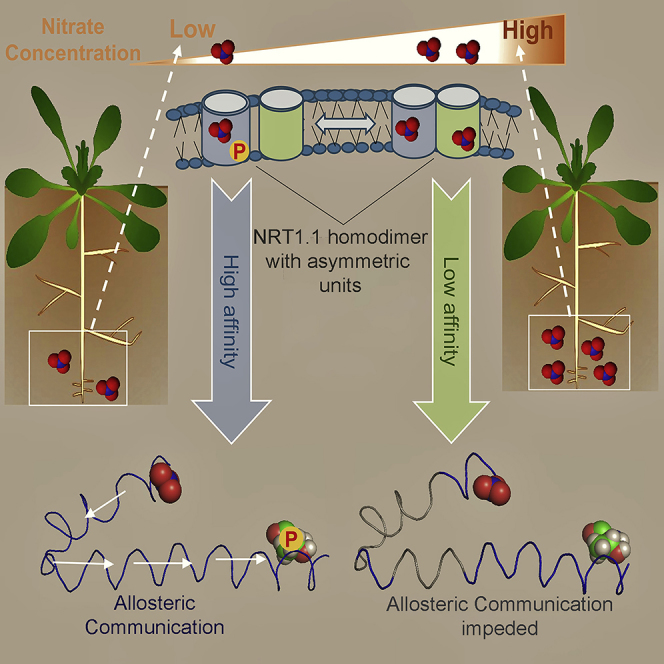
Summary
Plant adaptation in variable soil nitrate concentrations involves sophisticated signaling and transport systems that modulate a variety of physiological and developmental responses. However, we know very little about their molecular mechanisms. It has recently been reported that many of these responses are regulated by a transceptor NRT1.1, a transporter cum receptor of nitrate signaling. NRT1.1 displays dual-affinity modes of nitrate binding and establishes phosphorylated/non-phosphorylated states at the amino acid residue threonine 101 in response to fluctuating nitrate concentrations. Here we report that intrinsic structural asymmetries between the protomers of the homodimer NRT1.1 provide a functional basis for having dual-affinity modes of nitrate binding and play a pivotal role for the phosphorylation switch. Nitrate-triggered local conformational changes facilitate allosteric communications between the nitrate binding and the phosphorylation site in one protomer, but such communications are impeded in the other. Structural analysis therefore suggests the functional relevance of NRT1.1 interprotomer asymmetries.
Subject Areas: Computational Molecular Modeling, Data Analysis in Structural Biology, Plant Biology
Graphical Abstract
Highlights
-
•
NRT1.1 interprotomer asymmetry provides a functional basis for dual affinity
-
•
Nitrate-triggered conformational changes facilitate intraprotomer allostery
-
•
NRT1.1 interprotomer asymmetry is correlated with the phosphorylation switch
-
•
Allostery plays a critical role in regulating the phosphorylation
Computational Molecular Modeling; Data Analysis in Structural Biology; Plant Biology
Introduction
Nitrate is an essential mineral nutrient in plants and at the same time acts as a signaling molecule (Crawford, 1995, Wang et al., 2004). Its soil concentrations, however, fluctuate in several orders of magnitude from micromolar to millimolar range. To cope with these fluctuations, plants have developed sophisticated sensing and transport systems (Krouk et al., 2010). Rigorous molecular studies on ammonium and nitrate uptake have demonstrated the existence and functioning of two distinct uptake systems in plants referred to as high-affinity transport system (HATS) and low-affinity transport system (LATS) (Crawford and Glass, 1998, von Wirén et al., 2000). In low nutrient concentration, HATS is ON to scavenge ions and allows plants to maintain a normal uptake rate (Liu and Tsay, 2003, Nacry et al., 2013). In high nutrient concentration, LATS is ON, leading to increased uptake along increasing nitrate gradient (Wang et al., 1993, Nacry et al., 2013). HATS usually follows Michaelis-Menten kinetics and displays saturation characteristics relative to LATS that increase linearly with concentrations. These differences primarily indicate the involvement of distinct sets of genes. Indeed, there are two distinct families of nitrate transporter genes, NRT1 and NRT2, associated with LATS and HATS, respectively (Williams and Miller, 2001). With an interesting exception, recent studies have revealed that the nitrate transporter NRT1.1 (also known as NPF6.3 or CHL1), which is distinct from most of the members of both HATS and LATS gene family, contributes to both the systems and functions as transceptor (Ho et al., 2009, Giehl and von Wirén, 2015), a transporter cum receptor of changes in soil nitrate concentration. The dual-affinity modes of nitrate binding (Liu et al., 1999) and a phosphorylation switch allows NRT1.1 protein to control its capacity of switching between high- and low-affinity modes of uptake (Tsay, 2014). Detailed understanding of this molecular mechanism is essential for improving plant nutrient use efficiency (NUE) (Good et al., 2004, Gutiérrez, 2012) in a wide range of variation in soil nutrient availabilities, which, however, remains largely unknown.
