Short abstract
Objective
We aimed to investigate the relationships among chronic kidney disease (CKD), symptomatic ischaemic stroke, and carotid atherosclerosis.
Methods
We enrolled 455 patients who underwent carotid ultrasonography in our hospital, including 311 patients with symptomatic ischaemic stroke and 144 patients without symptomatic ischaemic stroke. Carotid intima–media thickness (IMT), the rate of internal carotid artery stenosis, and maximal plaque size were evaluated.
Results
The mean age of the patients was 68.5 ± 11.0 years and the mean estimated glomerular filtration rate (eGFR) was 68.8 ± 18.2 mL/min/1.73 m2. After adjustment for cardiovascular risk factors, the mean IMT was significantly higher in patients with CKD than in those without CKD. The IMT and eGFR were negatively correlated in patients with stroke (r = −0.169). Multiple logistic regression analyses showed that mean IMT, plaque size, and internal carotid artery stenosis were significant determinants of symptomatic ischaemic stroke after adjustment of multivariate risk factors. Furthermore, the eGFR was a negative determinant of symptomatic ischaemic stroke after adjusting for classical risk factors (odds ratio [95% confidence interval] = 0.868 [0.769–0.979]).
Conclusion
CKD might be associated with the carotid atherosclerosis and symptomatic ischaemic stroke.
Keywords: Chronic kidney disease (CKD), glomerular filtration rate (GFR), ultrasonography, ischaemic stroke, carotid atherosclerosis, intima–media thickness (IMT)
Introduction
Symptomatic stroke is the most prevalent cardiovascular and neurological disease in Asia. The incidence and mortality associated with stroke are higher than those associated with ischaemic heart disease in Japan.1 Moreover, the mortality rate or disability-adjusted life-year loss is significantly more affected by stroke than by ischaemic heart disease.2 Therefore, optimized management of risk factors is urgently required to prevent stroke. Ten major risk factors account for 90% of the risk of stroke. Accordingly, management of hypertension, cessation of smoking, treatment for diabetes and dyslipidaemia, and lifestyle modification are recommended to prevent stroke.3,4
A strategy to prevent stroke has been established. However, the prevalence of asymptomatic carotid artery stenosis, which is one of the major triggers of stroke, increases with age.5 Evaluation of residual risk factors is required to prevent stroke.
Chronic kidney disease (CKD), which is defined by a continuous reduction in the glomerular filtration rate and/or renal damage (i.e., albuminuria6) has been recognized as a global health problem of epidemic proportions.7,8 CKD is closely associated with cardiovascular disease (CVD); approximately 30% to 40% of patients with symptomatic stroke have CKD.9 Furthermore, a continuous decline in the estimated glomerular filtration rate (eGFR8,10) and the existence of proteinuria10,11 have been reported as independent risk factors for CVD. A lower eGFR on admission for stroke is an independent risk factor for mortality and new CVD.12
Recently, the strain vessel hypothesis has been advocated as a common pathological mechanism among stroke, CVD, and CKD.13 Strain vessels are defined as small vessels that branch off directly from large vessels, such as juxtamedullary afferent arterioles in the kidney, the perforating artery in the brain, and the coronary artery. These strain vessels are likely to be exposed to a high blood pressure gradient, leading to vascular impairment, including endothelial dysfunction. The pathophysiological similarity of these vascular structures has led to proposal of a new concept called “cardiorenal connection in chronic kidney disease”.
Several reports have shown the relationship among large vessel disease, carotid artery sclerosis, and small vessel diseases, such as CKD or asymptomatic ischaemic stroke.14,15 However, the relationship between carotid atherosclerosis and CKD in Japanese patients with symptomatic ischaemic stroke has not been fully investigated. Therefore, we performed a cross-sectional study to investigate the relationship among CKD, symptomatic ischaemic stroke, and carotid atherosclerosis.
