Abstract
Background
Adverse Childhood Experiences (ACEs) such as sexual and physical violence, serious illness, and bereavement have been linked to number of chronic pain conditions in adulthood, and specifically to urologic chronic pelvic pain syndrome (UCPPS).
Purpose
We sought to characterize the prevalence of ACEs in UCPPS using a large well-characterized cohort in comparison with a group of healthy controls. We also sought to determine the association of ACE severity with psychological factors known to impact pain and to determine whether ACEs are associated with patterns of improvement or worsening of symptom over a year of naturalistic observation.
Methods
For longitudinal analyses we used functional clusters identifying broad classes of (a) improved, (b) worsened, and (c) stable groups for genitourinary pain and urinary symptoms. We employed a mediation/path analysis framework to determine whether ACEs influenced 1 year outcomes directly, or indirectly through worse perceptions of physical well-being.
Results
ACE severity was elevated in UCPPS (n = 421) participants compared with healthy controls (n = 414; p < .001), and was most strongly associated with factors associated with complex chronic pain, including more diffuse pain, comorbid functional symptoms/syndromes, and worse perceived physical well-being (all p < .001). Finally, worse physical well-being mediated the relationship between ACE severity and less likelihood of painful symptom improvement (OR = .871, p = .007)) and a greater likelihood of painful symptom worsening (OR = 1.249, p = .003) at 1 year.
Conclusions
These results confirm the association between ACEs and UCPPS symptoms, and suggest potential targets for therapeutic interventions in UCPPS.
Clinical Trial registration
Keywords: Interstitial Cystitis/Painful Bladder Syndrome, Chronic Prostatitis with Chronic Pelvic Pain Syndrome, Psychological Trauma, Sexual Abuse
Urologic chronic pelvic pain patients with a history of adverse experiences in childhood have more comorbid pain and show less improvement over time.
Introduction
For more than three decades, researchers have investigated the association between the presence of chronic pain conditions and adverse childhood experiences (ACEs) such as sexual abuse, neglect, and family upheaval. The preponderance of case-control studies supports a relationship between self-reported ACEs and chronic pain conditions, a relationship since confirmed through meta-analysis [1, 2]. In addition to this evidence from retrospective designs, prospective analyses have found that ACEs are associated with a higher incidence of somatic and painful symptoms in adulthood [3, 4] though not all studies have confirmed this relationship [5]. Chronic pelvic pain in particular has been linked to multiple forms of ACEs [6–13]. For instance, a meta-analysis of case-control studies places the incidence of sexual abuse in pelvic pain at nearly three times that of control group [14].
A number of interleaving hypotheses have been advanced to explain the relationship between chronic pain and a history of ACEs, largely focusing on the concepts of biological embedding [15] in early life and the psychological sequelae of trauma. Biological embedding suggests that developmental trajectories change in response to trauma, perhaps by promoting a pro-inflammatory phenotype [16, 17]. ACEs have been consistently linked with increased levels of negative emotionality in adulthood [18, 19] and negative emotionality has been independently associated with painful symptoms and chronic pain conditions [20–28]. Concepts like pain catastrophizing and increased levels of perceived stress may be indicative of a stress reactive phenotype in chronic pain [29]. Similarly, the burden of somatic symptoms has been previously linked to greater pain sensitivity independently of depressive and anxious symptoms [30]. However, the vast majority of studies have considered the relationship between ACEs and chronic pain in a relatively static manner, and have not considered the broad range of potential associations between psychological and physical constructs and ACEs in the context of chronic pelvic pain.
The Multidisciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network is a multi-center study designed to provide information of symptomology and mechanisms of Urologic Chronic Pelvic Pain Syndrome (UCPPS) through deep phenotyping of patients [31, 32]. UCPPS is characterized by painful symptoms in the bladder or pelvic region (e.g., pain as the bladder fills) and urinary symptoms relating to a frequent or urgent need to urinate and/or getting up to urinate at night (e.g., needing to urinate less than 2 h after last urination) [33]. Recent investigations through the MAPP Network have examined longitudinal symptom patterns and uncovered novel risk factors for improvement and worsening of painful and urinary symptoms 1 year after study entry [34]. Specifically, a worse perception of physical well-being was associated with less favorable medium-term outcomes for UCPPS pain [34]. Perceived physical well-being is a composite measure of several aspects of perceived physical health including limitations in physical functioning, limitations in physical roles in work, interference and severity of bodily pain, energy or vitality, and general health [35]. The measure is related to both objective measures of physical fitness [36] and affective/cognitive processes [37]. The perception of physical well-being may have particular importance in the context of chronic pain, because worse perceived physical health is associated with less engagement in preventative health-related behaviors [38]. However, it is not yet known how a history of ACE relates to medium-term (i.e., 1 year) likelihood of improvement or worsening. Because UCPPS symptoms tend to be persistent [39, 40], any factors related to short- and medium-term change are important to understand for planning treatments and determining necessary windows of observation to determine treatment effectiveness.
The aim of this study is to determine how self-reported ACE severity is associated with painful and urinary symptoms in UCPPS, comorbid factors relating to physical and psychological health, including a variety of measures of negative mood and stress, somatic/functional symptoms, and the likelihood of symptom improvement/worsening at 1 year. We hypothesized that ACE severity is associated with both painful and urinary symptoms at study entry, greater levels of negative emotionality, more functional and somatic symptoms, and less likelihood of improvement over time. We also hypothesize that perceived physical well-being, previously shown to be associated with medium-term symptom outcomes [34], may mediate the effect of ACE severity on symptom improvement/worsening.
Methods
Participants
Inclusion/exclusion
UCPPS participants were recruited from clinics and local advertisements and healthy control participants were recruited at each MAPP Network Discovery Site. Complete information about the MAPP Network inclusion/exclusion criteria is available [31, 32]. Briefly, participants were eligible if they were at least 18 years of age and reported chronic pain/pressure/ discomfort associated with the bladder or pelvic region in the preceding 3 months in the presence of negative urine cultures for uropathogens. Exclusion criteria included pregnancy if female and conditions that might result in tissue damage to areas relevant to UCPPS symptomology (e.g., history of urethral stricture, neurological disorder affecting the bladder or bowel). UCPPS Participants were enrolled in a longitudinal study of symptom changes related to pelvic pain and urinary symptoms. Participants completed demographic and symptom information during a baseline visit following which genitourinary pain and urinary symptom data were collected biweekly for 48 weeks via an online module (25 time points, including baseline). At study entry, participants had a physical examination, urine collection, blood draw, and completed questionnaires regarding symptoms, and nonurologic factors such as mood, coping, and childhood adversity that are fully described elsewhere [32]. The same broad medical comorbidity exclusions were applied to healthy controls. In additon, healthy controls could not report any pain in the pelvic or bladder region or pain in more than one of the 42 nonurologic body regions on a body map or meet criteria for a chronic pain condition (described under the subsection ‘comorbid conditions’) [32]. At baseline the final sample consisted of 421 UCPPS participants (232 women) who completed the measure of ACE severity and 414 (233 women) healthy controls who completed measures of ACE severity. The final sample for longitudinal analyses included 421 (232 women) UCPPS participants. The study was approved by Institutional Review Boards at all participating sites.