Independent of its transporter function, NRT1.1 also acts as a nitrate sensor, leading to rapid transcriptional regulations of several transporters and assimilatory genes called primary nitrate response (PNR) (Krouk et al., 2006, Ho et al., 2009). In the face of a wide range of variation in extracellular nitrate availabilities, plant adaptation is accompanied by quantifiable changes in PNR mediated by NRT1.1. In vitro and in vivo studies showed a biphasic primary response; at low nitrate concentrations, protein kinase CIPK23 phosphorylates Thr101 of NRT1.1, which allows the maintenance of a low-level primary response relative to the PNR level at high nitrate concentration (Ho et al., 2009). PNR studies in transgenic plants suggest that dual-affinity binding of nitrate and phosphorylation switch jointly allow NRT1.1 to sense a wide range of extracellular nitrate availabilities and are mainly responsible for biphasic adjustment of PNR (Medici and Krouk, 2014; Krouk, 2017). In nrt1.1 loss-of-function mutant plant Arabidopsis thaliana, it has evidently been noted that NRT1.1 regulates the expressions of the dedicated high-affinity transporter nrt2.1. At high nitrate concentrations, the expression of nrt2.1 is not down-regulated when nrt1.1 function is lost, which indicates a critical role of NRT1.1 in the PNR (Bouguyon et al., 2015). However, it remains unknown how the biphasic states of the PNR are regulated by sensing extracellular availabilities of nitrate concentrations.
A key question about the biphasic states of NRT1.1 and their connection with dual-affinity nitrate binding and the phosphorylation at Thr101 has a potential structural basis. Recently reported apo- and nitrate-bound crystal structures of Arabidopsis thaliana NRT1.1 revealed a critical role of His 356 in nitrate binding and a phosphorylation-controlled dimerization switch that allows NRT1.1 to retain a dual-affinity mode of nitrate uptake (Sun et al., 2014, Parker and Newstead, 2014). This suggests that assembly and disassembly of the homodimer NRT1.1 controlled by the phosphorylation is responsible for toggling between low- and high-affinity modes of nitrate uptake (Sun et al., 2014). Despite this significant structural analysis, questions remain as to how the post-translational modifications associated with the nitrate sensing enables NRT1.1 to cope with a wide range of nitrate fluctuations. By comparative structural analyses of apo- and nitrate-bound X-ray crystallographic data of Arabidopsis thaliana NRT1.1 (Parker and Newstead, 2014), we report here that the intrinsic local asymmetries between the two protomers of NRT1.1 around the binding and Thr101 sites that are further enhanced by the nitrate binding provide a functional basis for having dual-affinity modes of nitrate binding. These asymmetries poise both the protomers for differential allosteric communications between the binding and phosphorylation sites, thereby regulating the phosphorylation-controlled dimerization of NRT1.1.
Results
Interprotomer Asymmetries and Differential Nitrate-Binding Affinities
To examine dual-affinity nitrate binding, comparative analyses were carried out between apo- and nitrate-bound crystal structures of Arabidopsis thaliana NRT1.1. The transporter protein NRT1.1 is a 590-amino-acid homodimer consisting of two asymmetric inward-facing units, protomer A and protomer B. While viewed from the side, the nitrate transporting tunnels in both the protomers are not in parallel but tilted at ∼15° angles with the central two-fold axis in opposite direction (Sun et al., 2014). Relative positions of the nitrate to its surrounding residues within the distance of 4.0 Å differ between the monomers. In the apo-protein, the protomer A nitrate-binding pocket consists of the residues Leu 49, His 356, Leu 359, Thr 360, Tyr 388, and Phe 511, with the minimum distances of 3.0 and 2.0 Å between the central nitrate atom and His 356 and Thr 360, respectively. However, the protomer B nitrate-binding pocket consists of Arg 45, Thr 48, Leu 49, Phe 82, and His 356, with minimum distances of 4.0 and 3.7 Å from the central nitrate atom to Arg 45 and His 356, respectively. Compared with the apo-protein structure, the nitrate-bound NRT1.1 protomer A neighborhood composition differs by the residues Val 53 and Leu 78, and in protomer B the composition differs by the residues Leu 78, Thr 360, and Phe 511 (Table S1, Figure S1). It is further noted that nitrate binds to Thr 360 and His 356 through H-bonding in protomer A, whereas in protomer B Thr 360 is replaced by Arg 45 (Figure S2). As observed by Parker and Newstead, (2014), the presence of His 356 in both the protomers seems to be necessary for nitrate binding following its protonation. Mutation of only His 356 has resulted in complete loss of nitrate binding.