Methods
Patients
We retrospectively recruited 498 patients who underwent carotid ultrasonography at Himeji Central Hospital between February 2005 and March 2009. Among them, 455 patients whose eGFR values were available were enrolled in this study. The patients were divided into two groups as follows: 311 patients who suffered from symptomatic ischaemic stroke (brain infarction [BI] group) and 144 patients who did not suffer from symptomatic ischaemic stroke (control group). All of the patients in the control group did not have a history of cerebrovascular disease or cardiovascular events. On the basis of physician’s charts, cardiovascular risk factors of each patient were evaluated, including body mass index (BMI), smoking, diabetes mellitus, hypertension, dyslipidaemia, atrial fibrillation, metabolic syndrome, and CKD. The eGFR was calculated using the Modification of Diet in Renal Disease (formula for Japanese individuals.16 CKD was defined as an eGFR of < 60 mL/min/1.73 m2.
Ultrasonography of the carotid artery
Carotid intima–media thickness (IMT), internal carotid artery stenosis, and the maximum size of plaques were measured using high-resolution B-mode ultrasonography (Aplio SSA-700; Toshiba, Otawara, Japan). Carotid IMT was defined as the average of three measurements of the distance from the edge of the lumen–intima interface to the edge of the collagen-containing upper layer of the adventitia at 1-cm intervals. The rate of internal carotid artery stenosis was calculated by square measurement in the short axis. All of the ultrasound data were obtained by one well-trained technician.
Ethics
The study was conducted in accordance with Declaration of Helsinki and the ethical principles of clinical studies in Japan. This study was approved by the local Ethics Committee at Himeji Central Hospital (UMIN000028744). Written consent was not required because this was a retrospective, observational study. The Institutional Review Board waived the requirement of written informed consent, but patients were provided the opportunity to reject enrolment into this study.
Statistical analyses
Data for clinical characteristics are expressed as mean ± standard deviation. Data for comparison of carotid atherosclerosis with cardiovascular risk factors or symptomatic stroke are expressed as mean ± standard error. Differences between the BI and the control groups were examined by the unpaired t-test or chi-square test as appropriate. Odds ratios were calculated to evaluate the risk factors for an increase in IMT and the presence of symptomatic BI. Univariate and multiple logistic regression analyses were carried out to examine the independent associations among risk factors. Pearson’s correlation analysis was performed to evaluate the relationships between IMT and the eGFR. A difference of P < 0.05 was considered statistically significant. All of the data were analysed using IBM SPSS Statistics for Windows, version 22.0 (IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics of the study population
In this study, 455 patients were analysed. The mean age was 68.5 ± 11.0 years and 38.5% of the patients were women (Table 1). Mean BMI was 23.2 ± 3.3 kg/m2 and the mean eGFR was 68.8 ± 18.2 mL/min/1.73 m2. In the whole study population, 40.4% of the patients had diabetes mellitus, 71.0% had hypertension, 45.9% had dyslipidaemia, 5.9% had atrial fibrillation, and 29.7% had CKD.
Table 1.
Clinical characteristics of all patients.
| Characteristic | |
|---|---|
| Number | 455 |
| Age (years) | 68.5 ± 11.0 |
| Sex (n; female) | 175 |
| BMI (kg/m2) | 23.2 ± 3.3 |
| Creatinine (mg/dL) | 0.8±0.3 |
| eGFR (mL/min/1.73 m2) | 68.8 ± 18.2 |
| SBP (mmHg) | 142 ± 23 |
| DBP (mmHg) | 80 ± 14 |
| Smoking (%; yes) | 53.2 |
| Diabetes mellitus (%) | 40.4 |
| Hypertension (%) | 71.0 |
| Dyslipidaemia (%) | 45.9 |
| Atrial fibrillation (%) | 5.9 |
| Metabolic syndrome (%) | 35.2 |
| Chronic kidney disease (%) | 29.7 |
Values are number, %, or mean ± standard deviation. BMI; body mass index, eGFR; estimated glomerular filtration rate, SBP; systolic blood pressure, DBP; diastolic blood pressure.
