Abstract
The aim of the study was to compare the effect of intermittent hypoxic training (IHT) and the live high, train low strategy on aerobic capacity and sports performance in off-road cyclists in normoxia. Thirty off-road cyclists were randomized to three groups and subjected to 4-week training routines. The participants from the first experimental group were exposed to normobaric hypoxia conditions (FiO2 = 16.3%) at rest and during sleep (G-LH-TL; n=10; age: 20.5 ± 2.9 years; body height 1.81 ± 0.04 m; body mass: 69.6 ± 3.9 kg). Training in this group was performed under normoxic conditions. In the second experimental group, study participants followed an intermittent hypoxic training (IHT, three sessions per week, FiO2 = 16.3%) routine (G-IHT; n=10; age: 20.7 ± 3.1 years; body height 1.78 ± 0.05 m; body mass: 67.5 ± 5.6 kg). Exercise intensity was adjusted based on the lactate threshold (LT) load determined in hypoxia. The control group lived and trained under normoxic conditions (G-C; n=10; age: 21.8 ± 4.0 years; body height 1.78 ± 0.03 m; body mass: 68.1 ± 4.7 kg; body fat content: 8.4 ± 2.4%). The evaluations included two research series (S1, S2). Between S1 and S2, athletes from all groups followed a similar training programme for 4 weeks. In each research series a graded ergocycle test was performed in order to measure VO2max and determine the LT and a simulated 30 km individual time trial. Significant (p<0.05) improvements in VO2max, VO2LT, WRmax and WRLT were observed in the G-IHT (by 3.5%, 9.1%, 6.7% and 7.7% respectively) and G-LH-TL groups (by 4.8%, 6.7%, 5.9% and 4.8% respectively). Sports performance (TT) was also improved (p<0.01) in both groups by 3.6% in G-LH-TL and 2.5% in G-IHT. Significant changes (p<0.05) in serum EPO levels and haematological variables (increases in RBC, HGB, HCT and reticulocyte percentage) were observed only in G-LH-TL. Normobaric hypoxia has been demonstrated to be an effective ergogenic aid that can enhance the exercise capacity of cyclists in normoxia. Both LH-TL and IHT lead to improvements in aerobic capacity. The adaptations induced by both approaches are likely to be caused by different mechanisms. The evaluations included two research series (S1, S2). Between S1 and S2, athletes from all groups followed a similar training programme for 4 weeks. In each research series a graded ergocycle exercise test was performed in order to measure VO2max and determine the lactate threshold as well as a simulated 30 km individual time trial.
Keywords: Hypoxia, Intermittent hypoxic training, Live high train low, Cycling, Endurance training
INTRODUCTION
Among the most popular altitude environment strategies used in recent years to fully activate adaptive reserves and improve athletic performance is intermittent hypoxic training (IHT), and the live high, train low training strategy (LH-TL). In IHT, athletes train in simulated normobaric hypoxia or, less often, in a natural high-altitude environment under hypobaric conditions, while living under normoxic conditions [1]. Compared to the well-known LH-TL strategy, IHT presents a few essential advantages that can be utilized as an integral component of modern athletic training. Among them the most evident are: 1) IHT prevents athletes from sleeping disorders and dehydration, which are typical symptoms seen during an extended stay at altitude during the LH-TL strategy [2], 2) recovery following IHT training sessions occurs under normoxic conditions, which protects athletes from deleterious effects of prolonged hypoxia and shortens the post-training recovery time, and 3) the time spent apart from training under hypoxic conditions may be used for normal training activity [3].
Both passive exposure to a hypoxic environment and combining hypoxia conditions with physical exercise contribute to activation of numerous exercise-induced adaptations that are beneficial to sports performance [2,4]. The mechanisms underlying the improvement in athletes’ performance at sea level with altitude training are generally attributed to either cardiovascular (5), haematological (3), or ventilatory (6) effects and peripheral adaptations, i.e. muscle buffering capacity (7), glycolytic enzyme activity (8) and mechanical efficiency (15).
Although a number of publications have demonstrated the effectiveness of the LH-TL routines [10, 11, 12, 13] and IHT procedures [14, 15, 16, 3, 10], the effects of hypoxia on aerobic capacity and sports performance of athletes remains debatable. These contradictions result from the methodological differences in study designs. It should be noted that the prerequisite for effective stimulation of adaptive mechanisms is the choice of adequate exposure time, hypoxia level and training stimuli that are proportional to expected adaptive changes. The appropriate combination of these variables ensures an increase in exercise capacity following hypoxic training [17]. The examinations presented in this study were conducted in a group of athletes involved in the same sport and during the same training period. The same hypoxic conditions and the same well-designed training plan were used in both experimental groups. This study design allowed for the comparison of the effect of normobaric hypoxia (LH-TL) and IHT on aerobic capacity and sports performance in off-road cyclists under normoxic conditions.
MATERIALS AND METHODS
Study participants
The study examined 30 competitive off-road cyclists. The basic inclusion criteria were a minimum of six years of training experience and at least a six-month washout period from previous altitude training. Study participants were randomized to three groups: two experimental groups and a control group. The first experimental group (G-LH-TL) (n=10; age: 20.5 ± 2.9 years; body height 1.81 ± 0.04 m; body mass: 69.6 ± 3.9 kg; body fat content: 8.4 ± 2.6%; lean body mass: 63.8 ± 4.1 kg) was exposed to normobaric hypoxia at rest and during sleep for 11 to 12 hours a day. Training in this group was performed exclusively under normoxic conditions. In the second experimental group (G-IHT) (n=10; age: 20.7 ± 3.1 years; body height 1.78 ± 0.05 m; body mass: 67.5 ± 5.6 kg; body fat content: 10.6 ± 2.0%; lean body mass: 60.3 ± 5.1 kg), participants followed an IHT routine three times a week under conditions of normobaric hypoxia. The control group (G-C) (n=10; age: 21.8 ± 4.0 years; body height 1.78 ± 0.03 m; body mass: 68.1 ± 4.7 kg; body fat content: 8.4 ± 2.4%; lean body mass: 62.4 ± 4.8 kg) lived and trained under normoxic conditions. Each participant had current valid medical examinations and showed no contraindications that would exclude him from participation in the experiment. The project was approved by the Bioethics Commission for Scientific Research of the Jerzy Kukuczka Academy of Physical Education in Katowice, Poland.
Study design
The evaluations included two research series (S1, S2). Between S1 and S2, athletes from all groups followed a similar training programme for 4 weeks (three basic microcycles and one recovery microcycle). The only factor that differentiated the training protocols in particular study groups was the exposure of the G-IHT group to normobaric hypoxia.