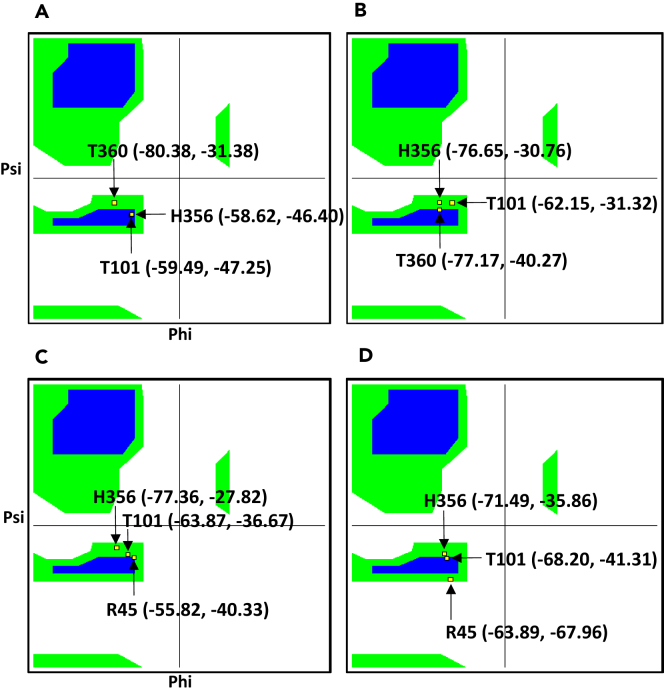
The phosphorylation site Thr101 is entirely buried in a hydrophobic pocket surrounded by the residues Gly 88,97,162; Ile 91,102,104; Ala 92,103; Phe 105; Leu 100 within 4.0 Å neighborhood of apo-protein protomer A. In contrast, the Thr 101 site in protomer B is surrounded by the additional residues Ala 106, 165 and Val 163 within the same neighborhood. In the nitrate-bounded structure, the Thr101 neighborhood composition in protomer A consists of additional residues Ala 106 and Val 163, whereas protomer B consists of additional residues Ala 165 with respect to the apo-structure. Ramachandran plot clearly shows significant conformational changes of both the nitrate-binding residues and phosphorylation sites Thr 101 located at the region of the right-handed helix (Figure 1, Table S2). Moreover, in the interface of the apo-structure with the interfacing area A.1093 Å2 and B.1099 Å2, besides the non-bonded contacts, the only bonded contacts present are four hydrogen bonds: A.Thr111–B.Val229, A.Thr111–B.Ser233, A.Thr111–B.Ser233, A.Val229–B.Thr11. After nitrate binding, all the four interactions are completely lost with reduced interfacing surface area, and a single new H-bond is built between A.Ser233 and B.Thr111 (Table S3). This analysis therefore indicates nitrate-triggered local conformational changes, enhancing asymmetries between the protomers.
Figure 1.
Ramachandran Plots Showing Differences in Phi and Psi Angles before and after Nitrate Binding
(A) Protomer A nitrate unbounded; (B) protomer A nitrate bounded. In (A) and (B), H356 and T360 are the ligand-binding pocket residues and T101 is the phosphorylation site. (C) Protomer B nitrate unbounded; (D) protomer B nitrate bounded. In (C) and (D), H356 and R45 are the binding pocket residues.
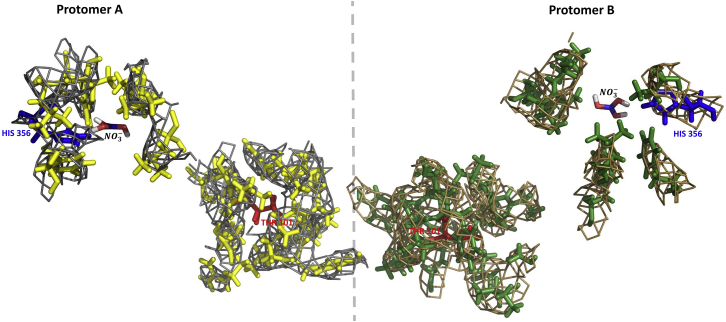
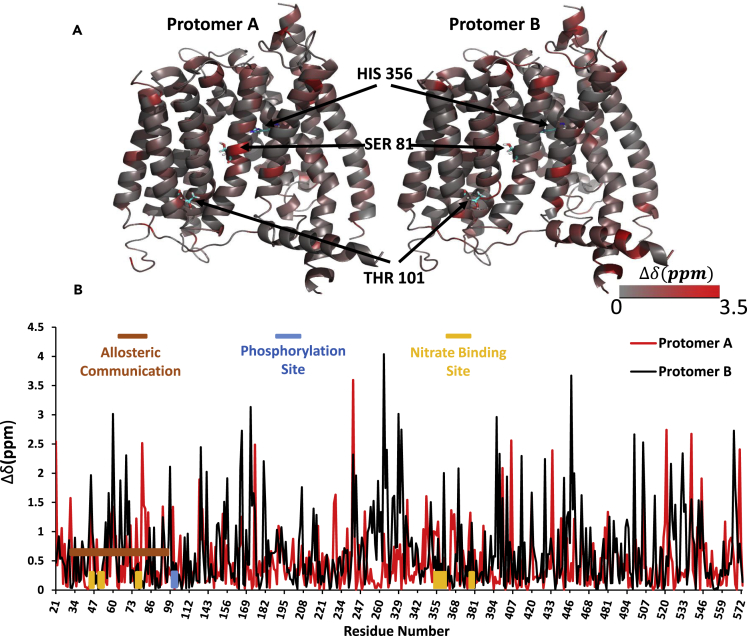
The observed interprotomer local conformational asymmetry is corroborated by the residual electron density within the 4.0-Å neighborhood of the nitrate-binding site and the phosphorylation site Thr 101 (Figure 2). The electron density maps contoured at 2.0 sigma, representing local conformational asymmetries, are calculated for the apo- (Figure S3) and nitrate-bounded (Figure 2) NRT1.1 crystals. To correlate this intrinsic asymmetry with the nitrate-bounded states, differences between backbone chemical shifts, 13, of protomers A and B are predicted with SHIFTX2 (Han et al., 2011) (Figure 3A), which combines ensemble machine learning methods with sequence alignment-based methods. It shows a wide range of variation of in both protomers A (0.003–3.6 ppm) and B (0.003–4.0 ppm). Several regions are shown to exhibit larger chemical shift differences associated with nitrate-triggered allostery: region of allosteric communication [30–94], nitrate-binding pocket, and phosphorylation sites Thr 101 (Figure 3B).
