and Wang (Jia and Wang, 2016) (Scheme 30). In these cases, substituted α,β-unsaturated aldehydes 1 reacted with the dienolate precursors 89 in the presence of pre-catalyst C1 or C14. The reaction worked well for various enones 89 with different β-aryl or alkyl groups and a broad range of α,β-unsaturated aldehydes with several functional groups (e.g., aryl, alkyl, alkenyl, alkynyl groups, ether, and ester) at the β-carbon position. The proposed reaction mechanism includes initiation by the Michael addition of dienolate 92 to the α,β-unsaturated acyl azolium 91 to afford the Michael adduct. Subsequent α-deprotonation leads to the azolium enolate 93. The aldol/lactonization furnishes β-lactone-fused cyclohexene 94 and regenerates the NHC catalyst. The following decarboxylation leads to the cyclohexadiene 95, which was further oxidized to afford the final benzonitriles 90.
Scheme 30.
Domino Michael/Aldol/Lactonization/Decarboxylation/Aromatization Reaction
The concept of α,β-unsaturated acyl azolium enolate activation modes is considerably less explored for 1,6-additions to the α,β-unsaturated acyl azoliums. One of the first examples using this concept was reported by Chi and co-workers (Scheme 31) (Zhu et al., 2015), who described the synthesis of similar aromatic species using α,β,γ,δ-unsaturated aldehydes 96 and 1,3-dicarbonyl compounds 97 as substrates. This approach involves steps similar to those of the previous transformation. The first reaction is the 1,6-addition of 1,3-dicarbonyl compounds 97 to the α,β-unsaturated acyl azoliums 100, followed by aldol/lactonization, decarboxylation, and aromatization.
Scheme 31.
Domino Reactions via 1,6-Addition to the α,β-Unsaturated Acyl Azoliums
Interestingly, when R is a reactive aryl ester group, the intramolecular transesterification takes place and the 5-membered lactone 104 forms. The following isomerization leads to the adduct 105, which forms the final 3-ylidenephthalide product 99 via an aldol lactonization/decarboxylation/aromatization sequence.
Shortly after these studies, another example of a Michael/aldol/lactonization/decarboxylation reaction for the synthesis of 1,2-dihydronaphthalenes was reported by Fang and co-workers (Scheme 32) (Perveen et al., 2017). The process is catalyzed by the pre-catalyst C7 to furnish a series of 1,2-dihydronaphthalenes 108 bearing two adjacent stereocenters from simple and readily available diketones 107 and α,β-unsaturated aldehydes 1 in up to 99% yield, with >20:1 diastereoselectivity ratio (d.r.), and up to 99% ee.
Scheme 32.
Domino Michael/Aldol/Lactonization/Decarboxylation between Diketones and α,β−Unsaturated Aldehydes
Domino Reactions Initiated by the Michael/Michael Reaction
The concept of α,β-unsaturated acyl azolium enolate activation modes was used by Li and co-workers in a Michael/Michael/lactonization reaction (Scheme 33) (Zhou et al., 2013). This sequence, starting from the oxindole 110 and the alkynyl aldehydes 109, permits the efficient synthesis of tricyclic oxindole systems with four consecutive stereogenic centers in the presence of pre-catalyst C1. The proposed mechanism involves the α,β-unsaturated acyl azolium intermediate for a Michael addition with the oxindole 110 followed by intramolecular Michael addition of the resulting azolium enolate. Lactonization then furnishes the final products 111 in good yields with moderate to good diastereoselectivities. Unfortunately, no reaction was observed with 3-aliphatic-substituted alkynyl aldehydes as substrates.
Scheme 33.
Domino Michael/Michael/Lactonization Reaction of Alkynyl Aldehydes
In 2015, Studer and co-workers (Bera et al., 2015) and Ye and co-workers (Liang et al., 2015) independently reported the NHC-catalyzed Michael/Michael/lactonization reaction of α,β-unsaturated aldehydes 1 and bifunctional malonates 112. The process, performed in the presence of pre-catalyst C2 or C7 and LiCl as a co-catalyst, afforded the corresponding cyclopentane-fused δ-lactones 113 in good to high yields, excellent diastereoselectivities, and high enantioselectivities, as shown in Scheme 34. Worth mentioning is that LiCl is necessary to get a high enantioselectivity. Later, the same products were obtained by using α,β-unsaturated carboxylic esters as the α,β-unsaturated acyl azolium precursor (Fu et al., 2016).
Scheme 34.
Domino Michael/Michael/Lactonization Reaction of α,β-Unsaturated Aldehydes and Bifunctional Malonates
Likewise, Hui and co-workers reported the stereoselective aza-Michael/Michael/lactonization domino reaction of 2′-aminophenylenones 114 and 2-bromoenals 64 (Scheme 35) (Zhang et al., 2013). The process is catalyzed by the pre-catalyst C2 to furnish a series of functionalized tetrahydroquinolines 115 bearing three adjacent stereogenic centers in high yields with excellent enantioselectivities. Remarkably, only a single diastereomer was observed. Later, Xu and co-workers (Lu et al., 2017) used thioester 116 as substrates in a similar sulfa-Michael/Michael/lactonization sequence (Scheme 36). Utilizing the pre-catalyst C15, the highly functionalized thiochroman derivatives 117 were obtained in good to high yields with excellent diastereoselectivities and enantioselectivities with a broad range of thioesters. Again, only a single diastereomer was observed. The products could further be modified to the corresponding multisubstituted thiochroman derivatives and sulfones.
