Short abstract
Objectives
This study aimed to investigate the usefulness and tolerability of topical tacrolimus in paediatric vulvar lichen sclerosus (LS). We examined whether there was improvement of the most problematic symptoms, such as itching, pain, and vulvar constipation.
Methods
Ten girls, aged from 4 to 9 years old who were affected by vulvar LS, were enrolled in an open clinical study to confirm the efficacy of tacrolimus 0.03% ointment to treat LS. Tacrolimus was applied twice a day for 6 weeks and then stopped during the follow-up period. The study duration included 6 weeks of treatment and 6 weeks of follow-up. A literature search of the PubMed (MEDLINE) database was conducted of reports published since 1 January, 2004.
Results
Our study and previous studies indicated the potential effectiveness of tacrolimus in LS. Treatment with topical tacrolimus was well tolerated with significant improvement of itching, pain, and constipation.
Conclusion
Tacrolimus may be a safe and effective alternative treatment, without the risk of corticosteroid-related vulvar atrophy, for paediatric vulvar LS. LS could become a further indication of topical tacrolimus therapy if these promising results are confirmed in the future.
Keywords: Children, dermatology, vulvar lichen sclerosus, tacrolimus, itching, topical ointment
Introduction
Lichen sclerosus (LS) is a rare, chronic, inflammatory skin condition, involving the anogenital area of pre-pubertal girls and post-menopausal women. The prevalence of LS in the female paediatric population is estimated to be 1/900, with a mean age of onset of 5 years.1 LS is characterized by distinct, itchy, ivory-rose plaques, encircling the vagina and anus, with a figure of eight shape. Plaques can be sclerotic, with a shiny “cigarette paper” appearance, or thickened, for repeated excoriations. Scarring atrophy may cause labial fusion and loss of pigmentation.1 Dermatoscopic findings in LS are characterized by pink/yellow/white structureless areas that are associated with a diffuse whitish background and comedo-like lesions.2 Diagnosis of LS is clinical. However, in case of equivocal macroscopic findings, the suspicion of neoplastic change or disease resistance to adequate treatment, and the need to switch to a second-line therapy, biopsy is strongly recommended.3 A histological examination often shows nonspecific features, especially in the early stages of LS. Adnexal structures present with luminal hyperkeratosis and hypergranulosis, with mild, irregular, and psoriasiform acanthosis and focal dermo-epidermal membrane thickening (Figure 1).4 Although the aetiology of LS remains unclear, its genetic predisposition has been documented. Autoimmune conditions, such as alopecia areata, Hashimoto’s thyroiditis, vitiligo, pernicious anaemia, and morphea, are also related to LS.5 The first-line treatment for LS is ultra-potent corticosteroids, which are extremely effective in reducing clinical signs. However, long-term application of steroids can lead to irritation, erythema, allergic dermatitis, secondary infections, atrophy, telangiectasia, and tachyphylaxis.1 Several studies have reported the efficacy of topical immunomodulators in children by inhibiting T lymphocyte activity, which reduces inflammation.5
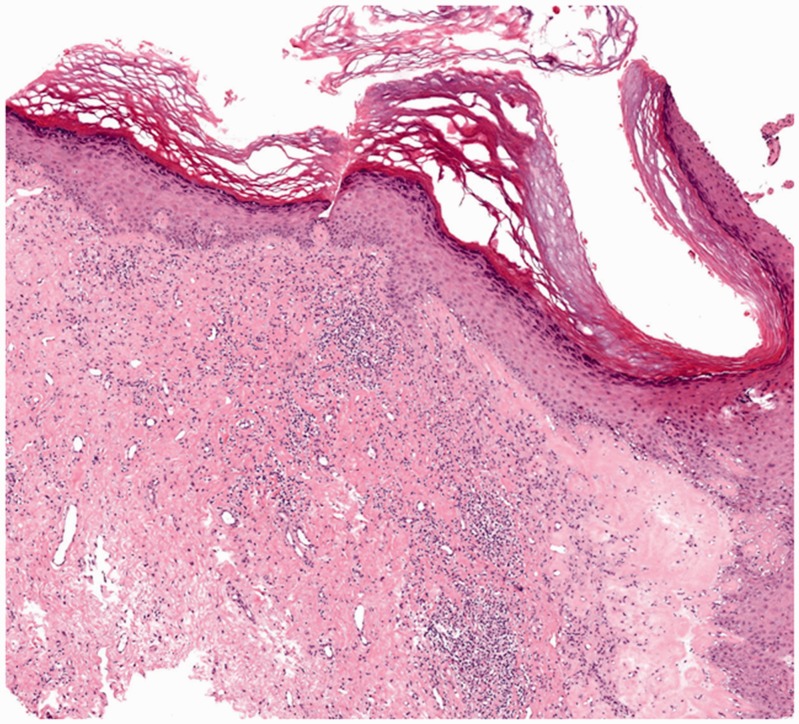
Figure 1.