Evaluations
Each research series was performed after an overnight fast, and was started by drawing venous blood (10 ml) from the basilic vein to determine red blood cell count (RBC), haemoglobin level (HGB) and haematocrit (HCT) (Advida 2120, Siemens, Germany), and biochemical fatigue indices: creatine kinase activity (CK), lactate dehydrogenase activity (LDH) and uric acid concentration (URIC). After obtaining the blood samples, body height, body mass and body composition were also measured (InBody 220, Biospace, Korea). Next, three hours after a light mixed meal, study participants performed a graded exercise test using the Excalibur Sport cycle ergometer (Lode, Netherlands) in order to measure VO2max and determine the lactate threshold. The graded exercise test began with a load of 40 W, with increments of 40 W every 3 minutes. The test was continued to exhaustion or until the participant was unable to maintain the minimal cadence of 60 rpm. Heart rate (HR), minute ventilation (VE), breathing frequency (BF), oxygen uptake (VO2) and carbon dioxide output (VCO2) (MetaLyzer 3B-2R, Cortex, Germany) were continuously recorded at rest and during the test. At the end of each load (last 15 s) and in the 3rd, 6th, 9th and 12th minute of recovery, capillary blood samples were obtained from fingertips in order to determine blood lactate concentration (Biosen C-line Clinic, EKF-diagnostic GmbH, Germany). These data were used to analyse the kinetics of concentration of this metabolite in blood, evaluate individual lactate thresholds based on the Dmax methodology [18] and determine individual exercise zones. Furthermore, before and immediately after completion of the test, blood was sampled from fingertips in order to determine the difference between resting and post-exercise blood pH (RapidLab 248, Bayer Diagnostics, Germany).
On the second day of measurements, study participants performed a simulated 30 km individual time trial in mountainous terrain (TT). The test trial was performed on personal bicycles of the studied athletes connected to an electromagnetic bicycle trainer (Cyclus 2, RBM elektronik-automation GmbH). Power generated during the TT (P) and heart rate (HR) were continuously recorded. After a warm-up and immediately after completion of the test, capillary blood samples were obtained in order to determine LA concentration and acid-base balance.
Furthermore, after 48 hours of rest, the G-IHT group performed an additional graded exercise test on the cycle ergometer under normobaric hypoxia conditions (FiO2 = 16%) in order to determine the lactate threshold load. These data were used to choose an individual training load for the experiment.
Venous blood samples (10 ml) were taken several times during the experiment in order to evaluate EPO levels (IMMULITE 1000, Siemens, Germany) and blood morphology. Blood samples were taken after fasting, not later than 30 minutes after waking up. In the G-LH-TL group, samples were obtained after the first and third night, as well as after the 1st, 2nd and 3rd week, whereas in the G-IHT and G-C groups, blood was sampled after each week. Furthermore, oxygen saturation of haemoglobin was evaluated during training sessions performed under hypoxia conditions (G-IHT) using a Pulsox-3 pulse oximeter (Minolta, Netherlands).
The values of SpO2 and resting heart rate (HRrest) (Pulsox-3) were also measured on each day after waking up (in a lying position) in the G-LH-TL group. HRrest was recorded on a daily basis in the G-IHT and G-C groups (S810i, Polar Electro). The information collected in the study was used to analyse the adaptive changes in the LH-TL group.
Training programme
All the groups followed the same training routines with individually adjusted intensity zones. The only factor that differentiated the training routines in particular study groups was the exposure of the G-IHT group to normobaric hypoxia (FiO2 = 16.3%, corresponding to the altitude of 2,100 m above sea level) in a hypoxic chamber. The intermittent hypoxic training programme in the G-IHT group was chosen based on our previous study [3]. Each training session in the G-IHT group was subdivided into a 15-min warm-up, 30 to 40-min main part and 15-minute cool-down. Intensity during these sessions was adjusted individually to each study participant based on the threshold load (WRLThyp), determined under normobaric hypoxia conditions. The warm-up during all IHT sessions was performed using the intensity at the level of 65-70% WRLThyp. In the main part, the intensity was increased to 100% WRLThyp. This level of intensity was maintained for 30 minutes (first week), 35 minutes (second week) and 40 minutes (third week). The cool-down included 15 min of continuous exercise with an intensity of 60% WRLThyp. After completion of the final part of the IHT session, cyclists performed a two-hour ride under normoxic conditions at an intensity of 65-75% WRLT.
The participants from the G-LH-TL and G-C groups followed the same training routine in the laboratory environment but under normoxic conditions. Training intensity (% WRLT) was the same as in the G-IHT group. However, it referred to the threshold load determined under normoxic conditions.
In the G-LH-TL group, participants spent from 11 to 12 hours (evenings and nights) during three basic microcycles (three weeks) under conditions of normobaric hypoxia (FiO2 = 16.3%). The Everest Summit II hypoxic generators and altitude tents (Hypoxico, USA) were used to ensure normobaric hypoxia conditions.
The training load was recorded using PowerTap technology (Cycle Ops, USA) [3]. It was calculated after each training session and expressed on the Training Stress Score point scale [19] using WKO+ 3.0 software (TrainingPeaks, USA).
Statistical Methods
The results of the study were analysed by means of Statistica 9.0 (StatSoft software). The results were presented as arithmetic means (x) with standard deviations (SD). Statistical significance was set at p<0.05. The Lilliefors test was used to demonstrate the consistency of the results obtained in the study with normal distribution. The intergroup differences between research series were determined using the multi-factor analysis of variance (MANOVA) for repeated measures. Significance of differences between individual research series in the study groups was calculated based on the post-hoc Tukey test.
RESULTS
The bivariate analysis of variance with repeated measures revealed statistically significant differences in the group × training interaction for absolute (F=21.091; p<0.001) and relative (F=10.15; p<0.001) values of maximal oxygen uptake (VO2max), and for absolute (F=3.90; p<0.05) and relative (F=4.1; p<0.05) values of oxygen uptake at the lactate threshold as well (VO2LT). Additionally, significant differences for this interaction were observed for minute ventilation (VEmax) (F=17.742; p<0.001), breathing frequency (BFmax) (F=5.484; p<0.01), respiratory exchange ratio (RERmax) (F=6.7; p<0.01), heart rate (HRmax) (F=4.49; p<0.05), and maximal power (WRmax) (F=13.5; p<0.001). Statistically significant differences in the group × training interaction were also found for maximal lactate concentration (LAmax) (F=4.2281, p<0.05), increments of this variable (ΔLA) during the graded exercise test (F=4.1110, p<0.05), post-exercise pH (F=6.141, p<0.05) and ΔpH (F=5.81, p<0.05). Analysis of the results from individual test trials showed statistically significant differences in the group × training interactions for trial time (TT) (F=6.84, p<0.001), average power (Pavg) expressed in both absolute (F=5.4823, p<0.01) and relative (F=4.1013, p<0.05) terms and average heart rate (HRavg) (F=8.42, p<0.001).
Furthermore, the type of group and training showed statistically significant differences in haematological variables: RBC (F=14.309, p<0.001), HGB (F=12.480, p<0.001), HCT (F=16.573, p<0.001), reticulocyte percentage (F=14.555, p<0.001), blood EPO levels (F=3.046, p<0.001), oxygen saturation of haemoglobin (SpO2) (F=73.198, p<0.001) and resting heart rate (HRrest) (F=52.1, p<0.001).