Figure 2.
Electron Density Maps Contoured at 2.0σ
It represents local conformational asymmetries, which are calculated for the nitrate-bounded NRT1.1 crystals (PDB: 5a2o).
Figure 3.
Differences between 13Cα Backbone Chemical Shift of Both the Protomers A and B
(A) Differences are projected onto the NRT1.1 secondary structure, and (B) several regions are identified in which larger differences are observed.
To examine whether nitrate-triggered structural asymmetries between the protomers have any functional consequences, CSM algorithm has been implemented for calculating the nitrate-binding affinities with the inputs of NRT1.1 nitrate-bounded structures in CSM-lig web server (Pires and Ascher, 2016). CSM is a class of graph-based signatures in which atoms are seen as nodes and binding interactions as edges. It extracts distance patterns between the interacting components, defining the complementarity between the proteins and binding molecule based on their shapes and chemistry. This examination has shown that the two protomers hold differential binding affinities. Protomer A has the nitrate-binding affinities of −78.7 kcal/mol, whereas protomer B has the affinity of −16.5 kcal/mol.
Intraprotomer Allosteric Communications
To prime Thr 101 site for phosphorylation with the initiation of nitrate binding, a certain amount of inflexibilities of both the protomers are essential. Rigidity analysis of protein structure based on the fundamental molecular theorem (Katoh and Tanigawa, 2011) is useful for determining and characterizing the mode and the nature of allostery. A network formed by considering all the types of chemical bonds (covalent, electrostatic, hydrophobic, and H-bonds) from a given protein conformation is used in forming rigid clusters. Rigidity-theory-based allostery analysis (Jacobs et al., 2001, Chubynsky and Thorpe, 2007) was carried out in the KINARI (http://kinari.cs.umass.edu), which uses pebble game algorithm (Jacobs and Hendrickson, 1997) with the inputs of apo- and nitrate-bound crystal structures. The KINARI outputs showed that the total degree of freedom reduces significantly with reduction in the number of rigid bodies of atoms (clusters) in protomer A after nitrate binding as compared with protomer B (Table 1). This indicates that nitrate triggered more changes in chemical interactions in protomer A, leading to redistribution of rigid clusters of atoms, making it relatively more rigid than protomer B.
Table 1.
Summary of Different Parameters of Rigidity Analysis
| Parameters | Protomer A |
Protomer B |
||
|---|---|---|---|---|
| Unbounded | Bounded | Unbounded | Bounded | |
| No. of H-bonds | 341 | 347 | 346 | 349 |
| No. of hinges | 1591 | 1362 | 1420 | 1434 |
| No. of bars | 278 | 230 | 224 | 248 |
| No. of bodies | 1532 | 1323 | 1379 | 1395 |
| No. of Degrees of Freedom | 953 | 892 | 944 | 946 |
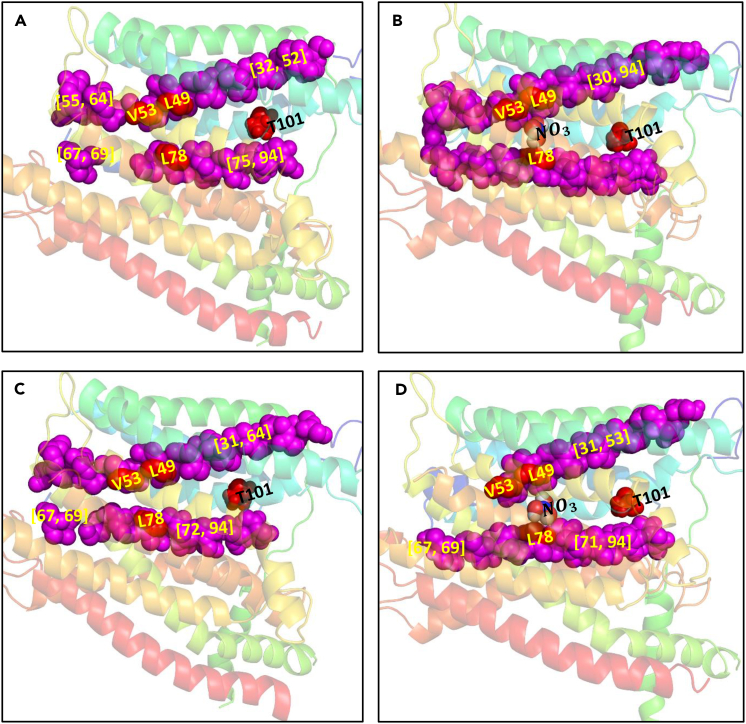
In particular, further analysis of the rigid clusters shows that there exists a largest rigid cluster (LRC) [30–94] in the nitrate-bound protomer A, which bridges the residues of the nitrate-binding pocket to the residues of the phosphorylation site (Figure 4, Table 2). Of relevance to allostery, this resulting rigid cluster measures the extent of allosteric communication. Specifically, a fraction of atoms that belongs to the LRC gives a quantitative measure of the degree of structural coupling and rigidity-based allostery within the protein: (N is the total number of atoms and is the number of atoms in the LRC). The difference (negative value) indicates that nitrate binding triggers rigidity-based allostery in the nitrate-bounded NRT1.1 (Rader and Brown, 2011). Such a rigid cluster has not been predicted in protomer B, indicating weak or absent allosteric communication between the binding and Thr 101 sites.
