Short abstract
Objective
This study was performed to systematically compare the safety and efficacy of total enteral nutrition (TEN) and total parenteral nutrition (TPN) for patients with severe acute pancreatitis (SAP).
Methods
The PubMed database was searched up to January 2017, and nine studies were retrieved. These studies were selected according to specific eligibility criteria. The methodological quality of each trial was assessed, and the study design, interventions, participant characteristics, and final results were then analyzed by Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
Nine relevant randomized controlled trials involving 500 patients (244 patients in the TEN group and 256 patients in the TPN group) were included in the meta-analysis. Pooled analysis showed a significantly lower mortality rate in the TEN than TPN group [odds ratio (OR), 0.31; 95% confidence interval (CI), 0.18–0.54]. The duration of hospitalization was significantly shorter in the TEN than TPN group (mean difference, −0.59; 95% CI, −2.56–1.38). Compared with TPN, TEN had a lower risk of pancreatic infection and related complications (OR, 0.41; 95% CI, 0.22–0.77), organ failure (OR, 0.17; 95% CI, 0.06–0.52), and surgical intervention (OR, 0.17; 95% CI, 0.05–0.62).
Conclusions
This meta-analysis indicates that TEN is safer and more effective than TPN for patients with SAP. When both TEN and TPN have a role in the management of SAP, TEN is the preferred option.
Keywords: Total parenteral nutrition, total enteral nutrition, severe acute pancreatitis, meta-analysis, larger statistical power, randomized controlled trial
Introduction
Among patients with severe acute pancreatitis (SAP), those who develop a systemic immunoinflammatory response exhibit a hypercatabolic state promoting nutritional deterioration.1 Consequently, SAP is usually accompanied by increased resting energy requirements and reductions in protein mass. This persistently negative nitrogen balance results in a higher mortality rate caused by the loss of function and structural integrity of vital organs.2 Thus, early nutritional support is very important in preventing serious complications and ensuring optimal recovery in patients with SAP.
Various techniques have been adopted for nutritional support in recent clinical studies, such as total parenteral nutrition (TPN), dual parenteral and enteral nutritional support, nasojejunal feeding, nasogastic feeding, and others. The optimal route of administering nutritional support is controversial. According to the assumption that resting the pancreas by avoiding irritation and production of pancreatic digestive enzymes is beneficial in patients with pancreatitis, TPN has become the standard route for providing exogenous nutrients since Feller et al.3 reported decreased complication and mortality rates in patients supported with parenteral nutrition in 1974. Parenteral nutrition can maintain lean body mass while avoiding adynamic ileus. However, it is limited by an increased risk of infection through the central venous catheter, may worsen the inflammatory process, alters gut permeability, and does not improve mortality.4
Preservation or restoration of the gut barrier function may have a beneficial impact on infectious morbidity from SAP and may reduce mortality. Additionally, recent studies of trauma and burn management have shown that enteral nutrition has fewer complications, offers the potential for immune modulation and disease attenuation, reduces the incidence of sepsis, and is less expensive.5 Thus, total enteral nutrition (TEN) is being used more frequently in patients with acute pancreatitis.6,7 In recent studies, however, TEN was started ≥48 h after admission to the hospital. One study showed no demonstrable effect of immediate TEN on the inflammatory response or intestinal permeability compared with conventional management (i.e., nothing per os or no nutritional support) in patients with predicted SAP.8 Enteral nutrition provides gut integrity with immune modulation, reduces the inflammatory response, is associated with fewer infectious complications, and is much less expensive; however, its widespread use in the clinical setting is limited by concern regarding adynamic ileus and pancreatic stimulation.
Comparisons between TEN and TPN for treatment of SAP have been performed in many clinical trials.9–17 However, the clinical outcomes were not completely consistent, and no study was large enough to provide definite conclusions about the safety of enteral nutrition. We therefore performed a meta-analysis of eligible comparative studies to evaluate the efficacy, tolerance, clinical outcome, and cost of TEN versus TPN for SAP.
Methods
Literature search for eligible studies
In January 2017, we searched the PubMed database, Embase, and Cochrane Library using the following search strategy: (enteral nutrition OR feeding) AND (parenteral nutrition OR feeding) AND (severe acute pancreatitis OR acute necrotizing pancreatitis). All retrieved articles and relevant reviews were manually searched to find other potentially eligible studies.