A statistically significant training effect was also demonstrated for power generated at lactate threshold (WRLT) (F=22.454, p<0.001), rate of blood lactate utilization after recovery (ΔLA12’res) (F= 9.514, p<0.001), changes in CK activity (F=18.375, p<0.001), and URIC concentration (F=21.465, p<0.001).
The training plan did not have a significant effect on changes in body mass or body composition.
Cardiorespiratory indices, maximal load and threshold load
A significant (p<0.001) increase in VO2max (by 4.8%) was observed in the G-LH-TL group and by 3.5% in the G-IHT group. Significant differences (p<0.01) were also found for the VO2LT (increase by 6.7% in the G-LH-TL group and by 9.1% in the G-IHT group) and WRmax (increases of 5.9% and 6.7%, respectively). The threshold load (WRLT) increased significantly by 4.8% for the G-LH-TL group and 7.7% in G-IHT. In the G-C group, the changes were statistically insignificant. Furthermore, significant (p<0.05) improvements in VEmax (7.1%), BFmax (9.2%) and RERmax (2.8 %) were observed in the G-IHT group. A significant post-test (p<0.01) reduction in HRmax (by 1.5%) was documented for the G-IHT group. No significant changes were found in HRLT. Mean values of indices recorded during the graded exercise test on the cycle ergometer are presented in Table 1.
TABLE 1.
Cardiopulmonary indices at maximal and threshold loads in the experimental groups (G-LH-TL, G-IHT) and the control group (G-C) before and after the intervention.
| Variable | G-LH-TL | G-IHT | G-C | |||
|---|---|---|---|---|---|---|
| Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | |
| WRmax (W) | 370 ± 18.6 |
391*** ± 18.6 |
385 ± 29.2 |
410*** ± 37.2 |
370 ± 24.2 |
374 ± 21.8 |
| WRLT(W) | 292 ±21.4 |
306* ±16.4 |
286 ± 25 |
308** ± 36.7 |
260 ± 21.1 |
268 ± 16.8 |
| VO2 max (l/min) | 4.58 ± 0.3 |
4.81*** ± 0.34 |
4.55 ± 0.28 |
4.71*** ± 0.33 |
4.54 ± 0.23 |
4.54 ± 0.21 |
| VO2max(ml/kg/min) | 66.0 ± 4.0 |
68.9*** ± 4.4 |
67.6 ± 2.7 |
69.9*** ± 1.4 |
67.0 ± 2.9 |
67.1 ± 2.7 |
| VO2LT(l/min) | 3.86 ±0.29 |
4.12*** ±0.26 |
3.72 ± 0.20 |
4.06*** ± 0.41 |
3.5 ± 0.18 |
3.59 ± 0.15 |
| VO2LT(ml/kg/min) | 55.9 ± 5.0 |
59.5*** ± 4.8 |
55.8 ± 3.5 |
60.0*** ± 1.6 |
51.6 ± 2.7 |
53.7 ± 3.0 |
| VEmax (l/min) | 165.1 ± 11.9 |
165.7 ± 12.7 |
169.6 ± 14.1 |
181.8*** ± 17.7 |
172.8 ± 14.5 |
169.8 ± 14.4 |
| BFmax (1/min) | 57.5 ± 3.9 |
58.8 ± 6 |
59.6 ± 6.5 |
65.0** ± 7.3 |
61.5 ± 7.2 |
60.4 ± 8.6 |
| RERmax | 1.09 ± 0.01 |
1.08 ± 0.02 |
1.09 ± 0.02 |
1.11*** ± 0.01 |
1.09 ± 0.01 |
1.09 ± 0.01 |
| HRmax (bpm) | 191 ± 3.1 |
192 ± 3.8 |
195 ± 5.1 |
192*** ± 3.7 |
196 ± 6.6 |
194 ± 4.9 |
p<0.05;
p<0.01;
<0.001 – statistically significant differences compared to the pre-test values.
WRmax – maximal power; WRLT – power at lactate threshold; VO2max – maximal oxygen uptake; VO2LT – oxygen uptake at lactate threshold; VEmax – maximal minute ventilation; BFmax – maximal breathing frequency; RERmax – respiratory exchange ratio; HRmax – maximal heart rate.
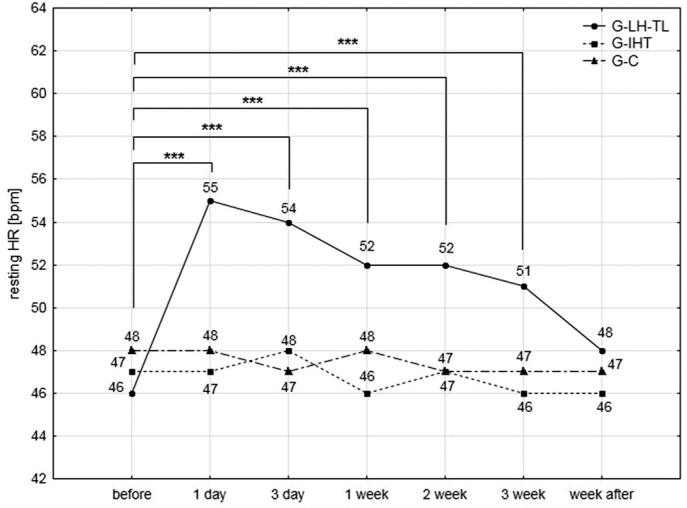
In the G-LH-TL group, HRrest increased significantly compared to the pre-test values. The highest rise (19.5%) was observed after the first night of staying in normobaric hypoxia conditions. During subsequent measurements, HRrest steadily decreased. After the recovery week, these values returned to the initial level. In other groups, changes were statistically insignificant (Fig. 1).
FIG. 1.
Changes in resting heart rates of studied athletes measured immediately after waking up in the lying position; ***<0.001 – statistically significant differences compared to the pre-test values.
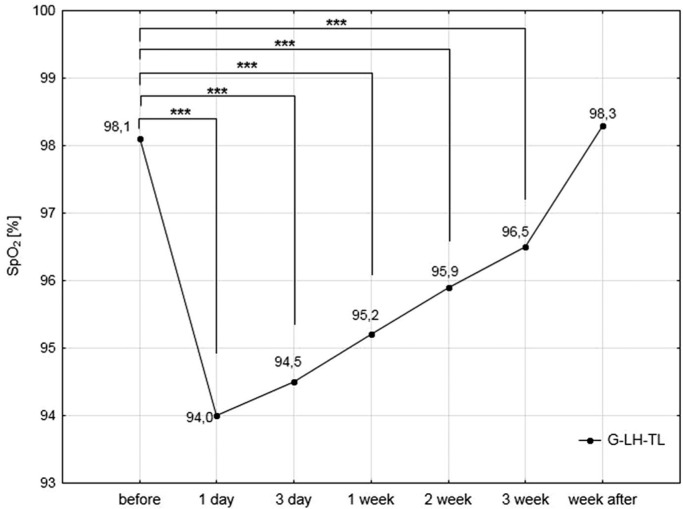
A significant (p<0.001) reduction in the SpO2 level was also documented in the G-LH-TL group during the experiment. The most substantial reduction (4.4%) was found after the first 12 hours of exposure to hypoxia. The next measurements showed a gradual increase in SpO2. However, the values of this variable during three weeks of exposure to hypoxia were significantly lower compared to initial measurements. No substantial differences in this range were observed after the recovery week in normoxic conditions (Fig. 2).