Figure 4.
Largest Rigid Cluster [30–94]
In protomer A, the LRC (magenta balls) connects the residues of the nitrate-binding pocket to the phosphorylation site. L49, V53, and L78 are the binding pocket residues, T101 is the phosphorylation site, and the residues within brackets are the clusters formed before and after nitrate binding in protomers A and B of NRT1.1. (A) Nitrate unbound protomer A. (B) Nitrate bound protomer A. (C) Nitrate unbound protomer B. (D) Nitrate bound protomer B.
Table 2.
Summary of the Rigidity-Based Allosteric Analyses
| Parameters | Nitrate-Unbound Protomer A [30, 94] | Nitrate-Bound Protomer A [30, 94] |
|---|---|---|
| Rigid clusters | [32, 52], [55, 64], [67, 69], [75, 94] | [30, 94] |
| No. of H-bonds | 46 | 49 |
| H-bonds broken in [30, 94] after binding | Ala32-Met36, Ser33-Ile37, Met36-Cys39, Glu44-Thr47, Arg45-Leu49, Thr48-Gly52, Thr57-Thr60, Tyr58-Thr62, Leu59-Leu65, Ala70-Thr73, Asn72-Thr75, Ile91-Thr94 | |
| Newly added H-bonds in [30, 94] after binding | Gly30-Ser33, Ile37-Cys39, Val43-Thr47, Glu44-Arg45, Glu44-Thr48, Asn54-Val56, Thr69-Thr73, Ala71-Val74, Ala71-Thr75, Phe77-Ser81, Leu78-Phe82, Ser81-Cys85, Leu86-Phe90, Phe90-Thr94, Phe90-Thr94 | |
| No. of H-bonds conserved in [30, 94] before and after binding | 34 | |
To determine the fraction of nitrate-binding site and Thr101 site residues in the LRC in either of the apo and nitrate-bound protomers, we calculated
The positive values of these expressions along with indicate that the nitrate binding is the main source of changes in the rigidity of protomer A (Rader and Brown, 2011). In contrast, there does not exist such an LRC for allostery in protomer B (Figure 4). This analysis, therefore, suggests that nitrate-induced conformational changes establish a rigidity-based allosteric communication between the nitrate-binding site and the Thr 101 site, which is responsible for priming Thr 101 for phosphorylation.
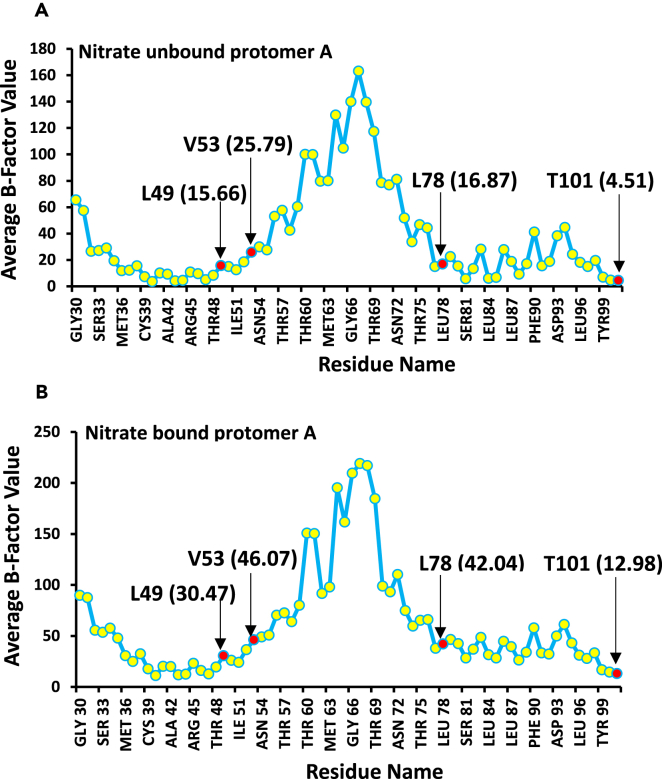
A study of the formation and dilution of H-bonds within the rigid cluster shows that nitrate binding has triggered the addition and re-distribution of H-bonds (Figures 5, S5, and Table 2) through conformational changes, resulting in strong allosteric communication between the distant sites in protomer A. This result is further supported by the crystallographic B-factors distribution within the clusters that shows rapid internal fluctuations upon the initiation of nitrate binding (Figure 6). The rigidity-based allosteric cluster remains rigid as the entropic cost (loss of energy from 276.2 to 218.63 kcal/mol) associated with the nitrate binding is compensated for by an increase in B-factors.