Inclusion and exclusion criteria
Articles were selected based on the following criteria: 1) the study was a randomized controlled trial, 2) the study included patients with SAP, 3) the study compared the efficacy and safety of TEN versus TPN for SAP, and 4) at least one of the following was assessed: mortality, length of hospital stay, infectious complications, organ failure, and surgical interventions.
The exclusion criteria were as follows: 1) patient age of <18 years, 2) pregnancy, 3) case reports and reviews, 4) non-English-language literature, and (5) studies that did not include participants.
Quality assessment
The quality of the included trials was assessed using the Physiotherapy Evidence Database (PEDro) score. Data were independently collected by two reviewers. The following information was collected: first author, country, year of publication, number of cases, patients’ baseline characteristics (mean age, sex ratio), and information regarding clinical outcomes (e.g., mortality, length of hospital stay, infectious complications, organ failure, and surgical interventions).
Statistical analysis
We used Review Manager 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) for the meta-analysis. Clinical outcomes were assessed with respect to mortality, pancreatic infections and related complications, organ failure, surgical interventions, and length hospital of stay. Binary outcome data (mortality, pancreatic infections and related complications, organ failure, and surgical intervention) were summarized using odds ratios (ORs) and 95% confidence intervals (CIs). For the successive variable (hospital stay), we used the weighted mean difference and its 95% CI. Funnel plots were employed to assess the possibility of publication bias. These plots showed the intervention effect of each study against the standard error. A symmetrical plot reveals no bias, and any asymmetry of the plot would suggest publication bias. If heterogeneity was either absent or low, we presented the results of only the fixed-effects model. If substantial heterogeneity was present (>50%), all analyses were based on the random-effects model. The sensitivity analysis was performed to test the strength and robustness of the pooled results by sequential omission of individual studies, and the results are expressed using P values.
Ethics statement
The need for ethics approval was waived because this was a meta-analysis and involved no people or animals.
Results
Characteristics of included studies
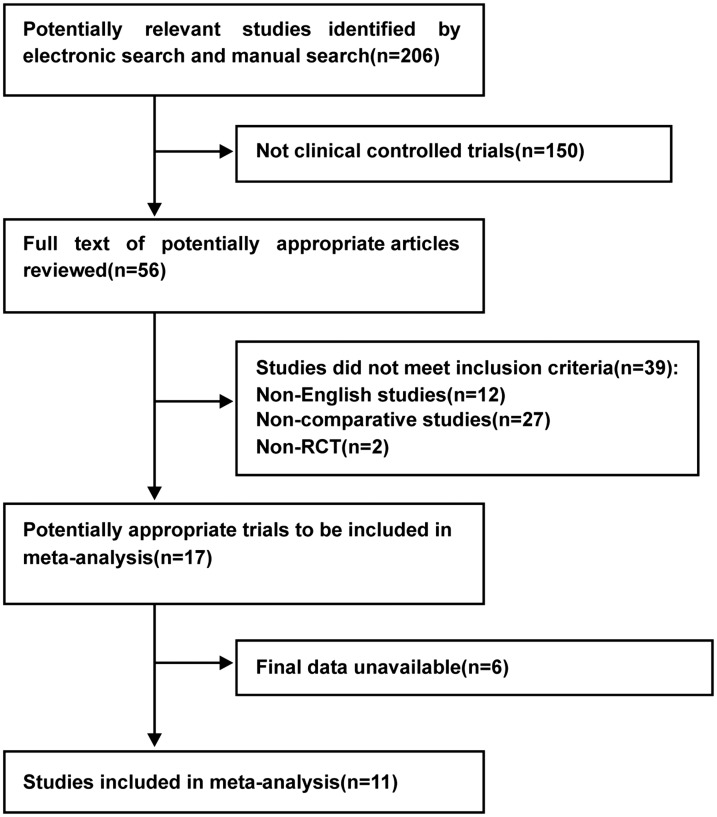
As shown in Figure 1, 206 articles were retrieved through the database search. After reviewing the abstracts and titles, 147 studies were excluded because they focused on an unrelated topic, and 50 were excluded because they did not meet the inclusion criteria. Finally, 9 relevant randomized controlled trials involving 500 patients (244 in the TEN group and 256 in the TPN group) were retrieved. The characteristics of these included studies are summarized in Table 1,9–17 and the quality of the trials as assessed by the PEDro score is shown in Table 2.
Figure 1.
Flow chart of selection of included studies.
Table 1.