FIG. 2.
Oxygen saturation of haemoglobin (SpO2) measured immediately after waking up in the lying position in the experimental group (G-LH-TL) during the study; ***<0.001 – statistically significant differences compared to the pre-test values.
Exercise capacity
The results of the time trial (Table 2) show that the time was significantly (p<0.001) improved by 3.6% in the G-LH-TL group and by 2.5% in the G-IHT group, which was accompanied by a substantial increase in mean power (Pavg), by 5.7% and 5.2%, respectively. Furthermore, a small (1.7%) yet statistically significant reduction in HRavg was observed in the G-IHT group. In the G-C group, changes in the above variable were statistically insignificant.
TABLE 2.
Trial time (TT), average power (Pavg), and average heart rate (HRavg) registered in the study groups during the time trial, before and after completion of the experiment.
| Variable | G-LH-TL | G-IHT | G-C | |||
|---|---|---|---|---|---|---|
| Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | |
| Time Trial (s) | 3052 ± 120.8 |
2943*** ± 159.3 |
3073 ± 110.6 |
2999*** ± 158.0 |
3154.9 ± 94.0 |
3116.3 ± 75.1 |
| Pavg (W) | 316.3 ± 14.1 |
334.3*** ± 15.2 |
307.8 ± 20.8 |
323.8*** ± 35.6 |
298.0 ± 26.8 |
303.7 ± 18.4 |
| HRavg (bpm) | 182 ± 2.9 |
181 ± 3.4 |
179 ± 5.0 |
176** ± 4.2 |
175 ± 4.7 |
175 ± 6.7 |
p<0.01;
<0.001 – statistically significant differences compared to the pre-test values.
Resting erythropoietin levels and haematological indices
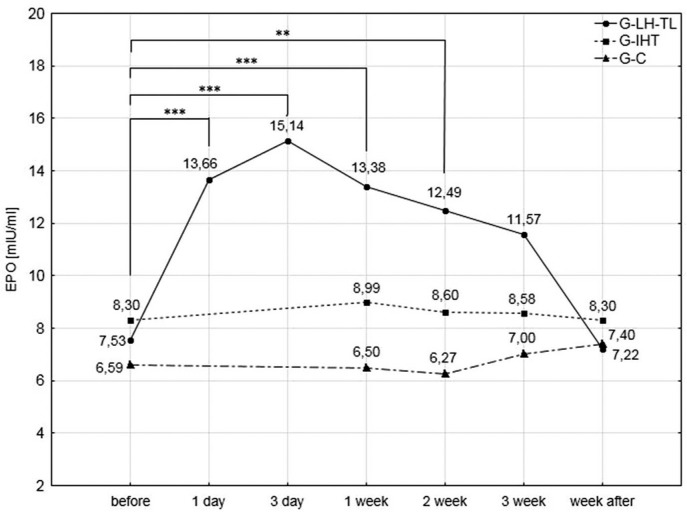
Analysis of blood serum erythropoietin (EPO) levels (Fig. 3) demonstrated the highest significant (p<0.001) increases after the first (81.4%) and third (101%) day of exposure to hypoxia in the G-LH-TL group. In subsequent measurements, serum EPO levels gradually declined. However, these changes were significantly (p<0.01) higher than the pre-test values (77.7% after a week, 66% after two weeks). After the third and the fourth week of the experiment, the changes were no longer statistically significant. No significant changes were demonstrated in the G-IHT and G-C groups in EPO levels.
FIG. 3.
Blood serum EPO levels in the experimental and control groups during the experiment; ** p<0.01; ***<0.001 – statistically significant differences with respect to initial examinations.
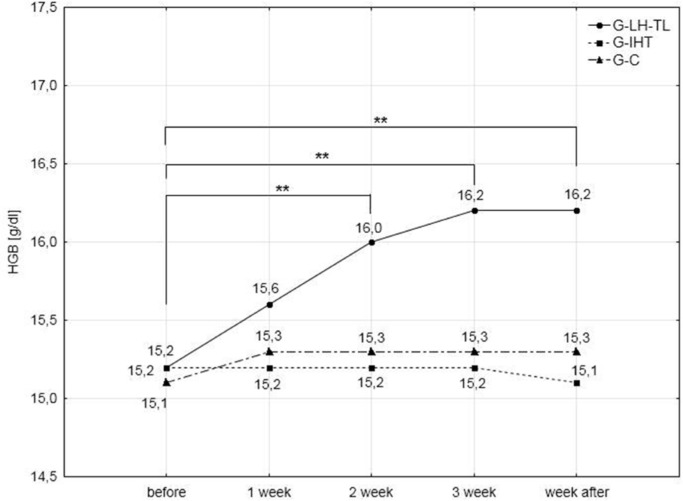
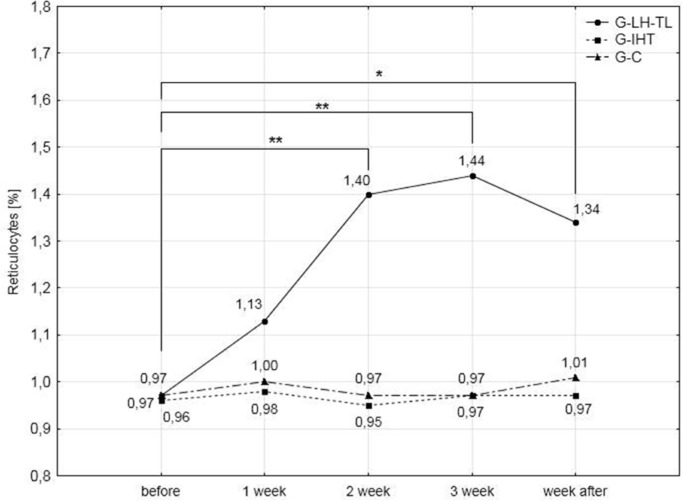
The results of the analysis of haematological variables (Table 3) demonstrated significant (p<0.01) improvements in RBC, HGB, HCT and blood reticulocyte percentage. Increases in RBC, HGB and HCT were observed after the second week of training. Continuation of the training procedure led to further insignificant increases in these variables. During the last series of examinations, the increase in RBC was 6.2%, HGB 6.6% and HCT 5.2% compared to pre-test values.
TABLE 3.