Relationships between IMT and cardiovascular risk factors
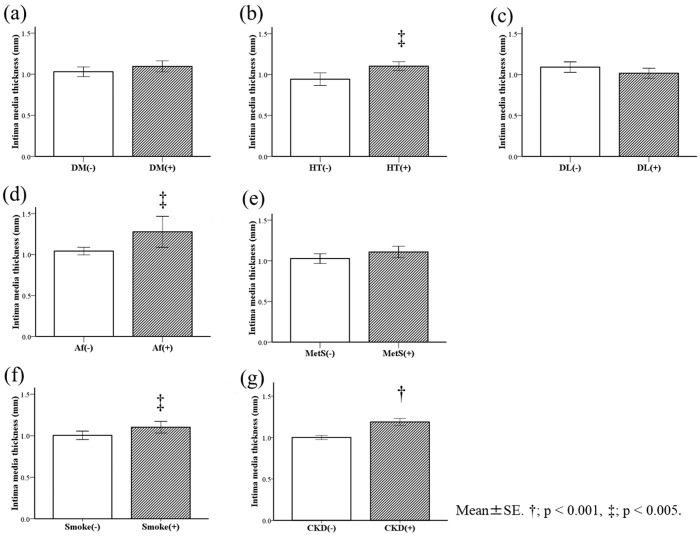
Initially, we examined the relationship between the IMT and cardiovascular risk factors in all of the patients (Figure 1). The mean IMTs in patients with hypertension (Figure 1b), atrial fibrillation (Figure 1d), and a smoking habit (Figure 1f) were significantly higher than those in patients without these risk factors (all P < 0.01). Moreover, the mean IMT in patients with CKD was significantly higher than that in patients without CKD (P < 0.001, Figure 1g). We performed multivariate analyses to further investigate the relationship between CKD and IMT. After adjustment for age, sex, and other cardiovascular risk factors, such as smoking habit, diabetes mellitus, hypertension, and dyslipidaemia, CKD tended to be correlated with an increase in IMT (P = 0.05, Table 2).
Figure 1.
Relationship between IMT and cardiovascular risk factors. The relationships between the IMT and cardiovascular risk factors are shown in all of the patients. Risk factors were a history of diabetes (a), hypertension (b), dyslipidaemia (c), atrial fibrillation (d), metabolic syndrome (e), a smoking habit (f), and patients with CKD (g). †P < 0.001, ‡P < 0.01 IMT; intima–media thickness, DM; diabetes mellitus, HT; hypertension, DL; dyslipidaemia, Af; atrial fibrillation, MetS; metabolic syndrome, Smoke; smoking habit, and CKD; chronic kidney disease.
Table 2.
Univariate and multivariate logistic regression analyses between CKD and IMT.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Dependent factor: presence of CKD | ||||||
| IMT (per 0.1 mm) | 1.082 | 1.037–1.130 | <0.001 | 1.047 | 0.999–1.096 | 0.053 |
Adjusted for age, sex, smoking habit, history of diabetes mellitus, hypertension, and dyslipidaemia. OR; odds ratio, CI; confidence interval, IMT; intima–media thickness, CKD; chronic kidney disease, eGFR; estimated glomerular filtration rate.