Measures
Adverse childhood experiences
The Childhood Traumatic Events Scale (CTES) examines traumatic events that occurred before the age of 17 in the following categories: death of a close family member or friend, parental separation, physical abuse including sexual assault, serious illness, and other. For each category, severity of the trauma, age at the time of the trauma, and extent of confiding in others is assessed [41]. Each ACE is rated either 0 (“no trauma”) or from 1 (“not at all traumatic”) to 7 (“extremely traumatic”). The total CTES severity score is a simple sum of the severity rating for each ACE category (possible range 0–42). We used this summary measure rather than individual ACE categories (e.g., sexual trauma) as the primary predictor, as cumulative ACE burden across multiple domains, including bereavement and serious illness, has been shown to strongly predict complex symptoms in adulthood even when controlling for specific categories of ACE and particular ACE characteristics, such as age of exposure [42, 43].
Functional symptoms
Functional symptoms were assessed using the Complex Medical Symptoms Inventory checklist [44], a 41 item measure assessing symptoms commonly reported in functional syndromes. The checklist assesses symptoms present at least 3 months out of the preceding year, using binary responses. A sum of nonurinary symptoms (e.g., dry eye, photo-sensitivity, palpitations) was used as an assessment of the cumulative burden of these symptoms.
Genitourinary pain and urinary symptoms
Pain and Urinary Severity was assessed using a composite measure derived from the genitourinary pain index (GUPI) [45], and Interstitial Cystitis Symptom Index (ICSI) [46], based on psychometric analyses performed on MAPP baseline data, suggesting that pain and urinary symptoms should be measured separately. In this analysis the urinary and symptom severity scores were correlated (r = .56) [33]. Pain severity scores range from 0 to 28 (MAPP sample Cronbach’s α = .92) and urinary severity scores range from 0 to 25 (MAPP sample Cronbach’s α = .89).
Health, medical, and demographic information
Health, medical, and demographic information (e.g., age, duration of symptoms, use of medication) was collected by self-report. A 45 site body map was also administered (42 nonpelvic sites) and the sum of sites checked as painful, using binary responses, was used as an indication of the burden of widespread pain [47]. Headache was indicated by any of four head sites being checked as painful.
Comorbid conditions
Comorbid Conditions were assessed using standardized self-report criteria for fibromyalgia (FM) [48], chronic fatigue syndrome (CFS) [49], irritable bowel syndrome (IBS) [50], vulvodynia (VVD; females only) [44], and temporomandibular disorder (TMD) [51].
Perceived stress
The Perceived Stress Scale (PSS) is a 10-item scale (MAPP sample Cronbach’s α = .93) measuring the degree to which situations are perceived as being unpredictable, uncontrollable, and overwhelming during the last month. Responses range from 0 (“never”) to 4 (“very often”). Higher scores indicate more stress [52].
Catastrophizing
Catastrophizing refers to the appraisal of one’s pain as overwhelmingly awful and the extent to which a person believes that their pain will lead to catastrophic consequences. The 6-item (MAPP sample Cronbach’s α = .88) Catastrophizing subscale from the Coping Strategies Questionnaire (CSQ) was used to measure this characteristic, with higher scores indicating worse catastrophizing. Responses range from 0 (“never do that”) to 6 (“always do that”) when the participant feels pain [53].
Sleep and fatigue
Sleep (8 items; MAPP sample Cronbach’s α = .95) and fatigue (7 items; MAPP sample Cronbach’s α = .80) were assessed with the NIH Patient Reported Outcomes Measurement Information System (PROMIS) questionnaires. Responses range from 1 (“never”) to 5 (“always”) for each symptom experienced in the last 7 days [54].
Anxiety and depression
Anxiety (7 items; MAPP sample Cronbach’s α = .88) and depression (7 items; MAPP sample Cronbach’s α = .88) were measured using the Hospital Anxiety and Depression Scale (HADS). Responses range from 0 (e.g., “not at all”) to 3 (e.g., “most of the time”) for each item experienced over the last week [55].
Perceived physical and mental well-being
Perceived physical and mental well-being were measured using the SF-12 physical components score (PCS; a composite of all physical health subscales), and mental components score (MCS). These measures are scored using a proprietary algorithm. Responses range from 1 (“all of the time”) to 4 (“none of the time”). Higher scores indicated better physical and mental well-being [56].
Statistical Analyses
Statistical analyses were performed using SPSS v. 24, MPLUS v 5.2. Descriptive statistics were generated for each of the six CTES subscales and compared between the longitudinal MAPP UCPPS (n = 421) and healthy cohorts by analysis of variance (ANOVA) for severity scores and psychosocial/physical health characteristics, and Pearson χ2 difference testing for binary ACE categories. Total number of ACE types endorsed and total severity scores were also compared by ANOVA. Pearson correlation analyses were conducted between ACE severity scores and each of the psychosocial and physical health measures in each cohort. We also examined these relationships stratified by cohort and sex.
UCPPS severity outcome groupings
As described in a previous publication, a functional clustering algorithm was employed to classify subjects according to symptom trajectory status on pain and urinary severity at 48 weeks (improve, stable, worse) [34]. Briefly, an initial clustering step Ward’s minimum variance was used to assign each subject to a cluster based on a treatment of all the longitudinal data from weeks 4 to 48. In the second iterative classification step, one subject is removed and new mixed effects models are estimated by K-mixture functional mixed effects models [57]. The posterior probability of the excluded subject belonging to one of the groups is then calculated, and the steps are repeated until group assignments for each subject are stable. The number of clusters is selected based on Kullback–Leibler (KL) criterion [58].
In previous analyses it was determined that large study entry or regression to the mean type effects were present in this cohort [59], and that classification of “improved” or “worse” changed substantially when the first 4 weeks of data were excluded. For these reasons, the “baseline” measure of symptoms occurred at week four. The algorithm subsequently produced three broad classifications of symptom change: “improved,” “stable,” and “worse.”
For pain severity outcomes at 1 year, 87 (21.9%) were classified as improved, 230 (57.9%) as stable, and 80 (20.1%) as worse. For urinary severity 83 (20.9%) improved, 261 (65.7%), remained stable, and 53 (13.3%) were worse.
Mediation/path analysis
To address the question of how levels of ACE severity might be related to changes in in genitourinary pain and urinary symptoms at 1 year, we conducted mediation/path analyses using perceived physical well-being (the SF-12 PCS scale) as the mediator of interest. Model building procedures in our previous work using univariate then multivariate analyses identified the SF-12 PCS as the only significant predictor of pain and urinary functional outcomes [33].
All models controlled for patient age. Sex was not used as a covariate because it was not found to be associated with UCPPS symptom outcome group membership [33]—in secondary analyses we confirmed that sex did not influence the model. For the mediation/path analyses, a parallel mediation model was conducted to control for any relationships between ACEs, baseline pain or urinary severity, and UCPPS symptom outcome status. This was owing to the established association between baseline severity and UCPPS symptom outcome status [34] as well as association between ACE severity and baseline symptom severity (pain severity, r = .156, p < .001; urinary severity, r =.194, p < .001). A logistic regression framework was employed, with separate models tested for improvement status (“improved” vs. “worse/stable”) and worse status (“worse” vs. “stable/improved”). We chose this approach as each outcome may have unique associations that would not be captured by proportional odds models. Indirect effects were tested through the construction of bias-corrected 95% confidence intervals (20,000 resamples).