Haematological indices in the experimental and control groups during initial and final evaluations.
| Variable | G-LH-TL | G-IHT | G-C | |||
|---|---|---|---|---|---|---|
| Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | |
| RBC (mln/μl) | 4.98 ± 0.22 |
5.29** ± 0.25 |
4.94 ± 0.29 |
4.96 ± 0.22 |
5.01 ± 0.25 |
5.09 ± 0.23 |
| HGB (g/dl) | 15.2 ±0.68 |
16.2** ±0.75 |
15.2 ± 0.58 |
15.1 ± 0.30 |
15.1 ± 0.84 |
15.3 ± 0.66 |
| HCT (%) | 44.2 ±2.5 |
46.5** ±2.5 |
43.7 ±1.2 |
43.9 ±1.1 |
44.2 ±2.1 |
44.6 ±1.5 |
| Reticulocytes (%) | 0.97 ± 0.23 |
1.34* ± 0.15 |
0.96 ± 0.17 |
0.97 ± 0.12 |
0.97 ± 0.16 |
1.01 ± 0.1 |
p<0.05;
<0.01 – statistically significant differences compared to the pre-test values.
RBC – red blood cell count; HGB – hemoglobin concentration; HCT – hematocrit; Reticulocytes (%) - blood reticulocyte percentage
Blood reticulocyte percentage values showed a similar tendency for change, but they were reduced after the recovery week, although compared to the pre-test values they continued to be significantly higher (36.7%). Changes in the above indices were not observed in the G-IHT and G-C groups. Haemoglobin levels and blood reticulocyte percentage values are presented in Figs. 4 and 5.
FIG. 4.
Hemoglobin concentrations in the experimental and control groups during the experiment; ** p<0.01; – statistically significant differences with respect to initial examinations.
FIG. 5.
Percentage reticulocyte concentration in the experimental and control groups during the experiment; * p<0.05; **<0.01 – statistically significant differences with respect to initial examinations.
Lactate concentration and blood pH
Lactate concentration and selected indices of acid-base balance were also evaluated during the experiment (Table 4). The result demonstrated significant changes (p<0.01) in the G-IHT group concerning the following indices: LAmax (increase by 13%), ΔLA (increase by 15.9%) and post-exercise pH (reduction by 0.5%). ΔpH was reduced by 22.7% (p<0.05). A statistically significant reduction in ΔLA12’res was found in all experimental groups (38% in G-LH-TL, 40.6% in G-IHT, 51.5% in G-C).
TABLE 4.
Lactate concentration (LA) and blood pH in study participants during the graded exercise test before and after the training programme.
| Variable | G-LH-TL | G-IHT | G-C | |||
|---|---|---|---|---|---|---|
| Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | Before (x±SD) | After (x±SD) | |
| LAmax (mmol/l) | 10.56 ± 1.6 |
10.46 ± 1.71 |
9.61 ± 0.55 |
10.87** ± 1.02 |
9.58 ± 1.06 |
10.1 ± 1.28 |
| ΔLA (mmol/l) | 8.94 ± 1.66 |
8.88 ± 1.69 |
7.79 ± 0.65 |
9.03** ± 0.98 |
7.74 ± 0.82 |
8.23 ± 1.07 |
| ΔLA12’res (mmol/l) | -2.71 ± 0.21 |
-3.74** ± 0.85 |
-1.82 ± 0.72 |
-2.56** ± 0.53 |
-1.9 ± 0.59 |
-2.88** ± 0.46 |
| pHafter | 7.246 ± 0.037 |
7.239 ± 0.034 |
7.264 ± 0.017 |
7.227** ± 0.027 |
7.251 ± 0.039 |
7.239 ± 0.053 |
| ΔpH | 0.167 ± 0.032 |
0.175 ± 0.027 |
0.158 ± 0.028 |
0.194* ± 0.033 |
0.163 ± 0.037 |
0.184 ± 0.051 |
p<0.05;
<0.01 – statistically significant differences compared to the pre-test values.
LAmax – maximal lactate concentration, ΔLA – difference between maximal and resting lactate levels; ΔLA12’res – difference between maximal lactate concentration and the concentration after 12 min of recovery; pHpo – post-exercise blood pH; ΔpH – difference between resting and post-exercise blood pH.
Training load and biochemical indices of muscle fatigue
Statistical analysis demonstrated a significant (p<0.05) increase in the activity of blood serum creatine kinase (CK) after the second and the third week of training in all study groups. Furthermore, a significant (p<0.05) increase in CK activity was also noted after the first week. Despite the significant rise in the activity of this enzyme, the values ranged within standard values in all groups.
Similar changes were observed in uric acid concentration (URIC). Statistically significant (p<0.05) changes were observed after the second and the third week of training in all study groups. After the recovery microcycle, CK activity and URIC concentration returned to initial values. Changes in these indices are shown in Table 5.
TABLE 5.
Training load and changes in selected biochemical indices of muscle fatigue in particular study groups during the experiment.
| Variable | Group | Measurement | ||||
|---|---|---|---|---|---|---|
| Before (x±SD) | After (x±SD) | |||||
| Week 1 | Week 2 | Week 3 | Week 4 | |||
| Training load (TSS) | G-LH-TL | - | 1094 ±63 |
1147 ±73 |
1283 ±69 |
412 ±27 |
| G-IHT | - | 1128 ±49 |
1164 ±69 |
1276 ±76 |
387 ±22 |
|
| G-C | - | 1152 ±51 |
1194 ±76 |
1308 ±86 |
426 ±31 |
|
| CK (U/I) | G-LH-TL | 90.1 ± 34.3 |
139.3 ± 48.9 |
159.7* ± 58.3 |
161.7* ± 62.1 |
98.7 ± 27.7 |
| G-IHT | 110.9 ± 32.1 |
151.8 ± 52.6 |
168.1* ± 64.1 |
175.1* ± 52.7 |
115.8 ± 34.1 |
|
| G-C | 89.3 ±28.4 |
147.2* ± 42.6 |
158.8* ± 49.4 |
161.7* ± 55.7 |
108.4 ±32.2 |
|
| LDH (U/I) | G-LH-TL | 274.3 ±41.8 |
289.4 ±23.1 |
305.7 ±27.1 |
312.1 ±37.8 |
248.1 ±35.1 |
| G-IHT | 282.4 ± 39.4 |
295.1 ± 45.1 |
291.6 ± 42.2 |
321.7 ±48.1 |
278.2 ±37.1 |
|
| G-C | 302.4 ± 52.4 |
298.4 ± 48.1 |
318.14 ±46.1 |
335.1 ±56.3 |
296.7 ±51.4 |
|
| URIC (mg/dl) | G-LH-TL | 4.75 ±0.34 |
4.98 ±0.24 |
5.65* ±0.26 |
5.81* ±0.31 |
4.79 ±0.34 |
| G-IHT | 4.83 ±0.29 |
5.12 ±0.34 |
5.74* ±0.38 |
5.97* ±0.42 |
4.89 ±0.29 |
|
| G-C | 4.91 ±0.38 |
5.21 ±0.41 |
5.84* ±0.46 |
6.01* ±0.52 |
4.87 ±0.31 |
|
p<0.05 – statistically significant differences compared to the pre-test values.