Figure 5.
Distribution of H-bonds before and after Nitrate Binding
Nitrate-binding has triggered the re-distribution of H-bonds through conformational changes, resulting in strong allosteric communication between the nitrate-binding and phosphorylation site T101 through the formation of a large rigid cluster. (A and B) (A) before Nitrate Binding, and (B) after Nitrate Binding.
Figure 6.
Crystallographic B-factor Distribution within the Rigid Cluster
It is responsible for allosteric communication in protomer A, indicating rapid internal fluctuations upon the initiation of nitrate binding. (A and B) (A) before Nitrate Binding, and (B) after Nitrate binding.
In Silico Mutational Analysis
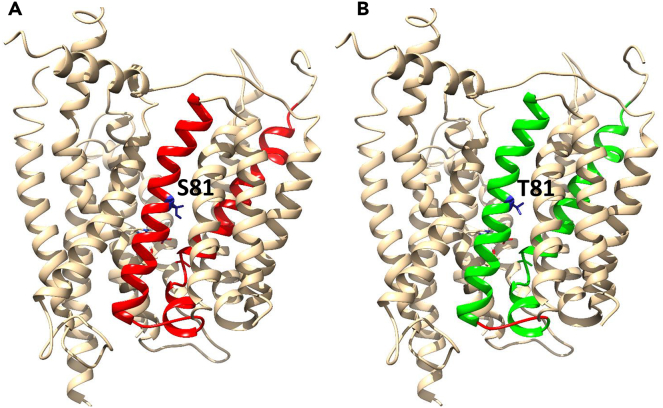
To identify key residues in the allosteric communication pathway [30–94], all possible in silico mutational analyses have been carried out in protomer A of the NRT1.1 crystallographic structure (Table S4). This method is calibrated with the experimental results of Ho et al. (2009) in which single amino acid mutants Thr101Asp (T101D) and Thr101Ala (T101A) mimicked phosphorylated and de-phosphorylated states of NRT1.1, respectively. In parallel to this experimental result, we showed that T101A breaks the rigid cluster that is responsible for allosteric communication into two distinct clusters, whereas the T101D retains the intact allosteric rigid cluster. It, therefore, suggests that priming of the T101 site in protomer A for the phosphorylation is allosterically triggered by the high-affinity nitrate binding, whereas in protomer B such allosteric communication is weak or absent. It has further been noted that most of the new H-bonds in protomer A are added at the sites 80–90 (Figure 5), from which residues are chosen for mutational analysis. The analysis showed that Ser 81 is one of the potential key residues for maintaining the allosteric communication pathway. With the mutations of Ser81Thr, Ser81Val, and Ser81Asp, the allosteric rigid cluster splits into two distinct clusters owing to the loss of H-bonds between Ser 81 and Phe 77, and Ser 81 and Cys 85, which were added through nitrate binding (Figure 7).
Figure 7.
Mutational Analysis of Ser81Thr
Mutation of Ser 81 to Thr splits the largest rigid cluster (LRC) (A: red) into two different pieces (B: green), indicating the key role of Ser 81 in maintaining the allosteric communication pathways within the LRC.
Discussion
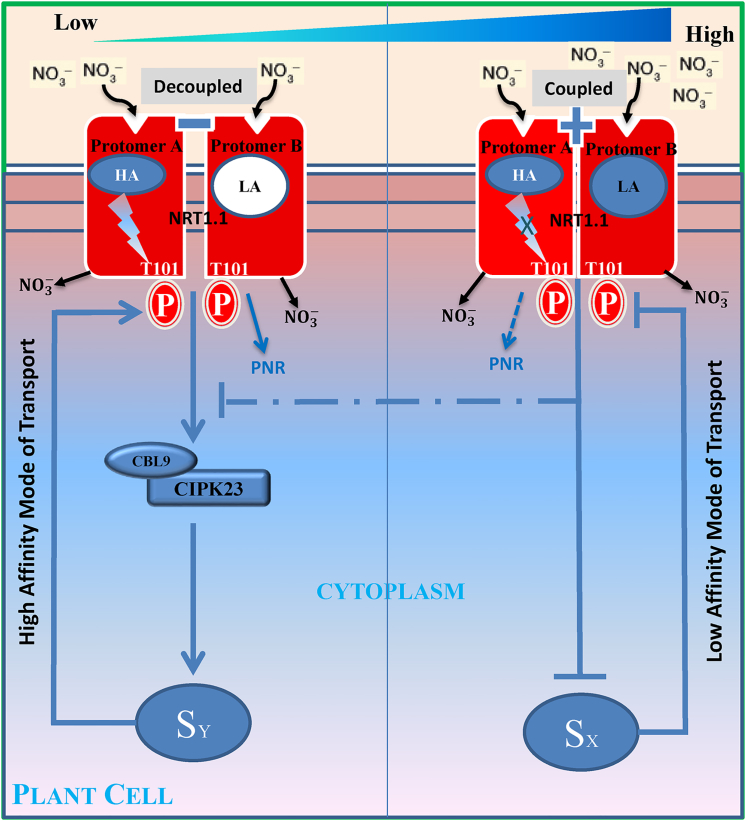
NRT1.1 acts like a toggle switch through the phosphorylation of Thr101, a functional switch for biphasic regulation of nitrate signaling and uptake. Phosphorylation of NRT1.1 at T101 leads to switching from low-affinity to high-affinity transport modes (Liu and Tsay, 2003). Besides, it is also responsible for down-regulating the PNR at low soil nitrate concentrations. For this phosphorylation, activation of calcineurin B-like protein CBL9-interacting kinase CIPK23 is essential at the downstream nitrate singling pathways (Leran et al., 2015). Nitrate binding to NRT1.1 is responsible for creating calcium waves through the action of an unknown phospholipase C, and blocking these waves severely affects several nitrate-induced responses (Riveras et al., 2015, Armijo and Gutiérrez, 2017). Activities of the CBL9.CIPK23 complex toward NRT1.1 depend on these calcium waves (Ho et al., 2009, Leran et al., 2015). Our structural analysis further indicates that the intrinsic asymmetries between the two protomers of NRT1.1 may also differentially affect the magnitude of this calcium wave via the dimerization switch and thereby the activities of the CIPK23 complex, as these asymmetries are differentially enhanced by the high- and low-affinity modes of nitrate binding controlling dimer disassembly and assembly, respectively.