Results
Demographics
UCPPS participants were 43 (15 +/− SD) years old on average. Of the sample, 55% were female, 93% were non-Hispanic, 88% were Caucasian, and 65% were college graduates. Approximately 30% of the sample was using a pain medication at the time of study entry. Demographic information on the trans-MAPP cohort has been previously published [60].
ACEs and Psychosocial/Physical Health
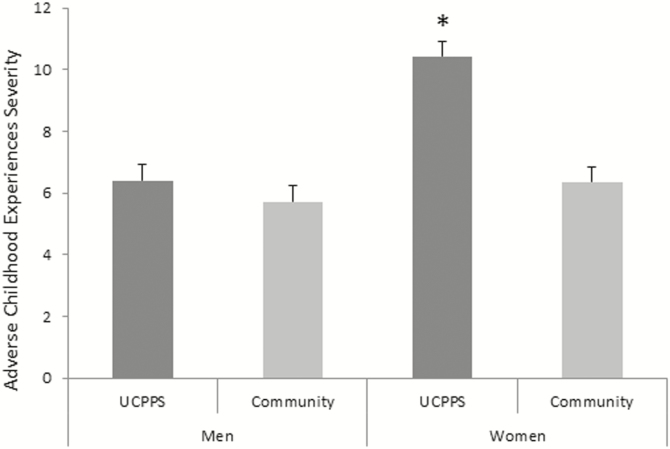
Compared with the healthy control group, UCPPS participants had elevated levels of depression, anxiety, perceived stress, pain catastrophizing, functional symptoms, painful body map sites, fatigue, sleep disturbance, head pain, and worse physical and mental health (all p < .001; see Table 1). Similarly, UCPPS participants reported experiencing more categories of ACEs than the control group and greater ACE severity (both p < .001). Comparing specific ACE categories, UCPPS participants were more likely to endorse sexual ACE, physical violence, illness/injury, and “other trauma” than healthy controls (all p < .01), while death of family members/close friends and parental separation/divorce were not more common for UCPPS participants (both p < .13). In addition, there was a significant effect of sex on ACE severity across both cohorts, such that women had higher ACE severity scores than men (p < .001) and significant group X sex interaction, such that women with UCPPS had higher ACE severity scores than healthy women and men from both groups (p < .001; see Fig. 1). UCPPS participants with one or more comorbid pain conditions had significantly higher ACE severity scores (UCPPS only, mean 0= 7.24, SD = 6.94; UCPPS comorbid, mean = 9.81, SD = 8.96; p = .001).
Table 1.
Trauma and psychological characteristics of UCPPS and healthy control participants
| Characteristic mean (SD) | UCPPS (n = 421) | Healthy controls (n = 414) | F value | DF | p |
|---|---|---|---|---|---|
| Depression (HADS-D) | 5.42 (4.20) | 1.80 (2.34) | 235.25 | 1,832 | < .001 |
| Anxiety (HADS-A) | 7.70 (4.54) | 3.63 (3.15) | 226.37 | 1,832 | < .001 |
| Catastrophizing (CSQ) | 12.63 (8.80) | 2.58 (4.58) | 421.23 | 1,826 | < .001 |
| Perceived stress (PSS) | 16.48 (7.89) | 10.44 (6.27) | 149.19 | 1,830 | < .001 |
| Physical health (SF-12 PCS) | 47.34 (10.31) | 57.14 (4.29) | 307.96 | 1,799 | <.001 |
| Mental health (SF-12 MCS) | 43.76 (10.42) | 53.76 (6.99) | 250.470 | 1,799 | <.001 |
| Number of functional symptoms | 8.74 (6.87) | 1.58 (2.80) | 386.98 | 1,833 | <.001 |
| Number of painful body map sites | 5.02 (6.47) | 1.24 (2.05) | 128.64 | 1,833 | <.001 |
| Fatigue (PROMIS-fatigue) | 19.12 (5.67) | 13.05 (3.74) | 333.09 | 1,833 | <.001 |
| Sleep disturbance (PROMIS –sleep disturbance) | 24.19 (7.75) | 15.09 (5.56) | 379.00 | 1,833 | <.001 |
| Adverse Childhood Experiences (ACEs) severity (CTES) |
8.63 (8.16) | 6.08 (6.68) | 24.51 | 1,833 | <.001 |
| ACE # categories | 1.69 (1.41) | 1.29 (1.22) | 32.81 | 1,833 | <.001 |
| Frequency (%) | Pearson χ2 | p | |||
| Body map head pain | 118 (28) | 47 (11) | 36.61 | <.001 | |
| ACEs | |||||
| Death of family member/close friend | 209 (50) | 184 (45) | 2.17 | .14 | |
| Divorce/separation of parents | 126 (30) | 123 (30) | .002 | .96 | |
| Traumatic sexual experience | 85 (20) | 41 (10) | 17.37 | <.001 | |
| Victim of violence | 65 (15) | 39 (9) | 6.94 | .008 | |
| Extremely ill or injured | 82 (20) | 48 (12) | 10.06 | .002 | |
| Other trauma | 143 (34) | 102 (25) | 9.04 | .003 | |
Fig. 1.
Means and S.E.s of ACE severity scores for men and women with UCPPS and healthy controls. *After adjustment for multiple comparisons with Sidak correction UCPPS women had higher ACE severity compared with all other groups (all p < .001), while no other groups differed significantly from one another (all p > .9).
In the UCPPS cohort, ACE severity was associated with higher levels of baseline depression, anxiety, perceived stress, pain catastrophizing, functional symptoms, painful body map sites, fatigue, sleep disturbance, and perceived physical well-being (all r > .108, all p < .05; see Table 2). The strongest relationships were with more functional symptoms (r = .370, p < .001), worse physical health (r = −.277, p < .001) and more painful body map sites (r = .267, p < .001). ACE severity was also associated with greater genitourinary pain severity (r = .118, p = .016) and urinary symptoms (r = .139, p = .005) at the time of study entry, though these associations were not significant after correction for multiple comparisons. In healthy controls, ACE severity was associated with worse depression, anxiety, perceived stress, pain catastrophizing, functional symptoms, painful body map sites, fatigue, sleep disturbance, and mental health (all r > .108, all p < .05) but not head pain (r = .059, p = .228) or perceived physical well-being (r = −.087, p = .084). There were some apparent sex differences, with females showing slightly stronger associations between ACE severity and some psychosocial variables.
Table 2.
Pearson correlations between psychosocial and physical health measures and adverse childhood experiences severity (CTES), for all participants in each group and stratified by sex
| Measure | UCPPS | HC | UCPPS | HC | ||
|---|---|---|---|---|---|---|
| r | r | r | r | |||
| All | All | Female | Male | Female | Male | |
| Depression (HADS-D) | .171b | .138 | .200b | .165 | .181 | .109a |
| Anxiety (HADS-A) | .205b | .162b | .248b | .128a | .110a | .219b |
| Catastrophizing (CSQ) | .199b | .108 | .209b | .095a | .098 | .111a |
| Perceived stress (PSS) | .246b | .175b | .279b | .138a | .159 | .191 |
| Perceived physical well-being (SF-12 PCS) | −.277b | −087a | −243b | −.222b | −.096a | −.076a |
| Perceived mental well-being (SF-12 MCS) | −.102 | −148 | −.149 | −.028a | −.184 | −.110a |
| Number of functional symptoms (CMSI) | .370b | .203b | .347b | .305b | .224b | .183 |
| Number of painful body map sites | .267b | .133 | .280b | .078a | .082a | .207 |
| Fatigue (PROMIS- fatigue) | .249b | .188b | .251b | .130a | .175 | .197 |
| Sleep disturbance (PROMISsleep disturbance) | .221b | .175b | .267b | .068a | .133 | .231b |
| Body map head pain | .109 | .059a | .088a | .001a | .012a | .121a |
| Genitourinary pain (Pain severity) | .118 | N/A | .051a | .144 | N/A | N/A |
| Urinary symptoms (Urinary severity) | .139 | N/A | .082a | .110a | N/A | N/A |
All p < .05 unless otherwise noted
a p > .05
b p < .05 after correction for multiple comparisons
Functional Outcomes at One Year
Total effect of ACE severity
The total effect of ACE severity was not significantly associated with changes in painful or urinary symptoms, adjusting for age (all p > .25; see Table 3).