CK – activity of creatine kinase, LDH – lactate dehydrogenase activity, URIC – uric acid activity.
DISCUSSION
The results of the study indicate that both LH-TL and IHT procedures combined with an individually selected training load of the same intensity for all participants, based on estimation of lactate threshold, led to substantial improvements in aerobic capacity (increases in VO2max by 4.8% in the G-LH-TL group and by 3.5% in the G-IHT group) and sports performance (TT improved by 3.6% and 2.5%, respectively) under normoxic conditions. However, various mechanisms of adaptation to hypoxia are stimulated depending on the altitude training regime. In the case of IHT the most important mechanisms involved were non-haematological, whereas in the case of LH-TL they were haematological. The second main outcome of our study is that haematological mechanisms were more effective.
The main benefit of the use of the LH-TL methodology is the improvement in oxygen capacity of blood. Levine and Stray-Gundersen [3], who developed the LH-TL method, demonstrated that this procedure speeds up erythropoiesis, thus improving aerobic capacity. It turns out that the important role that determines the final effect of erythropoiesis is played by an adequate dose of hypoxia (altitude and exposure time). Most studies that have used altitude training regimes with duration ranging from 100 to 200 hours have not found significant changes in either haemoglobin mass or red blood cell volume [20, 21, 22].
In our study, 30 hours of hypoxia (11-12 hours/day) was shown to be an effective stimulus leading to the improvement in haematological indices. During the last series of our evaluations, the increase in RBC was 6.2%, in HGB 6.6%, and in HCT 5.2%, compared to the pre-test values. These findings are consistent with previous studies which demonstrated that the minimal dose necessary for improvement in oxygen capacity of blood is 30 hours of exposure to hypoxia [5, 16, 23, 24, 25, 26].
The hypoxic dose in the IHT group was only 9 hours over the period of three weeks (3 times 60 min a week). No improvements in oxygen capacity of blood were found in this group. These findings and the previous studies [3, 8, 14 27, 28] provide evidence that this short exposure to hypoxia, although combined with intensive physical exercise, is insufficient to increase blood EPO levels and speed up erythropoiesis.
The strongest factor that contributes to the increased rate of erythropoietin secretion by kidneys is SpO2. It is known that the increase in EPO levels is directly proportional to the level of hypoxia and decline in oxygen saturation of haemoglobin [29, 30], which is consistent with our findings. During subsequent weeks of the experiment, SpO2 in the G-LH-TL group showed an upward tendency that was opposite to changes in EPO levels. Consequently, the effect of hypoxia on blood EPO levels became weaker with increasing acclimation, which was caused by the improvement in oxygen capacity of blood. However, it is worth emphasizing that, despite the decline in blood EPO levels, continuous production of red blood cells was observed, manifested by an increase in reticulocyte count, which is consistent with the findings of Mairbaurl [31].
In our study, reaction to the hypoxic stimulus was also noticeable in the changes of HRrest in the G-LH-TL group. Since the training load in individual weeks of our experiment and changes in biochemical indices of muscle fatigue (CK and URIC) in all study groups were similar, the changes in HRrest recorded for the G-LH-TL group lead to the conclusion that it is hypoxia dose rather than the training load or the accumulation of fatigue which provides the stimulus to modify resting heart rate.
The tendency for changes in HRrest, which was reversely proportional to changes in SpO2, provides evidence for the response to hypoxia and the related adaptations. This observation may be used in practice to control the adaptations of athletes to the training load typical of altitude exercise regimes.
It is assumed that hypoxia-induced stress combined with exercise-induced stress can contribute to greater adaptations in humans compared to the effects of training in normoxia [32]. This theory provides the basis for the intermittent hypoxic training concept (IHT). IHT does not cause an increase in oxygen capacity of blood, but it is likely to improve exercise capacity through reduction in energy expenditure, enhanced buffering capacity of muscle tissues and speeding up glycolytic enzyme activity [3, 33, 34]. The scope of these changes depends on the training load used during IHT.
The results of the experiment presented in this paper, with exercise intensity of the main part of each training session (IHT) at 100% WRLThyp and the hypoxic stimulus corresponding to the altitude of 2,100 m above sea level (FiO2 = 16.3%), showed a significant increase in VO2max (by 3.5%), and a shift in WRLT towards higher values (by 7.7%), which resulted in shorter trial times by 2.5%.
Most previous studies related to the IHT procedure focused on continuous exercise with low intensity, which did not improve aerobic capacity [35, 36, 37], or concerned high-intensity exercise with low training volume [38], which contributed only to the improvement in anaerobic capacity. Few studies have documented the effectiveness of IHT training based on high-intensity exercise (threshold and supra-threshold loads). In the theory of training methodology, this type of exercise is considered to be one of the most effective stimuli developing aerobic capacity. The results obtained in this study, our previous findings [3] and the findings documented by Dufour et al. [15] and Zoll et al. [16] have demonstrated the positive effect of the IHT methodology on aerobic capacity and sports performance. The characteristic feature of these studies was the use of threshold and supra-threshold intensity during training sessions in hypoxia.
Among the mechanisms behind the increase in sports performance induced by hypoxic training are enhanced aerobic metabolism in muscles and lower energy expenditure. However, previous studies are inconsistent as to whether altitude training has a beneficial effect on any of the above mechanisms [7, 16, 39]. Our previous studies [3] concerning hypobaric hypoxia showed improvements in the energy expenditure during exercise. However, hypoxia did not cause an increase in VO2max. The results obtained from the experiment do not lead to unequivocal conclusions that the energy expenditure improved following the exposure to hypoxia (LH-TL and IHT), since the increase in absolute WRLT values was accompanied by a significant increase in VO2LT. Furthermore, IHT is likely to have a positive effect on the cardiovascular system, expressed by reduced maximal heart rate (HRmax) and HR values observed during exercise.
Another potential mechanism whereby sports performance is enhanced after altitude training [40] is the ability of skeletal muscles to transport and buffer hydrogen ions (H+), which is an important regulator of pH and changes in acid-base balance. Publications by Mizuno et al. [41] and Saltin et al. [42] showed that hypoxic training can increase the buffering capacity of muscles in elite athletes.
In our study, the most significant changes in LA and pH during the graded exercise test were observed in the G-IHT group. Despite the largest increase in blood lactate concentration (ΔLA) and decline in blood pH (ΔpH), the largest increase was observed for WRmax (by ~7%). In the G-LH-TL group, an increase in WRmax (by ~6%) was also found, without changes in ΔLA and ΔpH. Furthermore, greater improvements in the rate of blood lactate utilization after recovery (ΔLA12’res) were observed in the experimental compared to the control group.
CONCLUSIONS
The results of the experiment show that normobaric hypoxia can be considered as an effective ergogenic aid that is likely to improve exercise capacity in normoxia. Both the live high, train low (LH-TL) method and intermittent hypoxic training (IHT) lead to improvements of aerobic capacity and sports performance in off-road cyclists. However, major changes in improvement in aerobic capacity and endurance performance occurred after LH-TL. This improvement is reflected by higher VO2max and haematological indices. In the case of IHT, the lactate threshold is also shifted towards higher training loads.