Relationship between carotid atherosclerosis and CKD in symptomatic BI
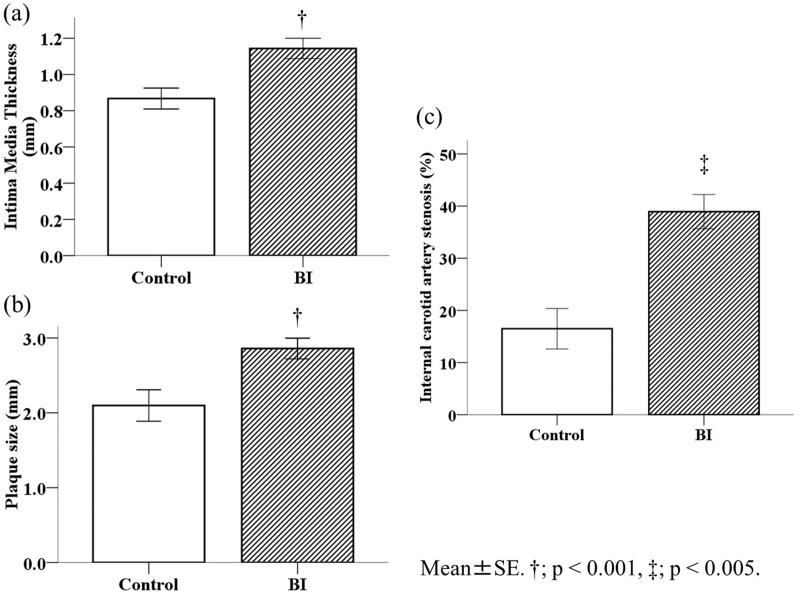
Because we found a relationship between carotid atherosclerosis and CKD, we subsequently examined the difference between the BI and control groups. The mean age was significantly older (P < 0.001) and the ratio of male participants was higher (P = 0.003) in the BI group compared with the control group (Table 3). Blood pressure in the BI group was significantly higher than that in the control group (P < 0.01). The eGFR was significantly lower in the BI group than in the control group (P = 0.005). The number of patients with CKD in the BI group (n = 103, 33.1%) was significantly higher than that of patients in the control group (n = 32, 22.2%, P = 0.02). The percentages of smokers, and patients with hypertension, atrial fibrillation, and metabolic syndrome in the BI group were significantly higher than those of patients in the control group (all P < 0.05). The percentage of patients with dyslipidaemia in the BI group was significantly lower than that of patients in the control group (P = 0.006, Table 3). The IMT in the BI group was significantly thicker than that in the control group (P < 0.001, Figure 2a). The percentage of patients with carotid plaques in the BI group (n = 272, 87.5%) was significantly higher than that of patients in the control group (n = 86, 59.7%, P < 0.001). The mean plaque size in the BI group was significantly larger than that in the control group (2.86 ± 1.14 vs. 2.10 ± 0.97 mm, P < 0.001) (Figure 2b). The area of internal carotid artery stenosis, which was determined using the area method, was significantly higher in the BI group than in the control group (P < 0.01, Figure 2c). The same tendency was observed using the North American Symptomatic Carotid Endarterectomy Trial and European Carotid Surgery Trial methods (data not shown).
Table 3.
Clinical characteristics of patients with symptomatic ischaemic stroke and control subjects.
| n = 455 | Brain infarction group | Control group | P value |
|---|---|---|---|
| Number | 311 | 144 | – |
| Age (years) | 70.7 ± 11.0 | 63.6 ± 10.9 | <0.001 |
| Sex (n; female) | 106 | 69 | 0.003 |
| BMI (kg/m2) | 23.1 ± 3.2 | 23.5 ± 3.5 | 0.201 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 0.8 ± 0.6 | 0.289 |
| eGFR (mL/min/1.73 m2) | 67.2 ± 17.6 | 72.4 ± 19.0 | 0.005 |
| SBP (mmHg) | 145 ± 23 | 135 ± 21 | <0.001 |
| DBP (mmHg) | 81 ± 15 | 77 ± 12 | 0.008 |
| Smoking (%; yes) | 57.6 | 43.8 | 0.006 |
| Diabetes mellitus (%) | 42.4 | 36.1 | 0.219 |
| Hypertension (%) | 78.1 | 55.6 | <0.001 |
| Dyslipidaemia (%) | 41.5 | 55.6 | 0.006 |
| Atrial fibrillation (%) | 7.7 | 2.1 | 0.018 |
| Metabolic syndrome (%) | 41.8 | 25.9 | 0.001 |
| Chronic kidney disease (%) | 33.1 | 22.2 | 0.020 |
Values are number, %, or mean ± standard deviation. BMI; body mass index, eGFR; estimated glomerular filtration rate, SBP; systolic blood pressure, DBP; diastolic blood pressure.