Scheme 35.
Domino Aza-Michael/Michael/Lactonization Reaction
Scheme 36.
Domino Sulfa-Michael/Michael/Lactonization Reaction
Domino Reactions Initiated by the Michael/Mannich Reaction
Further elegant domino sequences have also been developed via the combination of α,β-unsaturated acyl azolium and azolium enolate activation modes in the reaction with multifunctional reagents (Scheme 37) (Yang et al., 2014). This is the case of a Michael/Mannich/lactamization sequence between o-aminoarylideneaminomalonate imines 118 and 2-bromoenals 64, achieving the corresponding pyrrolo-[3,2-c]quinolines 119 bearing three consecutive stereocenters in good yields with excellent enantioselectivities.
Scheme 37.
Domino Michael/Mannich/Lactamization Reaction
Domino Reactions Initiated by the Michael/SN2 Reaction
Employing a sulfur ylide 120 as the substrate, highly substituted cyclopropyl carboxylic esters 121 could also be prepared with moderate to excellent selectivities (Scheme 38) (Biswas et al., 2012). The protocol utilized the pre-catalyst C2 to promote the domino Michael/SN2/esterification reaction between α,β-unsaturated aldehydes 1 and sulfur ylides 120. The selectivities of the reaction were strongly dependent on the electronic effects of the substrates, showing better performances with electron-poor substituents. Remarkably, it is crucial to choose suitable alcohols for this domino reaction, because the corresponding cinnamate will be formed as a major product in the presence of the more nucleophilic alcohols.
Scheme 38.
Domino Michael/SN2/Esterification Reaction
Domino Reactions via Bifunctional α,β-Unsaturated Acyl Azolium
As mentioned above, several groups have developed NHC-catalyzed domino reactions involving an α,β-unsaturated acyl azolium and the azolium enolate, whereas the corresponding reactions via bifunctional α,β-unsaturated acyl azoliums are far less developed. In 2011, Studer described a related domino reaction between β-diketones 97 and bifunctional enals 37 employing the bifunctional α,β-unsaturated acyl azoliums (Scheme 39) (Biswas et al., 2011). This Michael/Michael/lactonization domino reaction was complete after 16 hr in the presence of 20 mol% of the pre-catalyst C2 at room temperature, leading to the desired tricyclic δ-lactones 122 in good yields with excellent enantioselectivities. Their catalytic system showed a broad substrate scope. A variety of β-diketones and β-ketoesters all reacted smoothly with different substituted bifunctional enals.
Scheme 39.
Domino Michael/Michael/Lactonization Reaction via Bifunctional α,β-Unsaturated Acyl Azoliums
Similar to this strategy, Lupton and co-workers (Zhang and Lupton, 2017) realized a Michael/aldol/lactonization/decarboxylation domino reaction of bifunctional α,β-unsaturated acyl azolium precursors 123 and TMS enol ethers 124 (Scheme 40). In this case, both electron-rich and electron-poor acetophenone-derived TMS enol ethers proceeded smoothly to give the desired indenes 125 in moderate to good yields with good stereoselectivities. The yields of the reaction are influenced by the substituents, showing better results with electron-poor TMS enol ethers. Worth mentioning is that the TMS enol ether of acetone is also tolerable with high enantioselectivity.
Scheme 40.
Domino Michael/Michael/Lactonization/Decarboxylation Reaction via Bifunctional α,β-Unsaturated Acyl Azoliums
Domino Reactions via Dienolate-Enolate Activation Modes
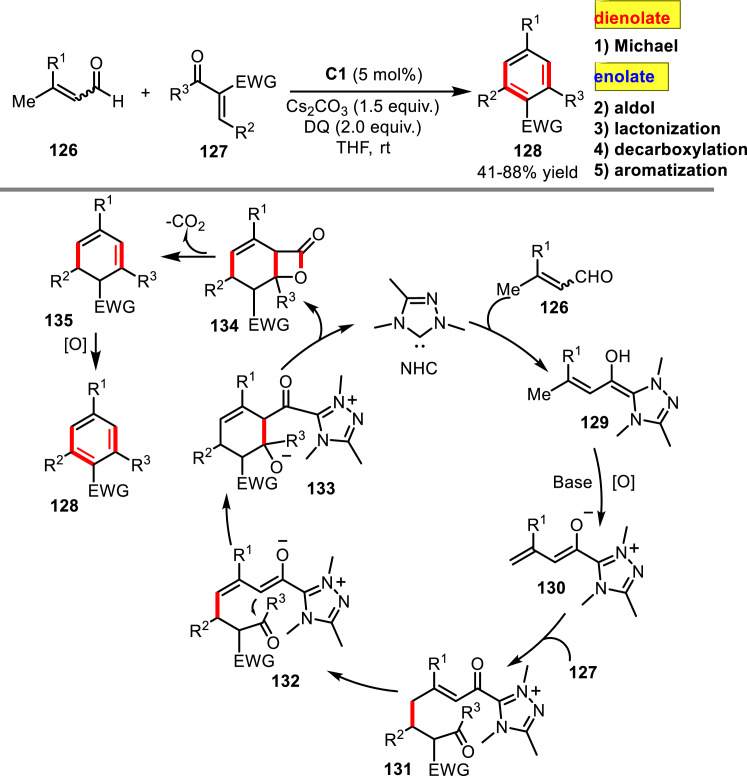
Since the early examples of NHC-catalyzed [4 + 2] cycloadditions of α,β-unsaturated acid chlorides and activated ketones via azolium dienolate intermediates reported by Ye and co-workers in 2011 (Shen et al., 2011), a number of azolium dienolate-mediated reactions have been successfully developed by several groups (Mo et al., 2012, Chen et al., 2013, Que et al., 2015, Poh et al., 2018). In 2014, Chi and co-workers (Zhu et al., 2014) first combined the azolium dienolate and azolium enolate activation modes for the synthesis of multi-substituted benzene derivatives (Scheme 41). The protocol utilized the pre-catalyst C1 to promote the Michael/aldol/lactonization/decarboxylation/aromatization reaction between α,β-unsaturated aldehydes 126 and activated enone 127. This domino reaction is proposed to start with the Michael addition of the azolium dienolate 130 to the enone 127. The resulting Michael adduct 131 then undergoes an intramolecular aldol lactonization through an azolium enolate intermediate to give the adduct 134 and regenerate the NHC catalyst. The following decarboxylation and oxidative aromatization leads to the final products 128.