Table 3.
Relationship between adverse childhood experiences (ACE) severity and functional outcomes adjusting for age
| Obs. used: 397 | Estimate | Standard Error | Estimate/Standard Error | p | Odds Ratio | 95% Confidence Interval, Lower Limit | Upper Limit |
|---|---|---|---|---|---|---|---|
| Pain improvement | |||||||
| ACE severity | −0.148 | 0.130 | −1.142 | 0.253 | 0.862 | 0.659 | 1.094 |
| Age | 0.006 | 0.008 | 0.840 | 0.401 | 1.006 | 0.991 | 1.021 |
| Pain worsening | |||||||
| ACE severity | −0.023 | 0.121 | −0.186 | 0.852 | 0.978 | 0.761 | 1.225 |
| Age | −0.016 | 0.009 | −1.745 | 0.081 | 0.984 | 0.967 | 1.002 |
| Urinary improvement | |||||||
| ACE severity | −0.113 | 0.116 | −0.972 | 0.331 | 0.893 | 0.706 | 1.119 |
| Age | −0.003 | 0.008 | −0.440 | 0.660 | 0.997 | 0.981 | 1.012 |
| Urinary worsening | |||||||
| ACE severity | 0.080 | 0.150 | 0.503 | 0.596 | 1.083 | 0.798 | 1.438 |
| Age | 0.008 | 0.010 | 0.843 | 0.399 | 1.009 | 0.988 | 1.028 |
Mediation/path analysis—painful symptoms
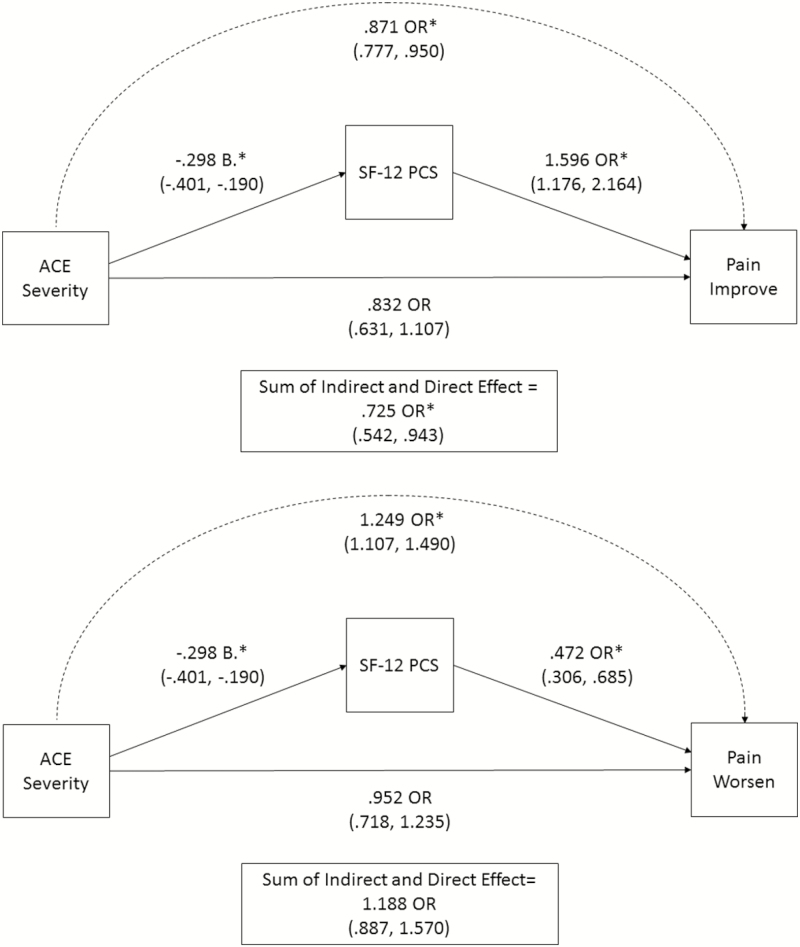
ACE severity was associated with less likelihood of improvement in pain symptoms at 1 year indirectly through worse perceived physical well-being (OR = .871, 95% CI = .777, .950, p = .007; see Fig. 2). This indicates that a 1 standard deviation increase in ACE severity is associated with an approximately 13% lower probability of 1-year improvement in pain scores through SF-12 PCS scores. The direct effect of ACE severity was not significant (p = .18), while the sum of the direct and indirect effect was significant (OR = .725, 95% CI = .542, .943, p = .023).
Fig. 2.
Mediation analyses showing the direct and indirect effects of ACE severity on pain functional outcomes. Odds ratios represent the increase or decrease in the likelihood of each pain outcome associated with a one standard deviation increase on the ACE severity measure. The association between ACE severity and SF-12 PCS scores is reported in standardized units. The sum of direct and indirect effects includes the mediated effect (by SF-12 PCS scores), and the direct effect of ACE severity on pain outcomes. Both models control for relationships between ACEs and early symptom severity and the effect of age (not shown). *p < .05.
Similarly, ACE severity was associated with a greater likelihood of worsening in pain symptoms at 1 year indirectly through worse perceived physical well-being (OR = 1.249, 95% CI = 1.107, 1.490, p = .003). This indicates that a one standard deviation increase in ACE severity is associated with an approximately 25% greater probability of worsening in pain symptoms at 1 year through SF-12 PCS scores. The direct effect of ACE severity was not significant (p = .71), nor was the sum of the direct and indirect effect (OR = 1.188, 95% CI = .887, 1.570, p = .23).
In summary, greater severity of self-reported ACEs is associated with worse perceived physical well- being, which in turn is associated with less likelihood of improvement and a greater likelihood of worsening in pain severity at 1 year. In secondary analyses, these relationships remained when sex was included as a covariate (data not shown).
Mediation/path analyses—urinary symptoms
ACE severity was not associated with urinary symptom improvement either directly (p = .07) or indirectly through perceived physical well-being (OR = .933, 95% CI = .839, 1.015, p = .15). Similarly, ACE severity was not associated with worsening of urinary symptoms either directly (p = .51) or indirectly through SF-12 PCS scores (OR = 1.107, 95% CI = .994, 1.241, p = .10).
Discussion
Our result show that the cumulative severity of ACEs is associated with substantially worse pain outcomes at 1 year, indirectly, through poorer perceived physical well-being. Identifying distinct pathways that impact symptom outcomes is particularly important when, as in these analyses, there is no clear association between the exposure (ACEs) and symptom outcomes in simple prediction models—this may be owing to the combination of different types of indirect effects not explicitly modeled [61]. These analyses confirm the relationship between ACEs and worse perceived physical well-being [62] and suggest that perceived physical well-being is important to evaluate in UCPPS sufferers with a history of ACEs. To our knowledge this is the first study in UCPPS to examine longitudinal outcomes within the context of ACEs.