Main characteristics of the studies.
| Study | Country | Number of patients (TPN/TEN) | Mean age, years (TPN/TEN) | Sex, M:F (TPN/TEN) | APACHE II score (TPN/TEN) | Key outcomes |
|---|---|---|---|---|---|---|
| Wang et al. 2013 | China | 60/61 | 41.7±11.4/43.7±13.7 | 17:13/32:29 | 14.63±3.67/13.27±2.86 | Pancreatic sepsis; MODS; mortality; plasma endotoxin levels; plasma cytokine levels |
| Wu et al. 2010 | China | 54/53 | 54±11.2/52±12.1 | 5:4/32:21 | 16±4.4/14±2.1 | Mortality; hospital stay; organ failure; surgical intervention; infection and complications |
| Doley et al. 2009 | India | 25/25 | 41.1±11.3/38.4±13.8 | - | -/- | Mortality; surgical intervention; hospital stay; infectious complications |
| Casas et al. 2007 | Spain | 11/11 | 55.6±15.6/61.2±16.6 | 8:3/8:3 | 8/8 | Mortality; surgical intervention; infection; organ failure |
| Petrov et al. 2006 | Russia | 34/35 | 52(41–70)/51(42–67) | 12:5/27:8 | 12.5/12 | Mortality; infectious complications; noninfectious complications; organ failure |
| Eckerwall et al. 2006 | Sweden | 25/23 | 68(60–80)/71(58–80) | 14:12/10:14 | 9/10 | Mortality; complications; organ failure; hospital stay |
| Louie et al. 2005 | Canada | 18/10 | 59.0±15.3/65.3±18.3 | 1:1/3:2 | 12.7/11.8 | Mortality; inflammation; infectious complications; cost |
| Gupta et al. 2003 | UK | 9/8 | 57(38–86)/65(56–89) | 1:1/1:2 | 3/4 | Hospital stay; infection; organ failure; mortality |
| Kalfarentzos et al. 1997 | Greece | 20/18 | 67/63 | 7:13/8:10 | 11.8/12.7 | Mortality; hospital stay; infection and complications; nutrition-related complications; surgical intervention; cost |
Data are presented as n, mean ± standard deviation, or mean (range).
TPN, total parenteral nutrition; TEN, total enteral nutrition; M, male; F, female; APACHE II, Acute Physiology and Chronic Health Evaluation II; MODS, multiple organ dysfunction syndrome.
Table 2.
Item PEDro score.
| Study |
Item PEDro score |
Total score | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Wang et al. 2013 | + | - | + | + | + | - | + | + | + | + | 8/10 |
| Wu et al. 2010 | + | - | + | - | - | - | + | + | + | + | 6/10 |
| Doley et al. 2009 | + | - | + | - | - | - | + | + | + | + | 6/10 |
| Casas et al. 2007 | + | + | + | - | - | - | + | + | + | + | 7/10 |
| Petrov et al. 2006 | + | - | + | - | - | - | + | + | + | + | 6/10 |
| Eckerwall et al. 2006 | + | + | + | - | - | - | + | + | + | + | 7/10 |
| Louie et al. 2005 | + | + | + | + | - | - | + | + | + | + | 8/10 |
| Gupta et al. 2003 | + | + | + | - | - | - | + | + | + | + | 7/10 |
| Kalfarentzos et al. 1997 | + | + | + | - | - | - | + | + | + | + | 7/10 |
PEDro, Physiotherapy Evidence Database.
Mortality rate
All nine studies assessed mortality. There were 244 patients in the TEN group and 256 patients in the TPN group. A heterogeneity test was performed on all clinical trials, and there was no statistically significant difference among the included studies (I2 = 25%); therefore, the fixed-effects model was used. The overall mortality rate for TEN and TPN were 7.0% and 20.7%, respectively. The meta-analysis of the mortality rate demonstrated that the mortality rate was significantly lower in the TEN than TPN group (OR, 0.31; 95% CI, 0.18–0.54; P < 0.0001) (Figure 2). The sensitivity analysis revealed that the result was robust and did not depend on any individual study.
Figure 2.
Meta-analysis of mortality.