Figure 2.
Carotid atherosclerotic changes in patients with symptomatic BI. Mean IMT (a), plaque size (b), and internal carotid artery stenosis (c) significantly progressed in the BI group. †P < 0.001, ‡P < 0.01. IMT; intima–media thickness, BI; brain infarction.
Correlation between IMT and CKD
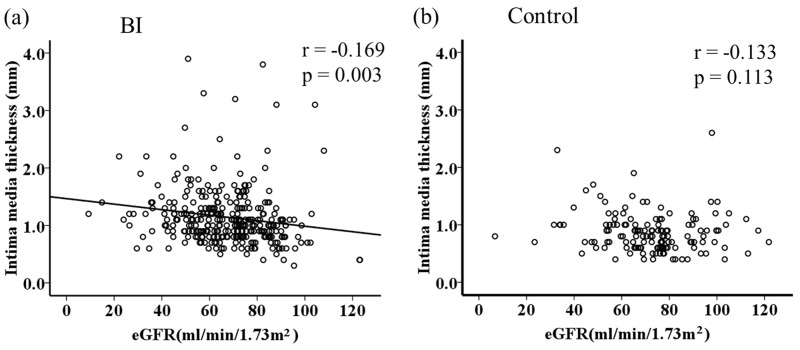
To investigate the relationship between carotid atherosclerosis and CKD in each group, simple correlation analysis was performed. In the BI group, IMT was significantly negatively correlated with the eGFR (r = −0.169, P = 0.003). However, this relationship was diminished and non-significant in the control group (Figure 3).
Figure 3.
Correlation between IMT and eGFR. The IMT and eGFR were only negatively correlated in the BI group. IMT; intima–media thickness, eGFR; estimated glomerular filtration rate, BI; brain infarction.
Multivariate analyses
We also performed multivariate analyses to evaluate the associations between symptomatic ischaemic stroke and the findings of carotid ultrasonography and renal function (Table 4). Multiple logistic regression analyses showed that internal carotid artery stenosis (P < 0.001) and the maximal plaque size (P < 0.001) were significant determinants of symptomatic ischaemic stroke after adjustment for multivariate risk factors. An increase in IMT was also independently associated with BI (P = 0.001). Furthermore, the eGFR was a negative determinant of symptomatic ischaemic stroke after adjustment of multivariate risk factors (P = 0.02).
Table 4.
Univariate and multivariate logistic regression analyses for symptomatic brain infarction.
|
Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Dependent factor: presence of symptomatic brain infarction | ||||||
| Internal carotid artery stenosis (per 1%) | 1.044 | 1.031–1.057 | <0.001 | 1.035 | 1.021–1.048 | <0.001 |
| Plaque size (per 1 mm) | 2.212 | 1.635–2.991 | <0.001 | 1.884 | 1.387–2.558 | <0.001 |
| IMT (per 0.1 mm) | 1.221 | 1.140–1.307 | <0.001 | 1.123 | 1.048–1.203 | 0.001 |
| eGFR (per 10 mL/min/1.73 m2) | 0.852 | 0.761–0.953 | 0.005 | 0.868 | 0.769–0.979 | 0.021 |
Adjusted for age, sex, smoking habit, history of diabetes mellitus, hypertension, and dyslipidaemia. OR; odds ratio, CI; confidence interval, IMT; intima–media thickness, CKD; chronic kidney disease, eGFR; estimated glomerular filtration rate.
Discussion
In the present study, we showed significant relationships among CKD, carotid atherosclerosis, and symptomatic ischaemic stroke. Moreover, advanced CKD, and significantly greater IMT thickness were observed in patients with symptomatic ischaemic stroke. Therefore, parameters determined by ultrasonography might be useful surrogate markers for symptomatic ischaemic stroke.