Scheme 41.
Domino Michael/Aldol/Lactonization/Decarboxylation/Aromatization Reaction
Very recently, our group has successfully developed an NHC-catalyzed [4 + 2] cycloaddition of enals 126 with electron-deficient 2,4-dienes 136 via a double vinylogous Michael addition (Scheme 42) (Chen et al., 2017). Applying a similar approach, a novel domino sequence initiated by the vinylogous Michael addition of enal 126 with furanone 138 has been disclosed by Chi and co-workers (Scheme 43) (Huang et al., 2017). Their catalytic system showed a broad substrate scope. A variety of enals 126 reacted well with different furanones 138 to give the desired products 139 in good yields. To demonstrate the synthetic utility of this novel organocatalytic domino strategy, this methodology was then applied for the total synthesis of several natural products (defucogilvocarcins M, E, and V) with coumarin as the key structural motif. Regarding the mechanism, it is postulated that the addition of the NHC to the enal 126, followed by further oxidation and deprotonation, leads to the α,β-unsaturated azolium dienolate intermediate 141, which undergoes a double vinylogous Michael addition to the electron-deficient 2,4-dienes 138 to give the adducts 142. The subsequent cyclization provides the cycloaddition products 144 and releases the NHC catalyst. The following enolization of 144 gives 145. The adduct 146 is then formed via an intramolecular transesterification. Finally, the dehydration of 146 yields the final product 139.
Scheme 42.
N-Heterocyclic-Carbene-Catalyzed [4 + 2] Cycloaddition of Enals via a Double Vinylogous Michael Addition
Scheme 43.
Proposed Catalytic Cycle for the Domino Michael/Acylation/Transesterification/Dehydration Reaction
Conclusions
In this review, we have shown that the combination of NHC catalysis with the concept of domino reactions is a powerful tool for the construction of highly functionalized molecular structures. The application of two or more activation modes allows the design of innovative NHC-catalyzed domino reactions to afford complex molecules bearing multiple stereogenic centers with an excellent level of stereocontrol under environment-friendly conditions. These domino reactions have several salient features, such as simple operational procedures and ready availability of NHC catalysts and substrates. Current studies involve two activation modes in which the initial step is intermolecular and the following steps are intramolecular. The development of novel and exciting applications of three and more activation modes and the extension to more intermolecular steps will be the target of further studies. We hope that this review will inspire the synthesis chemistry community to further develop new NHC-catalyzed domino reactions and to employ this concept in future valuable total syntheses.
Acknowledgments
Support from the European Research Council (ERC Advanced Grant 320493 “DOMINOCAT”) is gratefully acknowledged.
Author Contributions
Conceptualization, X. –Y. C.; writing: original draft, X. –Y. C. and S. L. contributed equally; writing: reviewing and editing, X. –Y. C., S. L., F. V., M. K., and D. E.; supervision, D. E.
References
- Ahrendt K.A., Borths C.J., MacMillan D.W.C. New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels−Alder reaction. J. Am. Chem. Soc. 2000;122:4243–4244. [Google Scholar]
- Albrecht Ł., Jiang H., Jørgensen K.A. A simple recipe for sophisticated cocktails: organocatalytic one-pot reactions-concept, nomenclature, and future perspectives. Angew. Chem. Int. Ed. 2011;50:8492–8509. doi: 10.1002/anie.201102522. [DOI] [PubMed] [Google Scholar]
- Bera S., Daniliuc C.G., Studer A. Enantioselective synthesis of substituted δ-lactones by cooperative oxidative N-heterocyclic carbene and Lewis acid catalysis. Org. Lett. 2015;17:4940–4943. doi: 10.1021/acs.orglett.5b01932. [DOI] [PubMed] [Google Scholar]
- Bera S., Samanta R.C., Daniliuc C.G., Studer A. Asymmetric synthesis of highly substituted β-lactones through oxidative carbene catalysis with LiCl as cooperative Lewis acid. Angew. Chem. Int. Ed. 2014;53:9622–9626. doi: 10.1002/anie.201405200. [DOI] [PubMed] [Google Scholar]
- Bhunia A., Patra A., Puranik V.G., Biju A.T. NHC-catalyzed reaction of enals with hydroxy chalcones: diastereoselective synthesis of functionalized coumarins. Org. Lett. 2013;15:1756–1759. doi: 10.1021/ol400562z. [DOI] [PubMed] [Google Scholar]