These findings are consistent with the notion that stressful early life events could play a role in exacerbation of painful symptomology in UCPPS. On average, the UCPPS participants had experienced 1.7 of 6 ACE categories, and approximately 78% of the sample endorsed at least one ACE. Interpersonal violence (both physical and sexual) was roughly twice as likely to be reported by UCPPS participants compared with healthy controls, a finding consistent with meta-analyses in other chronic pain conditions [2]. Although the cumulative impact of ACEs across domains are clearly associated with worse symptom outcomes in the medium-term, it may be true that ACEs involving personal threats during a critical developmental window are part of the pathogenesis of the syndrome. Longitudinal studies beginning in childhood are necessary to answer this question.
Worse perceived physical well-being appears to play an important role in the relationship between ACEs and worse functional pain outcomes. It has previously been shown that chronic pain patients with worse perceived physical well-being are likely to have experienced pain longer and to have more social difficulties related to their pain [63]. Longitudinal studies have demonstrated that individuals with poorer perceived physical health are less likely to engage in preventative health behaviors such as eating a balanced diet and receiving routine medical care [38], that ACEs prospectively predict more negative health behaviors in adulthood such as tobacco use and alcohol abuse [64] and a reduced sense of internal control around adversity [65]. These findings are consistent with conceptual models in which the link between ACEs and poor perceived physical health is due in part to worse overall health behaviors, worse objective measures of health, and a lack of social integration or support. It is possible then that individuals with a history of ACEs are more likely to engage in harmful health behaviors that exacerbate pain, may have less functional or perceived social support that would facilitate behavioral modification, or may have a reduced sense of control in managing symptoms. Our present findings suggest that in the context of medium-term pain outcomes, factors related to the perception of physical health are at least as important as the perception of mental health in individuals with a history of ACEs.
Extrapolating to estimates of UCPPS (3.3–7.9 million women [66] and .96–3.0 million men) [67] prevalence in the USA, our findings suggest that between 3.3 and 8.5 million UCPPS sufferers have experienced some form of ACE which in turn reduces the likelihood of medium-term improvement. By our estimates, a patient one standard deviation above the mean on ACE severity would be approximately 52% more likely to have pain worsen at 1 year after adjustment for age and baseline severity, and approximately 27% less likely to have pain improve, compared with a patient with no history of ACEs—an effect mediated through worse perceived physical well-being. Although ACE severity was higher in women than in men, levels of ACE severity were similarly associated with poorer perceived physical well-being. This is likely why sex did not appear to alter the mediation/path analysis relationship between ACE severity and 1 year painful outcomes. This also suggests that ACE severity confers vulnerability to worse symptom outcomes in the medium-term for both women and men.
The measures and conditions most strongly associated with ACE severity at study entry—spatial distribution of pain, comorbid functional syndromes, total number of functional symptoms, and poorer perceived physical well-being—are all associated with what is sometimes termed “centralized” pain, in recognition of the prominent role the central nervous system plays in the expression of nonspecific symptoms and widespread pain [68]. Interestingly, these constructs were also those more strongly associated with ACE severity in the UCPPS cohort than in the healthy control group. Hypotheses related to central nervous system disruption of sensory and affective pathways are strengthened by the exclusion of individuals with medical comorbidities of a known autoimmune or overtly inflammatory character. That UCPPS patients with more functional symptoms and bodily pain at study entry have higher levels of ACE severity is consistent with previous studies indicating that ACEs are both more prevalent in those with chronic pain conditions [1] and that the number/severity of ACEs are associated with a greater number of painful symptoms [69]. The concept of biological embedding has been advanced as a potential explanation for the cluster of physical and psychological symptoms seen in chronic pain patients with a history of ACEs [15], as a variety of neurobiological mechanisms appear to influence both sensory and affective pathways that may be relevant to pain. Along these lines, we have previously demonstrated that provoked immune responses are associated with both centralized pain characteristics [70, 71] and worse longitudinal outcomes in an exploratory MAPP cohort [72].
Our findings confirm previous investigations in chronic pain finding relationships between ACEs and affective vulnerabilities as well—at study entry, ACE severity was associated with higher levels of depression, catastrophizing, anxiety, and perceived stress. The associations between catastrophizing, depression, and ACEs at study entry is important, as previous research has found that both are important independent constructs in relation to pain reports [73]. Functional magnetic resonance imaging (fMRI) studies of pain catastrophizing suggest that neural networks related to pain anticipation, emotional elements of pain, and motor control are related to the construct [74, 75]. fMRI studies have revealed that the depressive symptoms are associated with activation of neural networks related to the emotional/affective salience of pain [76, 77], and that induced depressive symptoms enhance the unpleasantness of pain experienced [78]. These findings may have correlates in neuroimaging studies of individuals with a history of ACEs: limbic hyper-responsiveness to threat [79], hippocampal hyper-responsiveness to sad subliminal images [80], and overall reduced limbic-prefrontal connectivity [81]. Regarding pain processing, ACEs have also been associated with reduced activation in the hippocampus during pain–empathy tasks [82], and reduced activity of the anterior cingulate cortex during a visceral pain task [83] a result subsequently found to be modulated by affective symptoms [77]. It would therefore appear that the association of ACEs with pain may share similar neural pathways as affective vulnerabilities. The fact that the association between ACEs and neuroimaging outcomes was no longer significant once affective symptoms were controlled for supports the close relationship between affective and pain processing circuits, and their involvement in ACE-related pain modulation [77]. A recent MAPP analysis using resting-state functional connectivity analysis of MRI data on a subset of participants found that a broad frontoparietal network of connectivity predicted improvement and worsening at 3 months with substantial accuracy. A number of affective and pain processing regions were identified as having predictive value, including the hippocampus, parahippocampal gyrus, anterior cingulate cortex, nucleus accumbens, and insula [84].
Limitations
As with all self-reported measures of past events, reporting of ACEs may be subject to recall bias. Comparisons of prospective data on childhood maltreatment and self-report in adulthood reveal that a significant number of individuals do not report or recall previous abuse [85, 86], and while difficult to quantify, false-positive reports may be a non-negligible issue as well. Despite these limitations, there is general consensus to support the usefulness of retrospective reports of adverse early life events [86]. It is possible that affective disturbances such as depression and catastrophizing may influence current reporting and evaluations of ACE incidence and/or severity [87]. ACE severity and perceived physical well-being were measured at the same time point. This study was observational and therefore participants entered at different phases of the disease trajectory using different treatment modalities. A recent analysis of the same longitudinal dataset suggested however that self-reported changes in treatment at baseline appeared to have little impact on symptom trajectories [59].
Conclusions and Future Directions
These findings confirm the importance of ACEs in modulating the severity and persistence of UCPPS symptoms. Perhaps more importantly from a clinical perspective, these analyses suggest that ACEs may be associated with elements of the “centralized” pain phenotype. It remains to be determined if well-documented effects of early adversity on pain perception and stress responsiveness are responsible for these clinical observations. Both the change in inflammatory markers and brain systems modulating pain are being tracked in ongoing MAPP Network projects. In addition, projects are underway to identify the neural correlates of ACE severity in this MAPP Network cohort. Future research in UCPPS may focus on differential effects of common treatments among individuals with a history of ACE, particularly those targeting relevant neurotransmitter systems and cognitive and affective interventions targeting characteristics of chronic pain [88, 89].