A previous report on acute cerebral infarction showed that carotid atherosclerosis was significantly progressed in patients with CKD.17 Other studies have reported that CKD without hypertension has no involvement with carotid atherosclerosis.18 Therefore, further studies are required to examine whether CKD is independently involved in the development of carotid atherosclerosis. The important findings of the present study are that carotid artery ultrasonography showed significant progression of IMT and arterial stenosis in patients with CKD after adjustment for various classical risk factors for atherosclerosis. Additionally, CKD can be a risk factor for cerebrovascular diseases.19,20 Based on these results, the relationship between CKD and IMT, which is a marker of atherosclerosis, is important for determining the brain–renal connection.
Common risk factors of CKD and stroke are age, hypertension, diabetes mellitus, dyslipidaemia, obesity, and metabolic syndrome. Moreover, risk factors for CKD, such as endoplasmic reticulum stress and subsequent inflammation through activation of the renin–angiotensin system,21 oxidative stress,22 asymmetric dimethylarginine,22,23 and nitric monoxide abnormality,24 are associated with deterioration of atherosclerosis through vascular endothelial dysfunction in CKD. The phenotypic change of vascular smooth muscle cells to osteoplastic osteocytes or chondrocytes has been observed in lesions of atherosclerotic calcification in patients with renal disease.25 Furthermore, nitric oxide prevents osteoblast differentiation of vascular smooth muscle cells and calcification by attenuating transforming growth factor-β.26
Microalbuminuria27 and a lower eGFR28 are associated with asymptomatic lacunar infarction. According to an observational study of the Japanese general population over an average period of 7.76 years,29 the risk of the first stroke attack increases 1.9-fold in patients with a creatinine clearance of 40 to 70 mL/min, and 3.1-fold in patients with a creatinine clearance of < 40 mL/min compared with those with a creatinine clearance of >70 mL/min. Furthermore, the risk of stroke tends to increase in patients with proteinuria.29
In the present study, the number of patients with dyslipidaemia in the control group was significantly higher than that in the BI group. All participants were patients who underwent carotid ultrasonography because of some clinical reasons. Therefore, the prevalence of constant atherosclerotic risk factors was expected to be higher compared with that in the general population, even in control subjects. Treatment for dyslipidaemia, mainly with statins, contributes to prevention of BI in patients with atherosclerotic risk factors, such as CVD,30 a history of BI,31 and CKD.32 Consistent with previous studies,30–32 treatment for dyslipidaemia might have had a preventive effect against development of atherosclerotic disease in the control group.
In our study, multivariate analysis showed that carotid atherosclerosis was a common pathology of stroke and CKD. Additionally, IMT, one of the surrogate markers of atherosclerosis, could be a useful marker for evaluating not only the pathology of stroke, but also that of CKD.
This study has several limitations. One of these limitations is that CKD was defined only by the eGFR. Proteinuria is an independent risk factor for CVD.33,34 Another limitation is the possibility of coexistent diseases. We extracted the patients’ medical history from the records of each participant, but the patients’ treatment history, and the duration and severity of comorbidities were not fully extracted. Associations between some antihyperglycaemic drugs, statins, antihypertensive drugs, and progression of IMT or CVD have been reported, and thus differences between drugs should be taken into account.35–37 The number of patients with atrial fibrillation was relatively small, and thus the percentage of patients with cardioembolic stroke might have been smaller than epidemiological estimates. A smaller proportion of cardioembolic stroke could cause selection bias. A further limitation is that this was a cross-sectional study that was performed in a single institution. The small sample size might have affected the results. A longitudinal study in large number of participants should be conducted in the future to clarify the associations among CKD, IMT, and symptomatic ischaemic stroke in the Japanese population. However, in the present study, ultrasonography was performed by a trained engineer in a single institution, which represents an advantage because there is less deviation compared with multicentre studies. There are regional differences in the incidence of symptomatic ischaemic stroke. Because cerebrovascular disease is relatively common in Asian populations, more evidence is needed to investigate the epidemiology of stroke in Asian populations.
