Short abstract
Objective
To explore the etiology of human oocyte maturation arrest in two infertile Chinese sisters.
Methods
Clinical examination and genetic testing of all available family members were conducted, and the findings were used to create a pedigree. Mutation screening using PCR amplification and DNA Sanger sequencing of the entire tubulin beta 8 class VIII gene (TUBB8) including intron–exon boundaries was performed to identify mutations.
Results
A novel missense TUBB8 mutation (c.1054G > T, p.A352S) in the patient and her elder sister was detected and shown to be associated with oocyte maturation arrest.
Conclusion
Our findings expand the known mutation spectrum of TUBB8 and provide insights into the etiology of human oocyte maturation arrest.
Keywords: Oocyte maturation arrest, tubulin beta 8 class VIII, missense mutation, genetic pedigree, multiple sequence alignment, pathogenicity
Introduction
In the fetal ovary, oocytes in the primordial follicle initiate meiosis, and pause at prophase I. Until puberty, the oocytes begin the maturation process and resume meiosis in response to a surge of luteinizing hormone (LH), with germinal vesicle breakdown followed by spindle assembly, chromosome migration, asymmetric division, and extrusion of the first polar body. At this point, the oocytes are mature and arrest at metaphase II until fertilization.1,2
Successful mammalian reproduction requires the fusion of a mature oocyte with a sperm cell. Although it is common for a small number of oocytes to be immature after ovary stimulation, human chorionic gonadotropin (hCG) administration, and in vitro culture in assisted reproduction treatments, it is rare for all retrieved oocytes to remain immature. Indeed, only a few cases of human oocyte maturation arrest have been reported in the literature.3–10 Oocyte maturation arrest may occur at various stages of the cell cycle, such as the germinal vesicle, metaphase I, and metaphase II stage, but little is known about the underlying genetic pathology.
Recently, mutations in the primate-specific tubulin beta 8 class VIII gene (TUBB8) were identified as causative of human oocyte maturation arrest. TUBB8 is expressed specifically in oocytes and early embryos. A total of 26 TUBB8 mutations have been reported, including 21 heterozygous missense mutations, two homozygous missense mutations, and three homozygous deletion mutations. These mutations cause varying defects leading to arrest in human oocyte maturation, fertilization, or early embryonic development. Heterozygous missense mutations in TUBB8 may have dominant-negative effects that affect the formation of the α/β-tubulin heterodimer, disrupt microtubule dynamics, and interfere with spindle assembly, leading to maturation arrest of mouse and human oocytes.11–14
Here, we report a case of a patient and her sister with primary infertility caused by oocyte maturation arrest. Mutation screening of TUBB8 revealed a novel heterozygous missense mutation that was causative of disease.
Patients and methods
Patient
This study was approved by the institutional ethics committee of the Affiliated Suzhou Hospital of Nanjing Medical University. Written informed consent was obtained from all participants. All available members of the patient’s family were subjected to a full medical history evaluation and comprehensive physical examination. A pedigree of the family was constructed after clinical examination and genetic testing.
Mutational analysis
Peripheral blood was collected from the patient, her elder sister, and their mother. Genomic DNA was extracted from the blood samples using the QIAamp DNA Blood Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. All four exons and intron–exon boundaries of TUBB8 were amplified by PCR using Faststart Taq DNA polymerase (Roche, Basel, Switzerland). The PCR amplification program included an initial denaturation at 94°C for 5 minutes, followed by 16 cycles of denaturation at 94°C for 45 s, annealing for 45 s at 68°C then decreasing by 0.5°C each cycle, and extension at 72°C for 45 s, followed by 20 cycles of denaturation at 94°C for 45 s, annealing at 56°C for 45 s, extension at 72°C for 1 minute, a final extension at 72°C for 7 minutes, and holding at 4°C. The amplified DNA fragments were purified and sequenced in both directions using an ABI 3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The resulting sequences were compared with the reference sequence of TUBB8 (NM_177987.2) in the NCBI database.