Acknowledgments
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316.)
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare no conflicts of interest to disclose.
Ethical Approval The study was approved by the Institutional Review Boards of all participating institutions.
Informed Consent All subjects provided informed consent.
MAPP Research Network Study Group
MAPP Network Executive Committee: J. Quentin Clemens, MD, FACS, MSci, Network Chair, 2013–; Philip Hanno, MD; Ziya Kirkali, MD; John W. Kusek, PhD; J. Richard Landis, PhD; M. Scott Lucia, MD; Chris Mullins, PhD; and Michel A. Pontari, MD.
Northwestern University Discovery Site: David J. Klumpp, PhD, Co-Director; Anthony J. Schaeffer, MD, Co-Director; Apkar (Vania) Apkarian, PhD; David Cella, PhD; Melissa A. Farmer, PhD; Colleen Fitzgerald, MD; Richard Gershon, PhD; James W. Griffith, PhD; Charles J. Heckman II, PhD; Mingchen Jiang, PhD; Laurie Keefer, PhD; Darlene S. Marko, RN, BSN, CCRC; Jean Michniewicz; Todd Parrish, PhD; and Frank Tu, MD, MPH.
University of California, Los Angeles Discovery Site and PAIN Neuroimaging Core: Emeran A. Mayer, MD, Co-Director; Larissa V. Rodríguez, MD, Co-Director; Jeffry Alger, PhD; Cody P. Ashe-McNalley; Ben Ellingson, PhD; Nuwanthi Heendeniya; Lisa Kilpatrick, PhD; Jason Kutch, PhD; Jennifer S. Labus, PhD; Bruce D. Naliboff, PhD; Fornessa Randal; and Suzanne R. Smith, RN, NP.
University of Iowa Discovery Site: Karl J. Kreder, MD, MBA, Director; Catherine S. Bradley, MD, MSCE; Mary Eno, RN, RA II; Kris Greiner, BA; Yi Luo, PhD, MD; Susan K. Lutgendorf, PhD, ;Michael A. O’Donnell, MD; and Barbara Ziegler, BA.
University of Michigan Discovery Site: Daniel J. Clauw, MD, Co-Director; Network Chair, 2008–2013; J. Quentin Clemens, MD, FACS, MSci, Co-Director; Network Chair, 2013–; Suzie As-Sanie, MD; Sandra Berry, MA; Megan E. Halvorson, BS, CCRP; Richard Harris, PhD; Steve Harte, PhD; Eric Ichesco, BS; Ann Oldendorf, MD; Katherine A. Scott, RN, BSN; David A. Williams, PhD.
University of Washington, Seattle Discovery Site: Dedra Buchwald, MD, Director; Niloofar Afari, PhD, Univ. Of California, San Diego; John Krieger, MD; Jane Miller, MD; Stephanie Richey, BS; Susan O. Ross, RN, MN; Roberta Spiro, MS; T.J. Sundsvold, MPH; Eric Strachan, PhD; Claire C. Yang, MD.
Washington University, St. Louis Discovery Site: Gerald L. Andriole, MD, Co-Director; H. Henry Lai, MD, Co-Director; Rebecca L. Bristol, BA, BS, Coordinator; Graham Colditz, MD, DrPH; Georg Deutsch, PhD, University of Alabama at Birmingham; Vivien C. Gardner, RN, BSN, Coordinator; Robert W. Gereau IV, PhD; Jeffrey P. Henderson, MD, PhD; Barry A. Hong, PhD, FAACP; Thomas M. Hooton, MD, University of Miami; Timothy J. Ness, MD, PhD, University of Alabama at Birmingham; Carol S. North, MD, MPE, Univ. Texas Southwestern; Theresa M. Spitznagle, PT, DPT, WCS; Siobhan Sutcliffe, PhD, ScM, MHS.
University of Pennsylvania Data Coordinating Core (DCC): J. Richard Landis, PhD, Core Director; Ted Barrell, BA; Philip Hanno, MD; Xiaoling Hou, MS; Tamara Howard, MPH; Michel A. Pontari, MD; Nancy Robinson, PhD; Alisa Stephens, PhD; Yanli Wang, MS.
University of Colorado Denver Tissue Analysis & Technology Core (TATC): M. Scott Lucia, MD; Core Director, Adrie van Bokhoven, PhD; Andrea A. Osypuk, BS; Robert Dayton, Jr.; Karen R. Jonscher, PhD; Holly T. Sullivan, BS; R. Storey Wilson, MS.
Additional Sites: Drexel University College of Medicine, Garth D. Ehrlich, PhD; Harvard Medical School/Boston Children’s Hospital, Marsha A. Moses, PhD, Director; Andrew C. Briscoe; David Briscoe, MD; Adam Curatolo, BA; John Froehlich, PhD; Richard S. Lee, MD; Monisha Sachdev, BS; Keith R. Solomon, PhD; Hanno Steen, PhD.
Stanford University: Sean Mackey, MD, PhD, Director; Epifanio Bagarinao, PhD; Lauren C. Foster, BA; Emily Hubbard, BA; Kevin A. Johnson, PhD, RN; Katherine T. Martucci, PhD; Rebecca L. McCue, BA; Rachel R. Moericke, MA; Aneesha Nilakantan, BA; and Noorulain Noor, BS.
Queens University: J. Curtis Nickel, MD, FRCSC, Director.
National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH): Chris Mullins, PhD, John W. Kusek, PhD, Ziya Kirkali, MD; and Tamara G. Bavendam, MD.