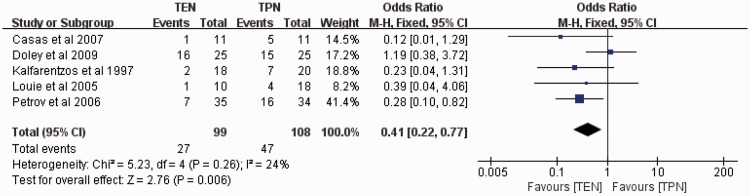
Pancreatic infections and related complications
Five studies assessed pancreatic infections and related complications with 99 patients in the TEN group and 108 patients in the TPN group. The fixed-effects model was used, and no significant heterogeneity was found among these studies (I2 = 24%). The meta-analysis of pancreatic infections and related complications demonstrated a significantly lower rate of pancreatic infections and related complications in the TEN than TPN group (OR, 0.41; 95% CI, 0.22–0.77; P = 0.006) (Figure 3). The sensitivity analysis revealed that the result was robust and did not depend on any individual study.
Figure 3.
Meta-analysis of pancreatic infections and related complications.
Organ failure
Six clinical studies had relevant data regarding organ failure, with 191 patients in the TEN group and 193 patients in the TPN group. Because there was evidence of heterogeneity among the included studies (P = 0.006, I2 = 70%), the random-effects model was used. The meta-analysis results showed that TEN was associated with a significantly lower risk of organ failure than TPN (OR, 0.17; 95% CI, 0.06–0.52; P = 0.002) (Figure 4). The sensitivity analysis revealed that the result was robust and did not depend on any individual study.
Figure 4.
Meta-analysis of organ failure.
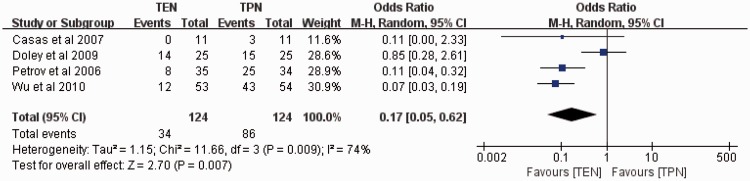
Surgical interventions
Four clinical studies assessed surgical interventions, with 124 patients in the TEN group and 124 patients in the TPN group. Because there was significant heterogeneity among these studies (P = 0.009, I2 = 74%), the random-effects model was used. The meta-analysis results revealed that the rate of surgical intervention was significantly lower in the TEN than TPN group (27.4% vs. 69.4%, respectively; OR, 0.17; 95% CI, 0.05–0.62; P = 0.007) (Figure 5). The sensitivity analysis revealed that the result was robust and did not depend on any individual study.
Figure 5.
Meta-analysis of surgical interventions.
Length of hospital stay
Five studies assessed the length of hospital stay, with 85 patients in the TEN group and 90 patients in the TPN group. A fixed-effects model was used to perform the pooled analysis with no significant heterogeneity among these studies (I2 = 34%). The meta-analysis of the length of hospital stay demonstrated that TEN was associated with a shorter hospitalization stay than TPN (mean difference, −0.59; 95% CI, −2.56–1.38) (Figure 6). The sensitivity analysis showed that the P value, which varied from 0.04 to 0.81, was not stable because of the study by Tao et al.18
Figure 6.
Meta-analysis of hospital stay.
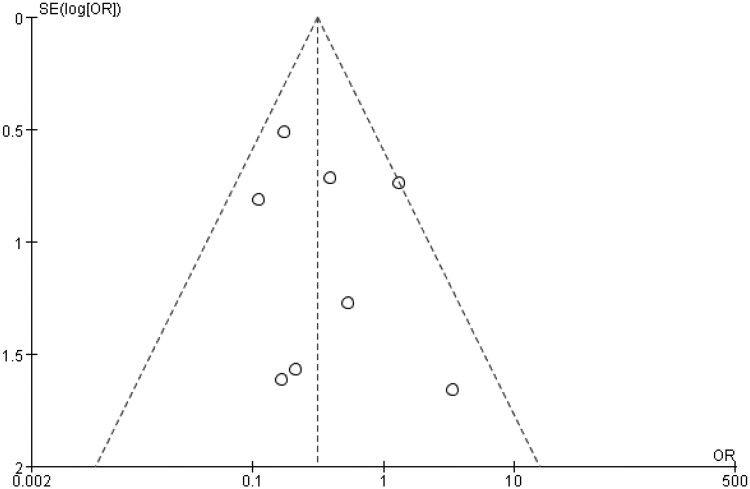
Publication bias
The funnel plot for outcome measurements among all included studies appeared to be symmetrical (Figure 7). The spots indicating single studies fell evenly on both sides of the inverted funnel, indicating no publication bias.