In silico analysis of mutations
Multiple sequence alignment of the TUBB8 protein and its orthologs was performed using MUSCLE alignment software (http://www.drive5.com/muscle/).15 The mutations were analyzed according to the Standards and Guidelines for the Interpretation of Sequence Variants released by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology.16 The corresponding mutations were searched in the following databases: dbSNP (http://www.ncbi.nlm.nih.gov/SNP/), Exome Aggregation Consortium (ExAC) (http://exac.broadinstitute.org/), the Genome Aggregation Database (gnomAD) (http://gnomad.broadinstitute.org/), the 1000 Genomes Project (http://www.1000genomes.org/), and Chinese genomes in diseaseDX (http://diseasedx.virgilbio.com/). Mutation pathogenicity was predicted by Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/)17 and Mutation Taster (http://www.mutationtaster.org/) prediction algorithms.18
Results
Clinical data
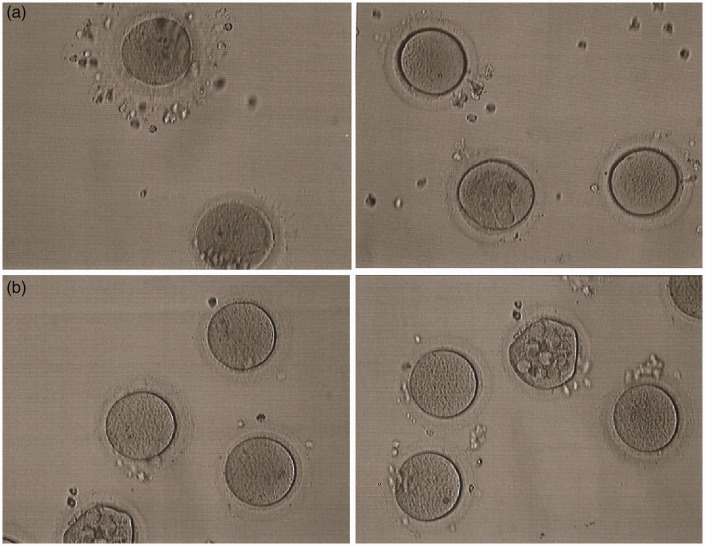
A 30-year-old female with a 6-year history of primary unexplained infertility was referred to our center. Routine infertility investigation revealed that her ovulatory cycle was regular, and that her fallopian tubes were patent. The concentrations of endocrine hormones in her serum were normal: follicle stimulating hormone (FSH) 7.14 mIU/mL, LH 4.89 mIU/mL, estradiol 55 pg/mL, testosterone 0.24 ng/mL, and anti-Mullerian hormone 1.95 ng/mL. The sperm concentration, motility, and morphology of her spouse were normal, and the karyotypes of both individuals were normal. The patient underwent a failed in vitro fertilization (IVF) attempt at another IVF center, in which 18 oocytes were retrieved but none were fertilized. On her second IVF attempt in our center, her pituitary was downregulated with gonadotropin-releasing hormone agonist, then her ovaries were stimulated for 10 days with a total dosage of 1725 IU gonadotropins, including 975 IU FSH and 750 IU human menopausal gonadotropin (HMG). Then, 6750 IU hCG was administered, at which point serum estradiol concentrations were 4335 pg/mL; 36 h later, 14 oocytes were aspirated by ultrasound-guided follicular puncture. The oocytes were divided into two equal groups; those in group I were stripped of their cumulus cells before intracytoplasmic sperm injection (ICSI), and were at the meiosis I stage. Twenty-four h after ICSI, no signs of fertilization were observed, and the seven oocytes were still at the meiosis I stage (Figure 1a). The remaining seven oocytes in group II were cultured overnight in culture medium supplemented with 75 mIU/mL HMG for in vitro maturation. However, 24 h later no polar body was extruded. After culture for a further 24 h, all seven oocytes were stripped of their cumulus cells and examined. Six oocytes were shown to have remained at the meiosis I stage, and one oocyte exhibited abnormal morphology (Figure 1b). Therefore ICSI was cancelled. The patient’s elder sister also presented with primary unexplained infertility and experienced a failed IVF attempt at another IVF center.
Figure 1.
Phenotypes of oocytes from the patient with maturation arrest. (a) The retrieved oocytes examined by light microscopy after stripping of cumulus cells. (b) The oocytes observed by light microscopy 48 h after in vitro maturation treatment
Genetic analysis
A pedigree of this family is shown in Figure 2a. Sequencing analysis identified the heterozygous TUBB8 mutation c.1054G > T in both sisters, which was not detected in their mother (Figure 2a). Because of a lack of genetic information regarding their father, the inheritance pattern of this mutation is unknown. c.1054G > T is a novel missense mutation found within exon 4 of TUBB8, and is responsible for converting a highly conserved alanine residue to a serine in the TUBB8 protein (p.A352S). This mutation is not found in dbSNP, ExAC, gnomAD, 1000 Genomes Project, or diseaseDX databases. Additionally, the alanine residue is evolutionarily conserved among primate species (Figure 2b), and the p.A352S mutation is predicted to be probably damaging by Polyphen-2 and disease-causing by Mutation Taster.
Figure 2.
Genetic analysis of the family. (a) The pedigree of the family. Slash indicates a deceased individual. Sanger sequencing chromatographs of TUBB8 in available family members revealing a heterozygous mutation in the patient and her elder sister, as indicated by red arrows. (b) Sequence alignment of the TUBB8 protein and its homologs from five primate species revealing conservation of the affected amino acid, as indicated by the blue frame
Discussion
In this study, the patient’s ovaries showed a normal response to gonadotropins, and the follicular growth rate was normal, suggesting that the oocyte maturation arrest could not be attributed to the ovarian stimulation protocol. Additionally, the regular menstrual cycle, patent fallopian tubes, and primary infertility of this patient whose spouse has normal semen suggested that the oocyte maturation arrest occurred in natural cycles. It has been reported that the supplementation of gonadotropins in culture medium augments the maturation and fertilization of human immature oocytes in vitro.19 However, in vitro maturation treatment by HMG supplementation did not promote maturation of the patient’s oocytes after a 48-h culture.