References
- 1. Afari N, Ahumada SM, Wright LJ, et al. . Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. 2014;76(1):2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Paras ML, Murad MH, Chen LP, et al. . Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302(5):550–561. [DOI] [PubMed] [Google Scholar]
- 3. van Tilburg MA, Runyan DK, Zolotor AJ, et al. . Unexplained gastrointestinal symptoms after abuse in a prospective study of children at risk for abuse and neglect. Ann Fam Med. 2010;8(2):134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: results from the 1958 British Birth Cohort Study. Pain. 2009;143(1-2):92–96. [DOI] [PubMed] [Google Scholar]
- 5. Raphael KG, Widom CS, Lange G. Childhood victimization and pain in adulthood: a prospective investigation. Pain. 2001;92(1–2):283–293. [DOI] [PubMed] [Google Scholar]
- 6. Lampe A, Sölder E, Ennemoser A, Schubert C, Rumpold G, Söllner W. Chronic pelvic pain and previous sexual abuse. Obstet Gynecol. 2000;96(6):929–933. [DOI] [PubMed] [Google Scholar]
- 7. Lampe A, Doering S, Rumpold G, et al. . Chronic pain syndromes and their relation to childhood abuse and stressful life events. J Psychosom Res. 2003;54(4):361–367. [DOI] [PubMed] [Google Scholar]
- 8. Walling MK, Reiter RC, O’Hara MW, Milburn AK, Lilly G, Vincent SD. Abuse history and chronic pain in women: I. Prevalences of sexual abuse and physical abuse. Obstet Gynecol. 1994;84(2):193–199. [PubMed] [Google Scholar]
- 9. As-Sanie S, Clevenger LA, Geisser ME, Williams DA, Roth RS. Pain catastrophizing in women with chronic pelvic pain and its relationship to pain experience and childhood sexual abuse. J Reprod Med. 2016;61(6):545–551. [Google Scholar]
- 10. As-Sanie S, Clevenger LA, Geisser ME, Williams DA, Roth RS. History of abuse and its relationship to pain experience and depression in women with chronic pelvic pain. Am J Obstet Gynecol. 2014;210(4):317.e1–317.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters KM, Carrico DJ, Diokno AC. Characterization of a clinical cohort of 87 women with interstitial cystitis/painful bladder syndrome. Urology. 2008;71(4):634–640. [DOI] [PubMed] [Google Scholar]
- 12. Peters KM, Kalinowski SE, Carrico DJ, Ibrahim IA, Diokno AC. Fact or fiction—is abuse prevalent in patients with interstitial cystitis? Results from a community survey and clinic population. J Urol. 2007;178(3 Pt 1):891–895; discussion 895. [DOI] [PubMed] [Google Scholar]
- 13. Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Curr Urol Rep. 2009;10(6):441–447. [DOI] [PubMed] [Google Scholar]
- 14. Paras ML, Murad MH, Chen LP, et al. . Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302(5):550–561. [DOI] [PubMed] [Google Scholar]
- 15. Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137(6):959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller GE, Chen E, Fok AK, et al. . Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106(34):14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coutinho SV, Plotsky PM, Sablad M, et al. . Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282(2):G307–G316. [DOI] [PubMed] [Google Scholar]
- 18. Allen B, Lauterbach D. Personality characteristics of adult survivors of childhood trauma. J Trauma Stress. 2007;20(4):587–595. [DOI] [PubMed] [Google Scholar]
- 19. Bunce SC, Larsen RJ, Peterson C. Life after trauma: personality and daily life experiences of traumatized people. J Pers. 1995;63(2):165–188. [DOI] [PubMed] [Google Scholar]
- 20. Geisser ME, Roth RS, Theisen ME, Robinson ME, Riley JL 3rd. Negative affect, self-report of depressive symptoms, and clinical depression: relation to the experience of chronic pain. Clin J Pain. 2000;16(2):110–120. [DOI] [PubMed] [Google Scholar]
- 21. Tosic-Golubovic S, Miljkovic S, Nagorni A, Lazarevic D, Nikolic G. Irritable bowel syndrome, anxiety, depression and personality characteristics. Psychiatr Danub. 2010;22(3):418–424. [PubMed] [Google Scholar]
- 22. Feldman SI, Downey G, Schaffer-Neitz R. Pain, negative mood, and perceived support in chronic pain patients: a daily diary study of people with reflex sympathetic dystrophy syndrome. J Consult Clin Psychol. 1999;67(5):776–785. [DOI] [PubMed] [Google Scholar]
- 23. Ward MM. Are patient self-report measures of arthritis activity confounded by mood? A longitudinal study of patients with rheumatoid arthritis. J Rheumatol. 1994;21(6):1046–1050. [PubMed] [Google Scholar]
- 24. Chou KL. Reciprocal relationship between pain and depression in older adults: evidence from the English Longitudinal Study of Ageing. J Affect Disord. 2007;102(1–3):115–123. [DOI] [PubMed] [Google Scholar]
- 25. Kindler S, Samietz S, Houshmand M, et al. . Depressive and anxiety symptoms as risk factors for temporomandibular joint pain: a prospective cohort study in the general population. J Pain. 2012;13(12):1188–1197. [DOI] [PubMed] [Google Scholar]
- 26. Chieng YJ, Chan WC, Klainin-Yobas P, He HG. Perioperative anxiety and postoperative pain in children and adolescents undergoing elective surgical procedures: a quantitative systematic review. J Adv Nurs. 2014;70(2):243–255. [DOI] [PubMed] [Google Scholar]
- 27. Gerrits MM, van Marwijk HW, van Oppen P, van der Horst H, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64–70. [DOI] [PubMed] [Google Scholar]
- 28. de Heer EW, Gerrits MM, Beekman AT, et al. . The association of depression and anxiety with pain: a study from NESDA. Plos One. 2014;9(10):e106907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Severeijns R, Vlaeyen JW, van den Hout MA, Weber WE. Pain catastrophizing predicts pain intensity, disability, and psychological distress independent of the level of physical impairment. Clin J Pain. 2001;17(2):165–172. [DOI] [PubMed] [Google Scholar]
- 30. Schrepf A, Harper DE, Williams DA, Hassett AL, Harte SE. Somatic awareness and tender points in a community sample. J Pain. 2016;17(12):1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clemens JQ, Mullins C, Kusek JW, et al. ; MAPP Research Network Study Group The MAPP research network: a novel study of urologic chronic pelvic pain syndromes. BMC Urol. 2014;14:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Landis JR, Williams DA, Lucia MS, et al. ; MAPP Research Network Study Group The MAPP research network: design, patient characterization and operations. BMC Urol. 2014;14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Griffith JW, Stephens-Shields AJ, Hou X, et al. . Pain and urinary symptoms should not be combined into a single score: psychometric findings from the MAPP Research Network. J Urol. 2016;195(4 Pt 1):949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naliboff BD, Stephens AJ, Lai HH, et al. ; MAPP Research Network Clinical and psychosocial predictors of urological chronic pelvic pain symptom change in 1 Year: a prospective study from the MAPP Research Network. J Urol. 2017;198(4):848–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. [DOI] [PubMed] [Google Scholar]
- 36. Olsson S, Ekblom‐Bak E, Ekblom B, et al. . Association of perceived physical health and physical fitness in two Swedish national samples from 1990 and 2015. Scand J Med Sci Sports. 2018;28(2):717–724. [DOI] [PubMed] [Google Scholar]
- 37. Okun MA, George LK. Physician-and self-ratings of health, neuroticism and subjective well-being among men and women. Personal Individ Differ. 1984;5(5):533–539. [Google Scholar]
- 38. Takahashi Y, Edmonds GW, Jackson JJ, Roberts BW. Longitudinal correlated changes in conscientiousness, preventative health-related behaviors, and self-perceived physical health. J Pers. 2013;81(4):417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suskind AM, Berry SH, Suttorp MJ, Elliott MN, Clemens JQ. Symptom persistence in a community cohort of women with interstitial cystitis/bladder pain syndrome (IC/BPS): 3-, 6-, 9-, and 12-month follow-up from the RICE cohort. Int Urogynecol J. 2014;25(12):1639–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Propert KJ, Schaeffer AJ, Brensinger CM, Kusek JW, Nyberg LM, Landis JR. A prospective study of interstitial cystitis: results of longitudinal followup of the interstitial cystitis data base cohort. The Interstitial Cystitis Data Base Study Group. J Urol. 2000;163(5):1434–1439. [DOI] [PubMed] [Google Scholar]