In summary, this cross-sectional study showed significant associations among CKD, symptomatic ischaemic stroke, and an increase in IMT. Furthermore, advanced CKD and a significant increase in IMT were observed in patients with symptomatic ischaemic stroke. A mutual relationship might be present between symptomatic ischaemic stroke, CKD, and carotid atherosclerosis, which is a surrogate marker of stroke.
Acknowledgements
The author would like to thank Brian Quinn, Japan Medical Communication, for his skilful English editing.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kubo M, Kiyohara Y, Kato I, et al. Trends in the incidence, mortality, and survival rate of cardiovascular disease in a Japanese community: the Hisayama study. Stroke 2003; 34: 2349–2354. [DOI] [PubMed] [Google Scholar]
- 2.Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 2011; 124: 314–323. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010; 376: 112–123. [DOI] [PubMed] [Google Scholar]
- 4.Park TH, Ko Y, Lee SJ, Lee KB, Lee J, Han M-K, et al. Identifying target risk factors using population attributable risks of ischemic stroke by age and sex. J Stroke 2015; 17: 302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannami T, Konishi M, Baba S, Nishi N, Terao A. Prevalence of asymptomatic carotid atherosclerotic lesions detected by high-resolution ultrasonography and its relation to cardiovascular risk factors in the general population of a Japanese city: the Suita study. Stroke 1997; 28: 518–525. [DOI] [PubMed] [Google Scholar]
- 6. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011) 2013; 3: 19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens LA, Levey AS. Current status and future perspectives for CKD testing. Am J Kidney Dis 2009; 53(3 Suppl 3): S17–S26. [DOI] [PubMed] [Google Scholar]
- 8.Eisen A, Hoshen M, Balicer RD, et al. Estimated glomerular filtration rate within the normal or mildly impaired range and incident cardiovascular disease. Am J Med 2015; 128: 1015–22.e2. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino H, Itoh Y, Yamada S, et al. Clinical features and neurologic severity in stroke patients with mild to moderate renal dysfunction. J Stroke Cerebrovasc Dis 2012; 21: 343–349. [DOI] [PubMed] [Google Scholar]
- 10.Chronic Kidney Disease Prognosis Consortium Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerstein HC, Mann JE, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA 2001; 286: 421–426. [DOI] [PubMed] [Google Scholar]
- 12.Tsagalis G, Akrivos T, Alevizaki M, et al. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol Dial Transplant 2009; 24: 194–200. [DOI] [PubMed] [Google Scholar]
- 13.Ito S, Nagasawa T, Abe M, et al. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res 2009; 32: 115–121. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka M, Abe Y, Furukado S, et al. Chronic kidney disease and carotid atherosclerosis. J Stroke Cerebrovasc Dis 2012; 21: 47–51. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, Ogawa T, Sugimoto H, et al. Relation of carotid intima-media thickness and silent cerebral infarction to cardiovascular events and all-cause mortality in chronic hemodialysis patients. Intern Med 2012; 51: 2111–2117. [DOI] [PubMed] [Google Scholar]
- 16.Imai E, Horio M, Nitta K, et al. Modification of the Modification of Diet in Renal Disease (MDRD) Study Equation for Japan. Am J Kidney Dis 2007; 50: 927–937. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K, Watanabe Y, Katsumata T, et al. Carotid intima-media thickness and cerebral white matter lesions are more advanced in acute ischemic stroke patients with renal dysfunction. Clin Nephrol 2011; 76: 290–295. [DOI] [PubMed] [Google Scholar]
- 18.Ohara T, Kokubo Y, Toyoda K, et al. Impact of chronic kidney disease on carotid atherosclerosis according to blood pressure category. The Suita study. Stroke 2013; 44: 3537–3539. [DOI] [PubMed] [Google Scholar]