- Biswas A., De Sarkar S., Fröhlich R., Studer A. Highly stereoselective synthesis of 1,2,3-trisubstituted indanes via oxidative N-heterocyclic carbene-catalyzed cascades. Org. Lett. 2011;13:4966–4969. doi: 10.1021/ol202108a. [DOI] [PubMed] [Google Scholar]
- Biswas A., De Sarkar S., Tebben L., Studer A. Enantioselective cyclopropanation of enals by oxidative N-heterocyclic carbene catalysis. Chem. Commun. (Camb.) 2012;48:5190–5192. doi: 10.1039/c2cc31501g. [DOI] [PubMed] [Google Scholar]
- Bode J.W. Carbene catalysis: an internal affair. Nat. Chem. 2013;5:813–815. doi: 10.1038/nchem.1766. [DOI] [PubMed] [Google Scholar]
- Breslow R. On the mechanism of thiamine action. IV. Evidence from studies on model systems. J. Am. Chem. Soc. 1958;80:3719–3726. [Google Scholar]
- Bugaut X., Glorius F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012;41:3511–3522. doi: 10.1039/c2cs15333e. [DOI] [PubMed] [Google Scholar]
- Bui T., Barbas C.F. A proline-catalyzed asymmetric Robinson annulation reaction. Tetrahedron Lett. 2000;41:6951–6954. [Google Scholar]
- Burstein C., Glorius F. Organocatalyzed conjugate umpolung of α,β-unsaturated aldehydes for the synthesis of γ-butyrolactones. Angew. Chem. Int. Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572. [DOI] [PubMed] [Google Scholar]
- Candish L., Forsyth C.M., Lupton D.W. N-tert-butyl triazolylidenes: catalysts for the enantioselective (3+2) annulation of α,β-unsaturated acyl azoliums. Angew. Chem. Int. Ed. 2013;52:9149–9152. doi: 10.1002/anie.201304081. [DOI] [PubMed] [Google Scholar]
- Candish L., Levens A., Lupton D.W. Enantioselective all-carbon (4+2) annulation by N-heterocyclic carbene catalysis. J. Am. Chem. Soc. 2014;136:14397–14400. doi: 10.1021/ja508542n. [DOI] [PubMed] [Google Scholar]
- Candish L., Levens A., Lupton D.W. N-heterocyclic carbene catalysed redox isomerisation of esters to functionalised benzaldehydes. Chem. Sci. 2015;6:2366–2370. doi: 10.1039/c4sc03726j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candish L., Lupton D.W. N-heterocyclic carbene-catalyzed Ireland–coates Claisen rearrangement: synthesis of functionalized β-lactones. J. Am. Chem. Soc. 2013;135:58–61. doi: 10.1021/ja310449k. [DOI] [PubMed] [Google Scholar]
- Cardinal-David B., Raup D.E., Scheidt K.A. Cooperative N-heterocyclic carbene/Lewis acid catalysis for highly stereoselective annulation reactions with homoenolates. J. Am. Chem. Soc. 2010;132:5345–5347. doi: 10.1021/ja910666n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells J., Llitjos H., Moreno-Mañas M. Nitrobenzene aldehyde oxidations catalyzed by the conjugate bases of thiazolium ions. Tetrahedron Lett. 1977;18:205–206. [Google Scholar]
- Chanda T., Zhao J.C.G. Recent progress in organocatalytic asymmetric domino transformations. Adv. Synth. Catal. 2018 [Google Scholar]
- Chauhan P., Mahajan S., Enders D. Achieving molecular complexity via stereoselective multiple domino reactions promoted by a secondary amine organocatalyst. Acc. Chem. Res. 2017;50:2809–2821. doi: 10.1021/acs.accounts.7b00406. [DOI] [PubMed] [Google Scholar]
- Chen X.Y., Gao Z.H., Song C.Y., Zhang C.L., Wang Z.X., Ye S. N-heterocyclic carbene catalyzed cyclocondensation of α,β-unsaturated carboxylic acids: enantioselective synthesis of pyrrolidinone and dihydropyridinone derivatives. Angew. Chem. Int. Ed. 2014;53:11611–11615. doi: 10.1002/anie.201407469. [DOI] [PubMed] [Google Scholar]
- Chen X.-Y., Liu Q., Chauhan P., Li S., Peuronen A., Rissanen K., Jafari E., Enders D. N-heterocyclic carbene catalyzed [4+2] annulation of enals via a double vinylogous Michael addition: asymmetric synthesis of 3,5-diaryl cyclohexenones. Angew. Chem. Int. Ed. 2017;56:6241–6245. doi: 10.1002/anie.201702881. [DOI] [PubMed] [Google Scholar]
- Chen X.Y., Liu Q., Chauhan P., Enders D. N-heterocyclic carbene catalysis via azolium dienolates: an efficient strategy for enantioselective remote functionalizations. Angew. Chem. Int. Ed. 2018 doi: 10.1002/anie.201709684. [DOI] [PubMed] [Google Scholar]
- Chen X.Y., Xia F., Cheng J.T., Ye S. Highly enantioselective γ-amination by N-heterocyclic carbene catalyzed [4+2] annulation of oxidized enals and azodicarboxylates. Angew. Chem. Int. Ed. 2013;52:10644–10647. doi: 10.1002/anie.201305571. [DOI] [PubMed] [Google Scholar]