Figure 7.
Publication bias of the included literature.
Discussion
SAP is a common acute surgical condition with high morbidity and mortality in many cases.19 The potential pathogenesis of acute pancreatitis involves premature activation of proteolytic enzymes, causing autodigestion of the pancreas.20 For the management of SAP, nutritional support should be started as soon as possible following admission (within 48 hours). The optimal nutritional support for SAP has been a subject of debate for decades. TPN was previously considered the standard nutritional support technique for SAP while avoiding stimulation of an already inflamed pancreas. In recent years, increasingly more investigators are recommending TEN instead of TPN because enteral nutrition ensures the integrity of the gut with immune modulation, a decreased inflammatory response, fewer septic complications, and lower cost. Some investigators have proposed that pancreatic stimulation should be maintained and that the stress response should be preserved to reduce the occurrence of nosocomial infections, multiorgan failure, and mortality.21 In 2015, experts in Italy proposed TEN as the recommended nutritional support technique in patients with SAP.22 However, the use of early TEN for patients with SAP has not been systematically evaluated.
Several randomized clinical trials12,14,15 and meta-analyses23–25 have been performed to compare the efficacy and safety of TEN and TPN in patients with SAP. By comparing similar published meta-analyses, we evaluated more clinical trials including those that assessed mortality, pancreatic infections and related complications, organ failure, surgical interventions, and length of hospital stay. Notably, our results differed from those of the previous meta-analysis with larger statistical power. In the present meta-analysis, pooled analysis of 11 studies involving 500 patients with SAP showed that mortality was significantly lower in the TEN than TPN group. Compared with TPN, TEN had a lower risk of pancreatic infections and related complications, organ failure, and surgical interventions, and the length of hospital stay in was significantly shorter in the TEN than TPN group. These findings indicate that TEN is superior to TPN with respect to the clinical outcomes studied.
Bacterial translocation from the gut or absorption of endotoxins might drive the inflammatory response in patients with SAP. In critically ill patients, especially those with SAP, a metabolically deprived gut absorbs endotoxins or other bacterial products, stimulating endogenous cytokines and finally resulting in an immunoinflammatory response.24,26,27 Additionally, intestinal permeability is increased in patients with SAP.27 After promoting gut barrier alterations, TPN may facilitate the development of severe nosocomial infections, sepsis, and organ failure.29,30 In contrast, TEN can maintain the integrity and function of the intestinal mucosa,31 reducing bacterial translocation and maintaining the immunocompetence of the host.
Clinical and experimental evidence have revealed higher levels of both local and systemic inflammatory mediators with TPN than enteral nutrition. In a study of 67 patients with SAP by Xu et al.,32, the serum endotoxin level, diamine oxidase level, and urinary lactulose to mannitol excretion ratio were significantly lower in the enteral nutrition group than in the TPN group (P < 0.05). Windsor et al.7 reported increased levels of serum IgM anti-endotoxin antibodies after TPN treatment in 34 patients with acute pancreatitis, whereas there was no change in patients who underwent TEN treatment, indicating ongoing exposure to endotoxin. Gupta et al.12 reported a consistent but non-significant rise in IgM antibodies in patients treated with TPN throughout the study period, while the IgM antibody level fell in patients treated with TEN. Furthermore, they reported a broadly similar change in IgG antibodies between the TPN and TEN groups. TPN appears to lead to increased endotoxin exposure, perhaps as a result of bacterial colonization of feeding catheters.