- 19.Makin SD, Cook FA, Dennis MS, et al. Cerebral small vessel disease and renal function: systematic review and meta-analysis. Cerebrovasc Dis 2015; 39: 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Lv P, Jin H, et al. Association between low estimated glomerular filtration rate and risk of cerebral small-vessel diseases: a m-analysis. J Stroke Cerebrovasc Dis 2016; 25: 710–716. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Wen Y, Lv LL, et al. Involvement of endoplasmic reticulum stress in angiotensin II-induced NLRP3 inflammasome activation in human renal proximal tubular cells in vitro. Acta Pharmacol Sin 2015; 36: 821–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldámiz-Echevarría L, Andrade F. Asymmetric dimethylarginine, endothelial dysfunction and renal disease. Int J Mol Sci 2012; 13: 11288–11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tripepi G, Kollerits B, Leonardis D, et al. Competitive interaction between fibroblast growth factor 23 and asymmetric dimethylarginine in patients with CKD. J Am Soc Nephrol 2015; 26: 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaysen GA, Eiserich JP. The role of oxidative stress–altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J Am Soc Nephrol 2004; 15: 538–548. [DOI] [PubMed] [Google Scholar]
- 25.Tyson KL, Reynolds JL, McNair R, et al. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arteriosclerosis, Thrombosis, and Vascular Biology 2003; 23: 489–494. [DOI] [PubMed] [Google Scholar]
- 26.Kanno Y, Into T, Lowenstein CJ, et al. Nitric oxide regulates vascular calcification by interfering with TGF-β signalling. Cardiovasc Res 2007; 77: 221–230. [DOI] [PubMed] [Google Scholar]
- 27.Wada M, Nagasawa H, Kurita K, et al. Microalbuminuria is a risk factor for cerebral small vessel disease in community-based elderly subjects. J Neurol Sci 2007; 255: 27–34. [DOI] [PubMed] [Google Scholar]
- 28.Wada M, Nagasawa H, Iseki C, et al. Cerebral small vessel disease and chronic kidney disease (CKD): Results of a cross-sectional study in community-based Japanese elderly. J Neurol Sci 2008; 272: 36–42. [DOI] [PubMed] [Google Scholar]
- 29.Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population—the Ohasama study. Nephrol Dial Transplant 2007; 22: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 30.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996; 335: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 31.Hosomi N, Nagai Y, Kohriyama T, et al. The Japan Statin Treatment Against Recurrent Stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine 2015; 2: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan YL, Qiu B, Wang J, et al. High-intensity statin therapy in patients with chronic kidney disease: a systematic review and meta-analysis. BMJ Open 2015; 5: e006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Z, Shahinfar S, Keane WF, et al. Importance of baseline distribution of proteinuria in renal outcomes trials: lessons from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study. J Am Soc Nephrol 2005; 16: 1775–1780. [DOI] [PubMed] [Google Scholar]
- 34.Halbesma N, Kuiken DS, Brantsma AH, et al. macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol 2006; 17: 2582–2590. [DOI] [PubMed] [Google Scholar]
- 35.Langenfeld MR, Forst T, Hohberg C, et al. Pioglitazone decreases carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus. Circulation 2005; 111: 2525–2531. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto K, Sera Y, Abe Y, et al. Metformin attenuates progression of carotid arterial wall thickness in patients with type 2 diabetes. Diabetes Res Clin Pract 2004; 64: 225–228. [DOI] [PubMed] [Google Scholar]
- 37.Barbieri M, Rizzo MR, Marfella R, et al. Decreased carotid atherosclerotic process by control of daily acute glucose fluctuations in diabetic patients treated by DPP-IV inhibitors. Atherosclerosis 2013; 227: 349–354. [DOI] [PubMed] [Google Scholar]