- Chen X.Y., Ye S. Enantioselective cycloaddition reactions of ketenes catalyzed by N-heterocyclic carbenes. Synlett. 2013;24:1614–1622. [Google Scholar]
- Cheng J., Huang Z., Chi Y.R. NHC organocatalytic formal LUMO activation of α,β-unsaturated esters for reaction with enamides. Angew. Chem. Int. Ed. 2013;52:8592–8596. doi: 10.1002/anie.201303247. [DOI] [PubMed] [Google Scholar]
- Chiang P.C., Kaeobamrung J., Bode J.W. Enantioselective, cyclopentene-forming annulations via NHC-catalyzed benzoin−oxy-Cope reactions. J. Am. Chem. Soc. 2007;129:3520–3521. doi: 10.1021/ja0705543. [DOI] [PubMed] [Google Scholar]
- Cohen D.T., Cardinal-David B., Scheidt K.A. Lewis acid activated synthesis of highly substituted cyclopentanes by the N-heterocyclic carbene catalyzed addition of homoenolate equivalents to unsaturated ketoesters. Angew. Chem. Int. Ed. 2011;50:1678–1682. doi: 10.1002/anie.201005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarkar S., Biswas A., Samanta R.C., Studer A. Catalysis with N-heterocyclic carbenes under oxidative conditions. Chemistry. 2013;19:4664–4678. doi: 10.1002/chem.201203707. [DOI] [PubMed] [Google Scholar]
- DeSarkar S., Studer A. NHC-catalyzed Michael addition to α,β-unsaturated aldehydes by redox activation. Angew. Chem. Int. Ed. 2010;49:9266–9269. doi: 10.1002/anie.201004593. [DOI] [PubMed] [Google Scholar]
- Douglas J., Churchill G., Smith A.D. NHCs in asymmetric organocatalysis: recent advances in azolium enolate generation and reactivity. Synthesis. 2012;44:2295–2309. [Google Scholar]
- Duguet N., Campbell C.D., Slawin A.M.Z., Smith A.D. N-heterocyclic carbene catalysed β-lactam synthesis. Org. Biomol. Chem. 2008;6:1108–1113. doi: 10.1039/b800857b. [DOI] [PubMed] [Google Scholar]
- Enders D., Balensiefer T. Nucleophilic carbenes in asymmetric organocatalysis. Acc. Chem. Res. 2004;37:534–541. doi: 10.1021/ar030050j. [DOI] [PubMed] [Google Scholar]
- Enders D., Breuer K., Runsink J., Teles J.H. The first asymmetric intramolecular Stetter reaction. Preliminary communication. Helv. Chim. Acta. 1996;79:1899–1902. [Google Scholar]
- Enders D., Grondal C., Hüttl M.R.M. Asymmetric organocatalytic domino reactions. Angew. Chem. Int. Ed. 2007;46:1570–1581. doi: 10.1002/anie.200603129. [DOI] [PubMed] [Google Scholar]
- Enders D., Niemeier O., Henseler A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007;107:5606–5655. doi: 10.1021/cr068372z. [DOI] [PubMed] [Google Scholar]
- Fan X.W., Cheng Y. N-heterocyclic carbene-catalyzed cascade reaction of 2-aroylvinylcinnamaldehydes with 2-aroylvinylchalcones: rapid assembly of six contiguous stereogenic centers with high diastereoselectivity. Org. Biomol. Chem. 2014;12:123–131. doi: 10.1039/c3ob41656a. [DOI] [PubMed] [Google Scholar]
- Fang X., Jiang K., Xing C., Hao L., Chi Y.R. A highly regio- and stereoselective cascade annulation of enals and benzodi(enone)s catalyzed by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2011;50:1910–1913. doi: 10.1002/anie.201007144. [DOI] [PubMed] [Google Scholar]
- Flanigan D.M., Romanov-Michailidis F., White N.A., Rovis T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015;115:9307–9387. doi: 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Jiang K., Zhu T., Torres J., Chi Y.R. Access to oxoquinoline heterocycles by N-heterocyclic carbene catalyzed ester activation for selective reaction with an enone. Angew. Chem. Int. Ed. 2014;53:6506–6510. doi: 10.1002/anie.201402620. [DOI] [PubMed] [Google Scholar]
- Fu Z., Wu X., Chi Y.R. Rapid access to bicyclic δ-lactones via carbene-catalyzed activation and cascade reaction of unsaturated carboxylic esters. Org. Chem. Front. 2016;3:145–149. [Google Scholar]
- Grondal C., Jeanty M., Enders D. Organocatalytic cascade reactions as a new tool in total synthesis. Nat. Chem. 2010;2:167–178. doi: 10.1038/nchem.539. [DOI] [PubMed] [Google Scholar]
- Grossmann A., Enders D. N-heterocyclic carbene catalyzed domino reactions. Angew. Chem. Int. Ed. 2012;51:314–325. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]
- Hayashi Y. Pot economy and one-pot synthesis. Chem. Sci. 2016;7:866–880. doi: 10.1039/c5sc02913a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Bode J.W. Enantioselective, NHC-catalyzed bicyclo-β-lactam formation via direct annulations of enals and unsaturated N-sulfonyl ketimines. J. Am. Chem. Soc. 2008;130:418–419. doi: 10.1021/ja0778592. [DOI] [PubMed] [Google Scholar]