Positive effects of the IHT method depend on training intensity. Exercise performed with individually adjusted threshold intensity depending on the altitude (hypoxia) is effective in improving aerobic capacity and sports performance.
The 250-hour exposure to hypoxia (11-12 hour/day for three weeks) used in the LH-TL group is a sufficient stimulus to improve oxygen capacity of blood. During the IHT protocol, the exposure to hypoxia was too short (9 hours for three weeks), and insufficient to modify haematological indices.
Since the adaptive changes induced by the use of the LH-TL strategy and IHT protocol are attributable to different mechanisms, adequate combination of both approaches may lead to substantially greater improvements in aerobic capacity and performance in normoxia, particularly in sport disciplines that utilize both anaerobic and aerobic energy systems.
Acknowledgments
Supported by the grants No. 2013/09/B/NZ7/00726 from the National Science Centre of Poland and N RSA3 04153 from the Ministry of Science and Higher Education of Poland
REFERENCES
- 1.Czuba M, Zając A, Maszczyk A, Roczniok R, Poprzęcki S, Garbaciak W, et al. The effects of high intensity interval training in normobaric hypoxia on aerobic capacity in basketball players. J Hum Kinet. 2013;39(4):103–114. doi: 10.2478/hukin-2013-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michalczyk M, Czuba M, Zydek G, Zając A, Langfort J. Dietary recommendations for cyclists during altitude training. Nutrients. 2016;8(6):377. doi: 10.3390/nu8060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czuba M, Waskiewicz Z, Zajac A, Poprzecki S, Cholewa J, Roczniok R. The effects of intermittent hypoxic training on aerobic capacity and endurance performance in cyclists. J Sports Sci Med. 2011;10:175–183. [PMC free article] [PubMed] [Google Scholar]
- 4.de Paula P, Niebauer J. Effect of high altitude training on exercise capacity:fact or myth. Sleep Breath. 2012;16(1):233–239. doi: 10.1007/s11325-010-0445-1. [DOI] [PubMed] [Google Scholar]
- 5.Naeije R. Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis. 2010;52(6):456–66. doi: 10.1016/j.pcad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Townsend NE, Gore CJ, Ebert TR, Martin DT, Hahn AG, Chow CM. Ventilatory acclimatisation is beneficial for high-intensity exercise at altitude in elite cyclists. Eur J Sport Sci. 2016;16(8):895–902. doi: 10.1080/17461391.2016.1139190. [DOI] [PubMed] [Google Scholar]
- 7.Gore CJ, Hahn AG, Aughey RJ, Martin DT, Ashenden MJ, Clark SA, Garnham AP, Roberts AD, Slater GJ, McKenna MJ. Live high:train low increases muscle buffer capacity and submaximal cycling efficiency. Acta Physiol Scand. 2001;173:275–286. doi: 10.1046/j.1365-201X.2001.00906.x. [DOI] [PubMed] [Google Scholar]
- 8.Katayama K, Sato K, Matsuo H, Ishida K, Iwasaki K, Miyamura M. Effect of intermittent hypoxia on oxygen uptake during submaximal exercise in endurance athletes. Eur J Appl Physiol. 2004;92:75–83. doi: 10.1007/s00421-004-1054-0. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt L, Millet G, Robach P, Nicolet G, Brugniaux JV, Fouillot JP, Richalet JP. Influence of living high training low on aerobic performance and economy of work in elite athletes. Eur J Appl Physiol. 2006;97:627–636. doi: 10.1007/s00421-006-0228-3. [DOI] [PubMed] [Google Scholar]
- 10.Levine BD, Stray-Gundersen J. „Living high-training low”:effect of moderate-altitude acclimatization with low-altitude training on performance. J Appl Physiol. 1997;83:102–112. doi: 10.1152/jappl.1997.83.1.102. [DOI] [PubMed] [Google Scholar]
- 11.Chapman RF, Stray-Gundersen J, Levine BD. Individual variation in response to altitude training. J Appl Physiol. 1998;85:1448–1456. doi: 10.1152/jappl.1998.85.4.1448. [DOI] [PubMed] [Google Scholar]
- 12.Rusko H, Tikkanen H, Hamalainen I, Kalliokoski K, Puranen A. Effect of living in hypoxia and training in normoxia on sea level VO2max and red cell mass. Med Sci Sports Exerc. 1999;31:86. [Google Scholar]
- 13.Robertson EY, Saunders PU, Pyne DB, Gore CJ, Anson JM. Effectiveness of intermittent training in hypoxia combined with live high/train low. Eur J Appl Physiol. 2010;110:379–387. doi: 10.1007/s00421-010-1516-5. [DOI] [PubMed] [Google Scholar]
- 14.Roels B, Millet GP, Marcoux CJL, Coste O, Bentley DJ, Candau RB. Effects of hypoxic interval training on cycling performance. Med Sci Sports Exerc. 2005;37:138–146. doi: 10.1249/01.mss.0000150077.30672.88. [DOI] [PubMed] [Google Scholar]
- 15.Dufour SP, Ponsot E, Zoll J, Doutreleau S, Lonsdorfer-Wolf E, Geny B, Lampert E, Flück M, Hoppeler H, Billat V, Mettauer B, Richard R, Lonsdorfer J. Exercise training in normobaric hypoxia in endurance runners. I. Improvements in aerobic performance capacity. J Appl Physiol. 2006;100:1238–1248. doi: 10.1152/japplphysiol.00742.2005. [DOI] [PubMed] [Google Scholar]
- 16.Zoll J, Ponsot E, Dufour S, Doutreleau S, Ventura-Clapier R, Vogt M, Hoppeler H, Richard R, Fluck M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol. 2006;100:1258–1266. doi: 10.1152/japplphysiol.00359.2005. [DOI] [PubMed] [Google Scholar]
- 17.Bonetti DL, Hopkins WG. Sea-level exercise performance following adaptation to hypoxia:a meta-analysis. Sports Med. 2009;39:107–127. doi: 10.2165/00007256-200939020-00002. [DOI] [PubMed] [Google Scholar]
- 18.Cheng B, Kuipers H, Snyder AC, Keizer HA, Jeukendrup A, Hesselink M. A new approach for the determination of ventilator and lactate thresholds. Int J Sports Med. 1992;13:518–522. doi: 10.1055/s-2007-1021309. [DOI] [PubMed] [Google Scholar]
- 19.Hunter A, Coggan A. Training and Racing with a Power Meter. 2nd. USA: Velopress:Boulder, CO; 2010. [Google Scholar]