TUBB8 is composed of four exons and three introns, and most of the previously identified mutations in TUBB8 are located in exon 4. In this study, a novel heterozygous mutation, c.1054G > T, in exon 4 of TUBB8 was identified in the patient with oocyte maturation arrest and her elder sister. c.1054G > T caused an amino acid change from a hydrophobic alanine residue to a hydrophilic serine residue in the TUBB8 protein (p.A352S), which is predicted to be damaging. Furthermore, the alanine residue is highly conserved among primate species, while p.A352S is located next to the previously identified heterozygous missense mutation p.V353I which was responsible for arresting four out of eight oocytes at meiosis I.14 A352 and V353 are buried within the β-tubulin structure, and mutations at these amino acids could affect the folding or stability of β-tubulin, thus impairing microtubule behavior and spindle assembly by dominant negative effects. Therefore, we propose that the A352S mutation affects microtubule dynamics and spindle assembly in a similar way to the V353I mutation.
In conclusion, we identified a novel missense mutation of TUBB8 in a patient with oocyte maturation arrest. This finding not only substantiates the key role of TUBB8 in human oocyte maturation, but also extends the spectrum of TUBB8 mutations.
Acknowledgements
We thank the families for participating in this research project. This work is supported by Jiangsu Provincial Medical Innovation Team (CXTDB2017013), Jiangsu Provincial Medical Youth Talent (QNRC2016238), Jiangsu Provincial Commission of Health and Family Planning (H2017073), Suzhou Key Medical Center (Szzx201505), and the Suzhou Industry Technology Innovation Project (SYS201770).
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol 2013; 14: 141–152. [DOI] [PubMed] [Google Scholar]
- 2.Coticchio G, Dal Canto M, Mignini Renzini M, et al. Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization. Hum Reprod Update 2015; 21: 427–454. [DOI] [PubMed] [Google Scholar]
- 3.Rudak E, Dor J, Kimchi M, et al. Anomalies of human oocytes from infertile women undergoing treatment by in vitro fertilization. Fertil Steril 1990; 54: 292–296. [DOI] [PubMed] [Google Scholar]
- 4.Eichenlaub-Ritter U, Schmiady H, Kentenich H, et al. Recurrent failure in polar body formation and premature chromosome condensation in oocytes from a human patient: indicators of asynchrony in nuclear and cytoplasmic maturation. Hum Reprod 1995; 10: 2343–2349. [DOI] [PubMed] [Google Scholar]
- 5.Hartshorne G, Montgomery S, Klentzeris L. A case of failed oocyte maturation in vivo and in vitro. Fertil Steril 1999; 71: 567–570. [DOI] [PubMed] [Google Scholar]
- 6.Levran D, Farhi J, Nahum H, et al. Maturation arrest of human oocytes as a cause of infertility: case report. Hum Reprod 2002; 17: 1604–1609. [DOI] [PubMed] [Google Scholar]
- 7.Schmiady H, Neitzel H. Arrest of human oocytes during meiosis I in two sisters of consanguineous parents: first evidence for an autosomal recessive trait in human infertility: Case report. Hum Reprod 2002; 17: 2556–2559. [DOI] [PubMed] [Google Scholar]
- 8.Chen ZQ, Ming TX, Nielsen HI. Maturation arrest of human oocytes at germinal vesicle stage. J Hum Reprod Sci 2010; 3: 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergere M, Lombroso R, Gombault M, et al. An idiopathic infertility with oocytes metaphase I maturation block: case report. Hum Reprod 2001; 16: 2136–2138. [DOI] [PubMed] [Google Scholar]
- 10.Harrison KL, Sherrin DA, Keeping JD. Repeated oocyte maturation block. J Assist Reprod Genet 2000; 17: 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng R, Yan Z, Li B, et al. Mutations in TUBB8 cause a multiplicity of phenotypes in human oocytes and early embryos. J Med Genet 2016; 53: 662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng R, Sang Q, Kuang Y, et al. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med 2016; 374: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, Tong X, Luo L, et al. Mutation analysis of the TUBB8 gene in nine infertile women with oocyte maturation arrest. Reprod Biomed Online 2017; 35: 305–310. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Li B, Li D, et al. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod 2017; 32: 457–464. [DOI] [PubMed] [Google Scholar]
- 15.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 2004; 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods 2010; 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz JM, Cooper DN, Schuelke M, et al. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods 2014; 11: 361–362. [DOI] [PubMed] [Google Scholar]
- 19.Prins GS, Wagner C, Weidel L, et al. Gonadotropins augment maturation and fertilization of human immature oocytes cultured in vitro. Fertil Steril 1987; 47: 1035–1037. [DOI] [PubMed] [Google Scholar]