- He M., Uc G.J., Bode J.W. Chiral N-heterocyclic carbene catalyzed, enantioselective oxodiene Diels−Alder reactions with low catalyst loadings. J. Am. Chem. Soc. 2006;128:15088–15089. doi: 10.1021/ja066380r. [DOI] [PubMed] [Google Scholar]
- Huang X., Zhu T., Huang Z., Zhang Y., Jin Z., Zanoni G., Chi Y.R. Carbene-catalyzed formal [5+5] reaction for coumarin construction and total synthesis of defucogilvocarcins. Org. Lett. 2017;19:6188–6191. doi: 10.1021/acs.orglett.7b03102. [DOI] [PubMed] [Google Scholar]
- Jia Q., Wang J. N-heterocyclic carbene-catalyzed convenient benzonitrile assembly. Org. Lett. 2016;18:2212–2215. doi: 10.1021/acs.orglett.6b00844. [DOI] [PubMed] [Google Scholar]
- Jiang K., Tiwari B., Chi Y.R. Access to spirocyclic oxindoles via N-heterocyclic carbene-catalyzed reactions of enals and oxindole-derived α,β-unsaturated imines. Org. Lett. 2012;14:2382–2385. doi: 10.1021/ol3008028. [DOI] [PubMed] [Google Scholar]
- Kaeobamrung J., Mahatthananchai J., Zheng P., Bode J.W. An enantioselective Claisen rearrangement catalyzed by N-heterocyclic carbenes. J. Am. Chem. Soc. 2010;132:8810–8812. doi: 10.1021/ja103631u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapworth A. XCVI.-Reactions involving the addition of hydrogen cyanide to carbon compounds. J. Chem. Soc. Trans. 1903;83:995–1005. [Google Scholar]
- Levens A., Lupton D.W. All-carbon (4+2) annulations catalysed by N-heterocyclic carbenes. Synlett. 2017;13:415–424. [Google Scholar]
- Levens A., Zhang C., Candish L., Forsyth C.M., Lupton D.W. Enantioselective N-heterocyclic carbene catalyzed diene regenerative (4 + 2) annulation. Org. Lett. 2015;17:5332–5335. doi: 10.1021/acs.orglett.5b02693. [DOI] [PubMed] [Google Scholar]
- Liang Z.Q., Wang D.L., Zhang H.M., Ye S. Enantioselective synthesis of bicyclic δ-lactones via N-heterocyclic carbene-catalyzed cascade reaction. Org. Lett. 2015;17:5140–5143. doi: 10.1021/acs.orglett.5b02695. [DOI] [PubMed] [Google Scholar]
- List B., Lerner R.A., Barbas C.F. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000;122:2395–2396. [Google Scholar]
- Lu H., Zhang J.L., Liu J.Y., Li H.Y., Xu P.F. N-heterocyclic carbene-catalyzed atom-economical and enantioselective construction of the C–S bond: asymmetric synthesis of functionalized thiochromans. ACS Catal. 2017;7:7797–7802. [Google Scholar]
- Lu S., Poh S.B., Siau W.Y., Zhao Y. Kinetic resolution of tertiary alcohols: highly enantioselective access to 3-hydroxy-3-substituted oxindoles. Angew. Chem. Int. Ed. 2013;52:1731–1734. doi: 10.1002/anie.201209043. [DOI] [PubMed] [Google Scholar]
- Lu S., Poh S.B., Zhao Y. Kinetic Resolution of 1,1′-biaryl-2,2′-diols and amino alcohols through NHC-catalyzed atroposelective acylation. Angew. Chem. Int. Ed. 2014;53:11041–11045. doi: 10.1002/anie.201406192. [DOI] [PubMed] [Google Scholar]
- Mahatthananchai J., Bode J.W. On the mechanism of N-heterocyclic carbene-catalyzed reactions involving acyl azoliums. Acc. Chem. Res. 2014;47:696–707. doi: 10.1021/ar400239v. [DOI] [PubMed] [Google Scholar]
- Menon R.S., Biju A.T., Nair V. Recent advances in employing homoenolates generated by N-heterocyclic carbene (NHC) catalysis in carbon-carbon bond-forming reactions. Chem. Soc. Rev. 2015;44:5040–5052. doi: 10.1039/c5cs00162e. [DOI] [PubMed] [Google Scholar]
- Mo J., Chen X., Chi Y.R. Oxidative γ-addition of enals to trifluoromethyl ketones: enantioselectivity control via Lewis acid/N-heterocyclic carbene cooperative catalysis. J. Am. Chem. Soc. 2012;134:8810–8813. doi: 10.1021/ja303618z. [DOI] [PubMed] [Google Scholar]
- Mondal S., Yetra S.R., Patra A., Kunte S.S., Gonnade R.G., Biju A.T. N-heterocyclic carbene-catalyzed enantioselective synthesis of functionalized cyclopentenes via α,β-unsaturated acyl azoliums. Chem. Commun. (Camb.) 2014;50:14539–14542. doi: 10.1039/c4cc07433e. [DOI] [PubMed] [Google Scholar]
- Nair V., Menon R.S., Biju A.T., Sinu C.R., Paul R.R., Jose A., Sreekumar V. Employing homoenolates generated by NHC catalysis in carbon-carbon bond-forming reactions: state of the art. Chem. Soc. Rev. 2011;40:5336–5346. doi: 10.1039/c1cs15139h. [DOI] [PubMed] [Google Scholar]
- Nair V., Vellalath S., Poonoth M., Suresh E. N-heterocyclic carbene-catalyzed reaction of chalcones and enals via homoenolate: an efficient synthesis of 1,3,4-trisubstituted cyclopentenes. J. Am. Chem. Soc. 2006;128:8736–8737. doi: 10.1021/ja0625677. [DOI] [PubMed] [Google Scholar]