- 20.Ashenden MJ, Gore CJ, Dobson GP, Hahn AG. ‘Live high, train low’ does not change the total haemoglobin mass of male endurance athletes sleeping at a simulated altitude of 3000 m for 23 nights. Eur J Appl Physiol. 1999;80:479–484. doi: 10.1007/s004210050621. [DOI] [PubMed] [Google Scholar]
- 21.Ashenden MJ, Gore CJ, Martin DT, Dobson GP, Hahn AG. Effects of a 12-day ‘live high, train low’ camp on reticulocyte production and haemoglobin mass in elite female road cyclists. Eur J Appl Physiol. 1999;80:472–478. doi: 10.1007/s004210050620. [DOI] [PubMed] [Google Scholar]
- 22.Laitinen H, Alopaeus K, Heikkinen R, Hietanen H, Mikkelsson L, Tikkanen H. Acclimatization to living in normobaric hypoxia and training in normoxia at sea level in runners. Med Sci Sports Exerc. 1995;27:109. [Google Scholar]
- 23.Dehnert C, Hütler M, Liu Y, Menold E, Netzer C, Schick R, Kubanek B, Lehmann M, Böning D, Steinacker JM. Erythropoiesis and performance after two weeks of living high and training low in well trained triathletes. Int J Sports Med. 2002;23(8):561–566. doi: 10.1055/s-2002-35533. [DOI] [PubMed] [Google Scholar]
- 24.Wehrlin JP, Zuest P, Hallen J, Marti B. Live High - Train Low for 24 days increases hemoglobin mass and red cell volume in elite endurance athletes. J Appl Physiol. 2006;100:1938–1945. doi: 10.1152/japplphysiol.01284.2005. [DOI] [PubMed] [Google Scholar]
- 25.Clark AS, Quod MJ, Clark MA, Martin DT, Saunders PU, Gore CJ. Time course of haemoglobin mass during 21 days live high:train low simulated altitude. Eur J Appl Physiol. 2009;106(3):399–406. doi: 10.1007/s00421-009-1027-4. [DOI] [PubMed] [Google Scholar]
- 26.Gough CE, Saunders PU, Fowlie J, Savage B, Pyne DB, Anson JM, Wachsmuth N, Prommer N, Gore CJ. Influence of altitude training modality on performance and total haemoglobin mass in elite swimmers. Eur J Appl Physiol. 2012;112(9):3275–3285. doi: 10.1007/s00421-011-2291-7. [DOI] [PubMed] [Google Scholar]
- 27.Knaupp W, Khilnani S, Sherwood J, Scharf S, Steinberg H. Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol. 1992;73:837–840. doi: 10.1152/jappl.1992.73.3.837. [DOI] [PubMed] [Google Scholar]
- 28.Katayama K, Sato K, Matsuo H, Ishida K, Iwasaki K, Miyamura M. Effect of intermittent hypoxia on oxygen uptake during submaximal exercise in endurance athletes. Eur J Appl Physiol. 2004;92:75–83. doi: 10.1007/s00421-004-1054-0. [DOI] [PubMed] [Google Scholar]
- 29.Eckardt KU, Boutellier U, Kurtz A, Schopen M, Koller EA, Bauer C. Rate of erythropoietin formation in humans in response to acute hypobaric hypoxia. J Appl Physiol. 1989;66(4):1785–1788. doi: 10.1152/jappl.1989.66.4.1785. [DOI] [PubMed] [Google Scholar]
- 30.Berglund B. High-altitude training:aspects of hematological adaptation. Sports Med. 1992;14:289–303. doi: 10.2165/00007256-199214050-00002. [DOI] [PubMed] [Google Scholar]
- 31.Mairbaurl H. Red blood cell function in hypoxia at altitude and exercise. Inter J Sports Med. 1994;15(2):51–63. doi: 10.1055/s-2007-1021020. [DOI] [PubMed] [Google Scholar]
- 32.Wolski LA, McKenzie DC, Wenger HA. Altitude training for improvements in sea level performance:is the scientific evidence of benefit? Sports Med. 1996;22:251–263. doi: 10.2165/00007256-199622040-00004. [DOI] [PubMed] [Google Scholar]
- 33.Desplanches D, Hoppeler H, Linossier MT, Denis C, Claassen H, Dormois D, Lacour JR, Geyssant A. Effects of training in normoxia and normobaric hypoxia on human muscle ultrastructure. Pflug Arch Eur J Phy. 1993;425:263–267. doi: 10.1007/BF00374176. [DOI] [PubMed] [Google Scholar]
- 34.Vogt M, Puntschart A, Geiser J, Zuleger C, Billeter R, Hoppeler H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol. 2001;91:173–182. doi: 10.1152/jappl.2001.91.1.173. [DOI] [PubMed] [Google Scholar]
- 35.Vallier JM, Chateau P, Guezennec CY. Effects of physical training in a hypobaric chamber on the physical performance of competitive triathletes. Eur J Appl Physiol O. 1996;73(5):471–478. doi: 10.1007/BF00334426. [DOI] [PubMed] [Google Scholar]
- 36.Hendriksen IJM, Meeuwsen T. The effect of intermittent training in hypobaric hypoxia on sea-level exercise:a cross-over study in humans. Eur J Appl Physiol. 2003;88:396–403. doi: 10.1007/s00421-002-0708-z. [DOI] [PubMed] [Google Scholar]
- 37.Hamlin MJ, Marshall CH, Hellemans J, Ainslie PN, Anglem N. Effect of intermittent hypoxic training on a 20 km time trial and 30 s anaerobic performance. Scan J Med Sci Sports. 2010;20(4):651–661. doi: 10.1111/j.1600-0838.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 38.Morton JP, Cable NT. The effects of intermittent hypoxic training on aerobic and anaerobic performance. Ergonomics. 2005;48:1535–1546. doi: 10.1080/00140130500100959. [DOI] [PubMed] [Google Scholar]
- 39.Lundby C, Calbet JA, Sander M, van Hall G, Mazzeo RS, Stray-Gundersen J, Stager JM, Chapman RF, Saltin B, Levine BD. Exercise economy does not change after acclimatization to moderate to very high altitude. Scand J Med Sci Sports. 2007;17:281–291. doi: 10.1111/j.1600-0838.2006.00530.x. [DOI] [PubMed] [Google Scholar]
- 40.Gore CJ, Clark SA, Saunders PU. Nonhematological Mechanisms of Improved Sea-Level Performance after Hypoxic Exposure. Med Sci Sports Exerc. 2007;39(9):1600–1609. doi: 10.1249/mss.0b013e3180de49d3. [DOI] [PubMed] [Google Scholar]
- 41.Mizuno M, Juel C, Bro-Rasmussen T, Mygind E, Schibye B, Rasmussen B, Saltin B. Limb skeletal muscle adaptation in athletes after training at altitude. J Appl Physiol. 1990;68:496–502. doi: 10.1152/jappl.1990.68.2.496. [DOI] [PubMed] [Google Scholar]
- 42.Saltin B, Larsen H, Terrados N, Bangsbo J, Bak T, Kim CK, Svedenhag J, Rolf CJ. Morphology, enzyme activities and buffer capacity in leg muscles of Kenyan and Scandinavian runners. Scan J Med Sci Sports. 1995;5:222–230. doi: 10.1111/j.1600-0838.1995.tb00038.x. [DOI] [PubMed] [Google Scholar]