Histological image of a patient with vulvar lichen sclerosus. Orthokeratotic hyperkeratosis of the epithelium and vacuolization of keratinocytes of the basal layer can be seen. Additionally, focal homogenization of the papillary dermis, perivascular, often band-like, infiltrate of lymphocytes, and plasma cells in the dermis can be seen (haematoxylin–eosin stain, ×40).
We report a case series of 10 girls who were affected by vulvar LS and treated by tacrolimus 0.03% ointment for 6 weeks. Complete remission of signs and symptoms was obtained from the first week of treatment in all of the patients, without any local side effects. A literature search of the PubMed (MEDLINE) database was also conducted to search for relevant English literature published since 1 January, 2004.
Case series
Ten girls aged from 4 to 9 years who were affected by vulvar LS were enrolled in an open clinical study to examine the efficacy of tacrolimus 0.03% ointment to treat LS. The study was conducted following the ethical guidelines of the Helsinki Declaration and each family signed written informed consent for the patients. Tacrolimus was applied twice a day for 6 weeks and then stopped during the follow-up period. The study duration included 6 weeks of treatment and 6 weeks of follow-up. The girls complained of itching and burning pain in the vulvar area, dysuria, and constipation, with a mean duration of symptoms from 6 to 9 months. At physical examinations, we observed pink and whitish skin areas, erosions, ecchymosis, and lichenification. We collected the past medical and family history from each patient. Type-1 diabetes mellitus and annular granuloma were found in three patients, while lichen ruber planus and thyroiditis were found in the family history of two patients. Laboratory tests showed normal results. Patients were also consulted by a child psychiatrist who excluded sexual abuse. After obtained informed consent from the parents, the girls started local medication with tacrolimus twice a day for 6 weeks in association with emollient cream in affected areas. A clinical examination and recording of the patients’ symptoms were performed at baseline, at days 7, 14, and 28, at the end of the treatment, after 6 weeks, and during follow-up at 3 and 6 weeks. Itching and burning completely disappeared after 2 weeks, while skin lesions were in remission at 1 week after beginning treatment, with residual milia. No local and systemic side effects were recorded.
Discussion and literature review
LS is a chronic inflammatory fibrotic skin disorder of unknown aetiology. The most common clinical presentation in girls and women is vulvar or perianal itching, and other symptoms include purpura, dysuria, constipation, and genital erosions. Paediatricians should be alert to early diagnosis of LS and consider it in the differential diagnosis of other anogenital conditions.2 British guidelines for management of LS suggest use of corticosteroids as the first recommendation.6 However, at least six published reports have described the usefulness of tacrolimus in treatment of paediatric vulvar lichen.7–12
A previous study examined use of low-dose tacrolimus for treatment of paediatric vulvar LS for 10 months and showed its efficacy and no severe side effects.7 Funaro et al investigated inhibitory effects of inflammation mediated by tacrolimus, which avoided most of the well-known steroid-induced side effects.9 Recent multicentre, randomized studies reported safe and possibly efficient long- and short-term topical tacrolimus treatment of LS in both sexes.8,9 In a recent phase 2 study, 43% of the patients had total clearance and 34% had partial clearance of LS lesions, and 9% of lesions recurred during follow-up.10 The time to clearance varied considerably between 10 and 24 weeks and the total application period ranged from 1.5 to 10 months. These therapeutic studies indicate the potential effectiveness of tacrolimus in LS. In our patients, treatment with topical tacrolimus was well tolerated and it was not associated with rebound flare at the end of treatment. We found considerable improvement of the most problematic symptoms, such as itching, pain, and vulvar constipation.
The long-term use of calcineurin inhibitors and their safety have been well documented in many controlled trials. An observational retrospective study on 21,000 patients affected by atopic dermatitis showed the long term efficacy of calcineurin inhibitors and the absence of skin cancer and lymphoproliferative tumours in these patients.13 Recently, Ribero and Borradori evaluated the development of squamous cell carcinoma during use of topical tacrolimus in oral lichen planus and the risk of absorption after application, and reporting the same risk of squamous cell carcinoma as for the general population.14 Treatment failure frequently occurs in children with LS and is usually secondary to behavioural problems or parental anxiety. Use of this alternative therapeutic approach provides relief to patients and potentially avoids unnecessary testing.