- Pellissier H. Recent developments in asymmetric organocatalytic domino reactions. Adv. Synth. Catal. 2012;354:237–294. [Google Scholar]
- Perveen S., Zhao Z., Zhang G., Liu J., Anwar M., Fang X. Enantioselective synthesis of 1,2-dihydronaphthalenes via oxidative N-heterocyclic carbene catalysis. Org. Lett. 2017;19:2470–2473. doi: 10.1021/acs.orglett.7b00555. [DOI] [PubMed] [Google Scholar]
- Poh S.B., Ong J.Y., Lu S., Zhao Y. Highly regio- and stereodivergent access to 1,2-amino alcohols or 1,4-fluoro alcohols by NHC-catalyzed ring opening of epoxy enals. Angew. Chem. Int. Ed. 2018;57:1645–1649. doi: 10.1002/anie.201709823. [DOI] [PubMed] [Google Scholar]
- Que Y., Xie Y., Li T., Yu C., Tu S., Yao C. An N-heterocyclic carbene-catalyzed oxidative γ-aminoalkylation of saturated carboxylic acids through in situ activation strategy: access to δ-lactam. Org. Lett. 2015;17:6234–6237. doi: 10.1021/acs.orglett.5b03223. [DOI] [PubMed] [Google Scholar]
- Reyes E., Uria U., Carrillo L., Vicario J.L. Enantioselective cascade reactions under N-heterocyclic carbene catalysis. Synthesis. 2017;49:451–471. [Google Scholar]
- Reynolds N.T., Read de Alaniz J., Rovis T. Conversion of α-haloaldehydes into acylating agents by an internal redox reaction catalyzed by nucleophilic carbenes. J. Am. Chem. Soc. 2004;126:9518–9519. doi: 10.1021/ja046991o. [DOI] [PubMed] [Google Scholar]
- Ryan S.J., Candish L., Lupton D.W. N-heterocyclic carbene-catalyzed generation of α,β-unsaturated acyl imidazoliums: synthesis of dihydropyranones by their reaction with enolates. J. Am. Chem. Soc. 2009;131:14176–14177. doi: 10.1021/ja905501z. [DOI] [PubMed] [Google Scholar]
- Ryan S.J., Candish L., Lupton D.W. N-heterocyclic carbene-catalyzed (4 + 2) cycloaddition/decarboxylation of silyl dienol ethers with α,β-unsaturated acid fluorides. J. Am. Chem. Soc. 2011;133:4694–4697. doi: 10.1021/ja111067j. [DOI] [PubMed] [Google Scholar]
- Ryan S.J., Candish L., Lupton D.W. Acyl anion free N-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 2013;42:4906–4917. doi: 10.1039/c3cs35522e. [DOI] [PubMed] [Google Scholar]
- Sheehan J.C., Hunneman D.H. Homogeneous asymmetric catalysis. J. Am. Chem. Soc. 1966;88:3666–3667. [Google Scholar]
- Shen L.T., Shao P.L., Ye S. N-heterocyclic carbene-catalyzed cyclization of unsaturated acyl chlorides and ketones. Adv. Synth. Catal. 2011;353:1943–1948. [Google Scholar]
- Shu T., Ni Q., Song X., Zhao K., Wu T., Puttreddy R., Rissanen K., Enders D. Asymmetric synthesis of cyclopentanes bearing four contiguous stereocenters via an NHC-catalyzed Michael/Michael/esterification domino reaction. Chem. Commun. (Camb.) 2016;52:2609–2611. doi: 10.1039/c5cc09581f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn S.S., Rosen E.L., Bode J.W. N-heterocyclic carbene-catalyzed generation of homoenolates: γ-Butyrolactones by direct annulations of enals and aldehydes. J. Am. Chem. Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b. [DOI] [PubMed] [Google Scholar]
- Stetter H. Catalyzed addition of aldehydes to activated double bonds—a new synthetic approach. Angew. Chem. Int. Ed. 1976;15:639–647. [Google Scholar]
- Sun F.G., Sun L.H., Ye S. N-heterocyclic carbene-catalyzed enantioselective annulation of bromoenal and 1,3-dicarbonyl compounds. Adv. Synth. Catal. 2011;353:3134–3138. [Google Scholar]
- Tietze L.F., Beifuss U. Sequential transformations in organic chemistry: a synthetic strategy with a future. Angew. Chem. Int. Ed. 1993;32:131–163. [Google Scholar]
- Ukai T., Tanaka R., Dokawa T. A new catalyst for acyloin condensation. J. Pharm. Soc. Jpn. 1943;63:296–300. [Google Scholar]
- Volla C.M.R., Atodiresei I., Rueping M. Catalytic C–C bond-forming multi-component cascade or domino reactions: pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 2014;114:2390–2431. doi: 10.1021/cr400215u. [DOI] [PubMed] [Google Scholar]
- Vora H.U., Wheeler P., Rovis T. Exploiting acyl and enol azolium intermediates via N-heterocyclic carbene-catalyzed reactions of α-reducible aldehydes. Adv. Synth. Catal. 2012;354:1617–1639. doi: 10.1002/adsc.201200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Wang Z.Y., Niu S.S., Rao Y., Cheng Y. The reaction of 2-aroylvinylcinnamaldehydes with aromatic aldehydes by dual Catalysis with a chiral N-heterocyclic carbene and a Lewis acid: enantioselective construction of tetrahydroindeno[1,2-c]furan-1-ones. J. Org. Chem. 2016;81:8276–8286. doi: 10.1021/acs.joc.6b01362. [DOI] [PubMed] [Google Scholar]