Asymmetries between the two protomers of NRT1.1 are enhanced by nitrate binding and caused for holding dual-affinity binding of nitrate. Protomer A contains a high-affinity nitrate-binding site, whereas protomer B contains a relatively low-affinity binding site. Binding of nitrate ion triggers an allosteric communication between the binding site and the T101 site in protomer A that primes the Thr 101 site for phosphorylation and is responsible for activating the immediate downstream component of nitrate signaling CBL9.CIPK23 complex at low nitrate concentration. In contrast, such an allosteric communication pathway is absent in protomer B. This intramolecular allostery possibly generates two distinct signals: one that activates calcineurin B-like protein CBL9-interacting kinase CIPK23 complex by creating specific cytoplasmic calcium waves at low nitrate concentration (SY) and the other that negatively regulates the activity of the kinase complex at high nitrate concentration (SX). At low nitrate concentration, nitrate ion binds only at the high-affinity site of protomer A and activates the CBL9.CIPK23 complex. At a high nitrate concentration, nitrate binds to both the sites of protomer A and protomer B and then continuously inhibits the activity of the CBL9.CIPK23 complex along the increasing gradient of nitrate (Figure 8). It therefore may generate two distinct regulatory effects of nitrate binding: one that positively regulates the NRT1.1 phosphorylated state and the other that negatively controls this state.
Figure 8.
A Model of Phosphorylation Switch
Protomer A contains a high-affinity nitrate-binding site, whereas protomer B contains a relatively low-affinity binding site. Binding of nitrate ion triggers an allosteric communication between the binding site and the T101 site in protomer A that primes the T101 site for phosphorylation and is responsible for activating the immediate downstream component of the nitrate signaling CBL9.CIPK23 complex at low nitrate concentration. In contrast, such an allosteric communication pathway is absent in protomer B. At low nitrate concentrations, nitrate ion binds only at the high-affinity site of protomer A and activates the CBL9.CIPK23 complex. At high nitrate concentrations, nitrate binds to both the sites of protomer A and protomer B and then continuously inhibits the activity of the CBL9.CIPK23 complex along the increasing gradient of nitrate.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Our thanks to all our colleagues and the members of the School of Mathematics, Statistics and Computational Sciences, Central University of Rajasthan (CURaj) for their support in completing this work. We highly appreciate Dr. Yi-Fang Tsay, Institute of Molecular Biology, Academia Sinica, Taiwan, for her critical comments on the previous version of this manuscript. This research was partially supported by DST-SERB grant (file no. EMR/2015/001671) and by the Central University of Rajasthan; M.R. and S.B. received their PhD fellowships from the CURaj.
Author Contributions
M.R., S.B., A.C. designed the study and conducted computational experiment and analytical analysis. A.C., M.R., G.-Q.S., A.B.M. and B.-L.L. have reviewed and written the article.
Declaration of Interests
The authors declare no conflict of interest.
Published: April 27, 2018
Footnotes
Supplemental Information includes Transparent Methods, six figures, and four tables and can be found with this article online at https://doi.org/10.1016/j.isci.2018.03.007.