- 41. Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26(3):327–332. [DOI] [PubMed] [Google Scholar]
- 42. Briere J, Kaltman S, Green BL. Accumulated childhood trauma and symptom complexity. J Trauma Stress. 2008;21(2):223–226. [DOI] [PubMed] [Google Scholar]
- 43. Steine IM, Winje D, Krystal JH, et al. . Cumulative childhood maltreatment and its dose-response relation with adult symptomatology: findings in a sample of adult survivors of sexual abuse. Child Abuse Negl. 2017;65:99–111. [DOI] [PubMed] [Google Scholar]
- 44. Williams DA, Schilling S. Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):339–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Clemens JQ, Calhoun EA, Litwin MS, et al. ; Urologic Pelvic Pain Collaborative Research Network Validation of a modified National Institutes of Health chronic prostatitis symptom index to assess genitourinary pain in both men and women. Urology. 2009;74(5):983–987; quiz 987.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O’Leary MP, Sant GR, Fowler FJ Jr, Whitmore KE, Spolarich-Kroll J. The interstitial cystitis symptom index and problem index. Urology. 1997;49(5A Suppl):58–63. [DOI] [PubMed] [Google Scholar]
- 47. Tripp DA, Nickel JC, Wong J, et al. . Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol. 2012;62(6):1188–1194. [DOI] [PubMed] [Google Scholar]
- 48. Wolfe F, Clauw DJ, Fitzcharles MA, et al. . Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. [DOI] [PubMed] [Google Scholar]
- 49. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121(12):953–959. [DOI] [PubMed] [Google Scholar]
- 50. Drossman DA. Rome III: the new criteria. Chin J Dig Dis. 2006;7(4):181–185. [DOI] [PubMed] [Google Scholar]
- 51. Dworkin SF, Sherman J, Mancl L, Ohrbach R, LeResche L, Truelove E. Reliability, validity, and clinical utility of the research diagnostic criteria for Temporomandibular Disorders Axis II Scales: depression, non-specific physical symptoms, and graded chronic pain. J Orofac Pain. 2002;16(3):207–220. [PubMed] [Google Scholar]
- 52. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 53. Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. [DOI] [PubMed] [Google Scholar]
- 54. Cella D, Yount S, Rothrock N, et al. ; PROMIS Cooperative Group The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. [DOI] [PubMed] [Google Scholar]
- 56. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. [DOI] [PubMed] [Google Scholar]
- 57. Guo W. Functional mixed effects models. Biometrics. 2002;58(1):121–128. [DOI] [PubMed] [Google Scholar]
- 58. Kullback S, Leibler RA. On information and sufficiency. Ann Math Stat. 1951;22:79–86. [Google Scholar]
- 59. Stephens-Shields AJ, Clemens JQ, Jemielita T, et al. ; MAPP Research Network Symptom variability and early symptom regression in the MAPP Study: a prospective study of urological chronic pelvic pain syndrome. J Urol. 2016;196(5):1450–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Clemens JQ, Clauw DJ, Kreder K, et al. ; MAPP Research Network Comparison of baseline urological symptoms in men and women in the MAPP research cohort. J Urol. 2015;193(5):1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hayes AF. Beyond baron and kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009; 76:408–420. [Google Scholar]
- 62. Walker EA, Gelfand A, Katon WJ, et al. . Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999;107(4):332–339. [DOI] [PubMed] [Google Scholar]
- 63. Keeley P, Creed F, Tomenson B, Todd C, Borglin G, Dickens C. Psychosocial predictors of health-related quality of life and health service utilisation in people with chronic low back pain. Pain. 2008;135(1–2):142–150. [DOI] [PubMed] [Google Scholar]
- 64. Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37(3):268–277. [DOI] [PubMed] [Google Scholar]
- 65. Bolger KE, Patterson CJ. Pathways from child maltreatment to internalizing problems: perceptions of control as mediators and moderators. Dev Psychopathol. 2001; 13(4):913–940. [PubMed] [Google Scholar]
- 66. Berry SH, Elliott MN, Suttorp M, et al. . Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186(2):540–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Suskind AM, Berry SH, Ewing BA, Elliott MN, Suttorp MJ, Clemens JQ. The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: results of the RAND Interstitial Cystitis Epidemiology male study. J Urol. 2013;189(1):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Clauw DJ. Fibromyalgia: a clinical review. JAMA. 2014;311(15):1547–1555. [DOI] [PubMed] [Google Scholar]
- 69. Kempke S, Luyten P, Claes S, et al. . The prevalence and impact of early childhood trauma in Chronic Fatigue Syndrome. J Psychiatr Res. 2013;47(5):664–669. [DOI] [PubMed] [Google Scholar]
- 70. Schrepf A, Bradley CS, O’Donnell M, et al. . Toll-like Receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: a multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. 2015;49:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schrepf A, O’Donnell M, Luo Y, et al. . Inflammation and inflammatory control in interstitial cystitis/bladder pain syndrome: associations with painful symptoms. Pain. 2014;155(9):1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schrepf A, O’Donnell MA, Luo Y, Bradley CS, Kreder KJ, Lutgendorf SK. Inflammation and symptom change in interstitial cystitis or bladder pain syndrome: a multidisciplinary approach to the Study of Chronic Pelvic Pain Research Network Study. Urology. 2016;90:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 1994;59(1):79–83. [DOI] [PubMed] [Google Scholar]
- 74. Gracely RH, Geisser ME, Giesecke T, et al. . Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(Pt 4):835–843. [DOI] [PubMed] [Google Scholar]
- 75. Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120(3):297–306. [DOI] [PubMed] [Google Scholar]
- 76. Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52(5):1577–1584. [DOI] [PubMed] [Google Scholar]
- 77. Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. 2010;59(4):489–495. [DOI] [PubMed] [Google Scholar]
- 78. Berna C, Leknes S, Holmes EA, Edwards RR, Goodwin GM, Tracey I. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry. 2010;67(11):1083–1090. [DOI] [PubMed] [Google Scholar]
- 79. Dannlowski U, Stuhrmann A, Beutelmann V, et al. . Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286–293. [DOI] [PubMed] [Google Scholar]
- 80. Dannlowski U, Kugel H, Huber F, et al. . Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum Brain Mapp. 2013;34(11):2899–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Souza-Queiroz J, Boisgontier J, Etain B, et al. . Childhood trauma and the limbic network: a multimodal MRI study in patients with bipolar disorder and controls. J Affect Disord. 2016;200:159–164. [DOI] [PubMed] [Google Scholar]
- 82. Noll-Hussong M, Otti A, Laeer L, et al. . Aftermath of sexual abuse history on adult patients suffering from chronic functional pain syndromes: an fMRI pilot study. J Psychosom Res. 2010;68(5):483–487. [DOI] [PubMed] [Google Scholar]
- 83. Ringel Y, Drossman DA, Leserman JL, et al. . Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134(2):396–404. [DOI] [PubMed] [Google Scholar]
- 84. Kutch JJ, Labus JS, Harris RE, et al. . Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP Network Study. Pain. 2017;158(6):1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Williams LM. Recall of childhood trauma: a prospective study of women’s memories of child sexual abuse. J Consult Clin Psychol. 1994;62(6):1167–1176. [DOI] [PubMed] [Google Scholar]
- 86. Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry. 2004;45(2):260–273. [DOI] [PubMed] [Google Scholar]
- 87. Williams J. Depression and the specificity of autobiographical memory. In: Rubin DC, ed. Remembering our Past: Studies in Autobiographical Memory. New York: Cambridge University Press; 1996:244–267. [Google Scholar]
- 88. Hsu MC, Schubiner H, Lumley MA, Stracks JS, Clauw DJ, Williams DA. Sustained pain reduction through affective self-awareness in fibromyalgia: a randomized controlled trial. J Gen Intern Med. 2010;25(10):1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Williams DA. Cognitive—behavioral therapy in central sensitivity syndromes. Curr Rheumatol Rev. 2016;12(1):2–12. [DOI] [PubMed] [Google Scholar]