- Wang L., Li S., Blümel M., Puttreddy R., Peuronen A., Rissanen K., Enders D. Switchable access to different spirocyclopentane oxindoles by N-heterocyclic carbene catalyzed reactions of isatin-derived enals and N-sulfonyl ketimines. Angew. Chem. Int. Ed. 2017;56:8516–8521. doi: 10.1002/anie.201704210. [DOI] [PubMed] [Google Scholar]
- Wang M.H., Scheidt K.A. Cooperative catalysis and activation with N-heterocyclic carbenes. Angew. Chem. Int. Ed. 2016;55:14912–14922. doi: 10.1002/anie.201605319. [DOI] [PubMed] [Google Scholar]
- Wang Z.T., Zhao Y., Wang Z.Y., Cheng Y. N-heterocyclic carbene-catalyzed diastereoselective and enantioselective reaction of 2-aroylvinylcinnamaldehydes with α,β-unsaturated imines: complete control and switch of diastereoselectivity by N-substituents of catalysts. J. Org. Chem. 2015;80:1727–1734. doi: 10.1021/jo502668c. [DOI] [PubMed] [Google Scholar]
- Wu X., Hao L., Zhang Y., Rakesh M., Reddi R.N., Yang S., Song B.-A., Chi Y.R. Construction of fused pyrrolidines and β-lactones by carbene-catalyzed C−N, C−C, and C−O bond formations. Angew. Chem. Int. Ed. 2017;56:4201–4205. doi: 10.1002/anie.201700045. [DOI] [PubMed] [Google Scholar]
- Yang Y.J., Ji Y., Qi L., Wang G., Hui X.P. Asymmetric synthesis of cyclopenta[3,4]pyrroloindolones via N-heterocyclic carbene-catalyzed Michael/aldol/lactamization cascade reaction. Org. Lett. 2017;19:3271–3274. doi: 10.1021/acs.orglett.7b01411. [DOI] [PubMed] [Google Scholar]
- Yang Y.J., Zhang H.R., Zhu S.Y., Zhu P., Hui X.P. Highly stereoselective synthesis of functionalized pyrrolo[3,2-c]quinolines via N-heterocyclic carbene catalyzed cascade sequence. Org. Lett. 2014;16:5048–5051. doi: 10.1021/ol5023917. [DOI] [PubMed] [Google Scholar]
- Zhang C., Lupton D.W. Enantioselective N-heterocyclic carbene catalyzed synthesis of functionalized indenes. Org. Lett. 2017;19:4456–4459. doi: 10.1021/acs.orglett.7b01981. [DOI] [PubMed] [Google Scholar]
- Zhang C., Hooper J.F., Lupton D.W. N-heterocyclic carbene catalysis via the α,β-unsaturated acyl azolium. ACS Catal. 2017;7:2583–2596. [Google Scholar]
- Zhang C.L., Gao Z.H., Liang Z.Q., Ye S. N-heterocyclic carbene-catalyzed synthesis of multi-substituted benzenes from enals and α-cyano-β-methylenones. Adv. Synth. Catal. 2016;358:2862–2866. [Google Scholar]
- Zhang H.R., Dong Z.W., Yang Y.J., Wang P.L., Hui X.P. N-heterocyclic carbene-catalyzed stereoselective cascade reaction: synthesis of functionalized tetrahydroquinolines. Org. Lett. 2013;15:4750–4753. doi: 10.1021/ol4024985. [DOI] [PubMed] [Google Scholar]
- Zhang J.Q., Li N.K., Yin S.J., Sun B.B., Fan W.T., Wang X.W. Chiral N-heterocyclic carbene-catalyzed asymmetric Michael–intramolecular aldol-lactonization cascade for enantioselective construction of β-propiolactone-fused spiro[cyclopentane-oxindoles] Adv. Synth. Catal. 2017;359:1541–1551. [Google Scholar]
- Zhang Y.R., He L., Wu X., Shao P.L., Ye S. Chiral N-heterocyclic carbene catalyzed Staudinger reaction of ketenes with imines: highly enantioselective synthesis of N-boc β-lactams. Org. Lett. 2008;10:277–280. doi: 10.1021/ol702759b. [DOI] [PubMed] [Google Scholar]
- Zhang Z.F., Chen K.Q., Zhang C.L., Ye S. N-heterocyclic carbene-catalyzed synthesis of spirocyclopentene-oxindoles from bromoenals. Chem. Commun. (Camb.) 2017;53:4327–4330. doi: 10.1039/c6cc10304a. [DOI] [PubMed] [Google Scholar]
- Zhou B., Luo Z., Li Y. Assembly of spirooxindole derivatives containing four consecutive stereocenters by using cascade reactions catalyzed by an N-heterocyclic carbene. Chemistry. 2013;19:4428–4431. doi: 10.1002/chem.201203436. [DOI] [PubMed] [Google Scholar]
- Zhu T., Mou C., Li B., Smetankova M., Song B.-A., Chi Y.R. N-heterocyclic carbene-catalyzed δ-carbon LUMO activation of unsaturated aldehydes. J. Am. Chem. Soc. 2015;137:5658–5661. doi: 10.1021/jacs.5b02219. [DOI] [PubMed] [Google Scholar]
- Zhu T., Zheng P., Mou C., Yang S., Song B.-A., Chi Y.R. Benzene construction via organocatalytic formal [3+3] cycloaddition reaction. Nat. Commun. 2014;5:5027–5032. doi: 10.1038/ncomms6027. [DOI] [PubMed] [Google Scholar]