Conclusions
Tacrolimus may be a safe and effective alternative treatment, without the risk of corticosteroid-related vulvar atrophy, for paediatric vulvar LS. This topical ointment is well tolerated in the anogenital area, and improves quality of life and provides relief to patients and their family. LS could become a further indication of topical tacrolimus therapy if these promising results are confirmed in the future. However, long-term follow-up of this treatment for LS is mandatory and new controlled, clinical trials are required to confirm its effectiveness.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Kreuter A, Kryvosheyeva Y, Terras S, et al. Association of autoimmune diseases with lichen sclerosus in 532 male and female patients. Acta Derm Venereol 2013; 93: 238–241. [DOI] [PubMed] [Google Scholar]
- 2.Borghi A, Corazza M, Minghetti S, et al. Dermoscopic Features of Vulvar Lichen Sclerosus in the Setting of a Prospective Cohort of Patients: New Observations. Dermatology 2016; 232: 71–77. [DOI] [PubMed] [Google Scholar]
- 3.Regauer S, Liegl B, Reich O. Early vulvar lichen sclerosus: a histopathological challenge. Histopathology 2005; 47: 340–347. [DOI] [PubMed] [Google Scholar]
- 4.LeBoit PE. A thickened basement membrane is a clue to … lichen sclerosus!. Am J Dermatopathol 2000; 22: 457. [DOI] [PubMed] [Google Scholar]
- 5.Powell J, Wojnarowska F, Winsey S, et al. Lichen sclerosus premenarche: autoimmunity and immunogenetics. Br J Dermatol 2000; 142: 481–484. [DOI] [PubMed] [Google Scholar]
- 6.Neill SM, Lewis FM, Tatnall FM, Cox NH, British Association of Dermatologists. British Association of Dermatologists' guidelines for the management of lichen sclerosus 2010. Br J Dermatol 2010; 163: 672–682. [DOI] [PubMed] [Google Scholar]
- 7.Böhm M, Frieling U, Luger TA, Bonsmann G. Successful treatment of anogenital lichen sclerosus with topical tacrolimus. Arch Dermatol 2003; 139(7): 922–924. [DOI] [PubMed]
- 8.Li Y, Xiao Y, Wang H, et al. Low-concentration topical tacrolimus for the treatment of anogenital lichen sclerosus in childhood: maintenance treatment to reduce recurrence. J Pediatr Adolesc Gynecol 2013; 26: 239–242. [DOI] [PubMed] [Google Scholar]
- 9.Funaro D, Lovett A, Leroux N, et al. A double-blind, randomized prospective study evaluating topical clobetasol propionate 0.05% versus topical tacrolimus 0.1% in patients with vulvar lichen sclerosus. J Am Acad Dermatol 2014; 71: 84–91. [DOI] [PubMed] [Google Scholar]
- 10.Hennge UR, Krause W, Hofmann H, et al. Multicentre, phase II trial on safety and efficacy of topical tacrolimus ointment for the treatment of lichen sclerosus. Br J Dermatol 2006; 155: 1021–1028. [DOI] [PubMed] [Google Scholar]
- 11.Anderson K, Ascanio NM, Kinney MA, et al. A retrospective analysis of pediatric patients with lichen sclerosus treated with a standard protocol of class I topical corticosteroid and topical calcineurin inhibitor. J Dermatolog Treat 2016; 27: 64–66. [DOI] [PubMed] [Google Scholar]
- 12.Kim GW, Park HJ, Kim HS, et al. Topical tacrolimus ointment for the treatment of lichen sclerosus, comparing genital and extragenital involvement. J Dermatol 2012; 39: 145–150. [DOI] [PubMed] [Google Scholar]
- 13.Langley RG, Luger TA, Cork MJ, et al. An update on the safety and tolerability of Pimecrolimus cream 1%: evidence from clinical trials and post-marketing surveillance. Dermatology 2007; 215(Suppl 1): 27–44. [DOI] [PubMed] [Google Scholar]
- 14.Ribero S, Borradori L. Risk of malignancy and systemic absorption after application of topical tacrolimus in oral lichen planus. J Eur Acad Dermatol Venereol 2017; 31: e85–e86. [DOI] [PubMed] [Google Scholar]