Supplemental Information
References
- Armijo G., Gutiérrez R.A. Emerging players in the nitrate signaling pathway. Mol. Plant. 2017;10:1019–1022. doi: 10.1016/j.molp.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Bouguyon E., Brun F., Meynard D., Kubes M., Pervent M., Leran S., Lacombe B., Krouk G., Guiderdoni E., Zažímalová E. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants. 2015;1:1–8. doi: 10.1038/nplants.2015.15. [DOI] [PubMed] [Google Scholar]
- Chubynsky M.V., Thorpe M.F. Algorithms for three-dimensional rigidity analysis and a first-order percolation transition. Phys. Rev. E. Stat. Nonlin. Soft. Matter Phys. 2007;76:041135. doi: 10.1103/PhysRevE.76.041135. [DOI] [PubMed] [Google Scholar]
- Crawford N.M. Nitrate: nutrient and signal for plant growth. Plant Cell. 1995;7:859–868. doi: 10.1105/tpc.7.7.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N.M., Glass A.M. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998;3:1360–1385. [Google Scholar]
- Giehl R.F.H., von Wirén N. Functions of a nitrate transceptor. Nat. Plants. 2015;1:15021. doi: 10.1038/nplants.2015.21. [DOI] [PubMed] [Google Scholar]
- Good A.G., Shrawat A.K., Muench D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci. 2004;9:597–605. doi: 10.1016/j.tplants.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R.A. Systems biology for enhanced plant nitrogen nutrition. Science. 2012;336:1673–1675. doi: 10.1126/science.1217620. [DOI] [PubMed] [Google Scholar]
- Han B., Liu Y., Ginzinger S.W., Wishart D.S. SHIFTX2: significantly improved protein chemical shift prediction. J. Biomol. NMR. 2011;50:43–57. doi: 10.1007/s10858-011-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.H., Lin S.H., Hu H.C., Tsay Y.F. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–1194. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Jacobs D.J., Hendrickson B. An algorithm for two-dimensional rigidity percolation: the pebble game. J. Comput. Phys. 1997;137:346–365. [Google Scholar]
- Jacobs D.J., Rader A.J., Kuhn L.A., Thorpe M.F. Protein flexibility predictions using graph theory. Proteins. 2001;44:150–165. doi: 10.1002/prot.1081. [DOI] [PubMed] [Google Scholar]
- Katoh N., Tanigawa S.I. A proof of the molecular conjecture. Discrete Comput. Geom. 2011;45:647–700. [Google Scholar]
- Krouk G. Calcium bridges the nitrate gap. Nat. Plants. 2017;3:17095–17096. doi: 10.1038/nplants.2017.95. [DOI] [PubMed] [Google Scholar]
- Krouk G., Crawford N.M., Coruzzi G.M., Tsay Y.F. Nitrate signaling: adaptation to fluctuating environments. Curr. Opin. Plant Biol. 2010;13:266–273. doi: 10.1016/j.pbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Krouk G., Tillard P., Gojon A. Regulation of the high-affinity nitrate uptake system by NRT1.1-mediated NO3- demand signaling in Arabidopsis. Plant Physiol. 2006;142:1075–1086. doi: 10.1104/pp.106.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leran S., Edel K., Pervent M., Hashimoto K., Corratgé-Faillie C., Offenborn J., Tillard P., Gojon A., Kudla J., Lacombe B. Nitrate sensing and uptake in Arabidopsis are enhanced by ABI2, a phosphatase inactivated by the stress hormone abscisic acid. Sci. Signal. 2015;8:ra43. doi: 10.1126/scisignal.aaa4829. [DOI] [PubMed] [Google Scholar]
- Liu K.H., Huang C.Y., Tsay Y.F. CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell. 1999;11:865–874. doi: 10.1105/tpc.11.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K.H., Tsay Y.F. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003;22:1005–1013. doi: 10.1093/emboj/cdg118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medici A., Krouk G. The primary nitrate response: a multifaceted signalling pathway. J. Exp. Bot. 2014;65:5567–5576. doi: 10.1093/jxb/eru245. [DOI] [PubMed] [Google Scholar]
- Nacry P., Bouguyon E., Gojon A. Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant Soil. 2013;370:1–29. [Google Scholar]
- Parker J.L., Newstead S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature. 2014;507:68–72. doi: 10.1038/nature13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires D.E., Ascher D.B. CSM-lig: a web server for assessing and comparing protein-small molecule affinities. Nucleic Acids Res. 2016;44:557–561. doi: 10.1093/nar/gkw390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader A.J., Brown S.M. Correlating allostery with rigidity. Mol. Biosyst. 2011;7:464–471. doi: 10.1039/c0mb00054j. [DOI] [PubMed] [Google Scholar]
- Riveras E., Alvarez J.M., Vidal E.A., Oses C., Vega A., Gutierrez R.A. The calcium ion is a second messenger in the nitrate signalling pathway of Arabidopsis. Plant Physiol. 2015;169:1397–1404. doi: 10.1104/pp.15.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Bankston J.R., Payandeh J., Hinds T.R., Zagotta W.N., Zheng N. Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature. 2014;507:73–77. doi: 10.1038/nature13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay Y.F. How to switch affinity. Nature. 2014;507:44–45. doi: 10.1038/nature13063. [DOI] [PubMed] [Google Scholar]
- von Wirén N., Gazzarrini S., Gojon A., Frommer W.B. The molecular physiology of ammonium uptake and retrieval. Curr. Opin. Plant Biol. 2000;3:254–261. [PubMed] [Google Scholar]
- Wang R., Tischner R., Gutierrez R.A., Hoffman M., Xing X., Chen M., Coruzzi G., Crawford N.M. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136:2512–2522. doi: 10.1104/pp.104.044610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.Y., Siddiqi M.Y., Ruth T.J., Glass A.D.M. Ammonium uptake by rice roots. (I. Fluxes and subcellular distribution of 13NH4+) Plant Physiol. 1993;103:1249–1258. doi: 10.1104/pp.103.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.E., Miller A.J. Transporters responsible for the uptake and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:659–688. doi: 10.1146/annurev.arplant.52.1.659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.