This meta-analysis has some limitations. First, although we combined all available data to systematically evaluate the safety of TPN and TEM, the quality of some of the included studies was relatively low. Second, heterogeneity was present among the included studies and arose from differences in clinical samples, evaluation standards, and definitions of various infectious complications. Despite these limitations, we conclude that TEN is associated with a lower mortality rate, fewer infectious complications, a lower rate of organ failure, a lower surgical intervention rate, and a shorter hospital stay when compared with TPN. Third, through the sensitivity analysis, we found that the P value was not stable because of the study by Tao et al.18 The mixed population and the large sample size in this study might be the reasons for the lack of stability of the results. Hence, this meta-analysis supports more favorable outcomes for TEN than TPN. However, further well-designed clinical trials are necessary to support this conclusion.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Havala T, Shronts E, Cerra F. Nutritional support in acute pancreatitis. Gastroenterol Clin North Am 1989; 18: 525–542. [PubMed] [Google Scholar]
- 2.Pisters PW, Ranson JH. Nutritional support for acute pancreatitis. Surg Gynecol Obstet 1992; 175: 275–284. [PubMed] [Google Scholar]
- 3.Feller JH, Brown RA, Toussaint GP, et al. Changing methods in the treatment of severe pancreatitis. Am J Surg 1974; 127: 196–201. [DOI] [PubMed] [Google Scholar]
- 4.Kalfarentzos FE, Karavias DD, Karatzas TM, et al. Total parenteral nutrition in severe acute pancreatitis. J Am Coll Nutr 1991; 10: 156–162. [DOI] [PubMed] [Google Scholar]
- 5.Kudsk KA, Minard G, Wojtysiak SL, et al. Visceral protein response to enteral versus parenteral nutrition and sepsis in patients with trauma. Surgery 1994; 116: 516–523. [PubMed] [Google Scholar]
- 6.Moore FA, Feliciano DV, Andrassy RJ, et al. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann Surg 1992; 216: 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Windsor AC, Kanwar S, Li AG, et al. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut 1998; 42: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powell JJ, Murchison JT, Fearon KC, et al. Randomized controlled trial of the effect of early enteral nutrition on markers of the inflammatory response in predicted severe acute pancreatitis. Br J Surg 2000; 87: 1375–1381. [DOI] [PubMed] [Google Scholar]
- 9.Casas M, Mora J, Fort E, et al. [Total enteral nutrition vs. total parenteral nutrition in patients with severe acute pancreatitis]. Rev Esp Enferm Dig 2007; 99: 264–269. [DOI] [PubMed] [Google Scholar]
- 10.Doley RP, Yadav TD, Wig JD, et al. Enteral nutrition in severe acute pancreatitis. JOP 2009; 10: 157–162. [PubMed] [Google Scholar]
- 11.Eckerwall GE, Axelsson JB, Andersson RG. Early nasogastric feeding in predicted severe acute pancreatitis: A clinical, randomized study. Ann Surg 2006; 244: 965–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta R, Patel K, Calder PC, et al. A randomised clinical trial to assess the effect of total enteral and total parenteral nutritional support on metabolic, inflammatory and oxidative markers in patients with predicted severe acute pancreatitis (APACHEE II≥or = 6). Pancreatology 2003; 3: 406–413. [DOI] [PubMed] [Google Scholar]
- 13.Kalfarentzos F, Kehagias J, Mead N, et al. Enteral nutrition is superior to parenteral nutrition in severe acute pancreatitis: results of a randomized prospective trial. Br J Surg 1997; 84: 1665.–. [PubMed] [Google Scholar]
- 14.Louie BE, Noseworthy T, Hailey D, et al. 2004 MacLean-Mueller prize enteral or parenteral nutrition for severe pancreatitis: a randomized controlled trial and health technology assessment. Can J Surg 2005; 48: 298–306. [PMC free article] [PubMed] [Google Scholar]
- 15.Petrov MS, Kukosh MV, Emelyanov NV. A randomized controlled trial of enteral versus parenteral feeding in patients with predicted severe acute pancreatitis shows a significant reduction in mortality and in infected pancreatic complications with total enteral nutrition. Dig Surg 2006; 23: 336–344. [DOI] [PubMed] [Google Scholar]
- 16.Wang G, Wen J, Xu L, et al. Effect of enteral nutrition and ecoimmunonutrition on bacterial translocation and cytokine production in patients with severe acute pancreatitis. J Surg Res 2013; 183: 592–597. [DOI] [PubMed] [Google Scholar]
- 17.Wu XM, Ji KQ, Wang HY, et al. Total enteral nutrition in prevention of pancreatic necrotic infection in severe acute pancreatitis. Pancreas 2010; 39: 248–251. [DOI] [PubMed] [Google Scholar]
- 18.Tao Y, Tang C, Feng W, et al. Early nasogastric feeding versus parenteral nutrition in severe acute pancreatitis: A retrospective study. Pakistan Journal of Medicine Sciences 2016; 32: 1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003; 83: 713–721. [PubMed] [Google Scholar]
- 20.Greenberg JA, Hsu J, Bawazeer M, et al. Clinical practice guideline: management of acute pancreatitis. Can J Surg 2016; 59: 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClave SA, Spain DA, Snider HL. Nutritional management in acute and chronic pancreatitis. Gastroenterol Clin North Am 1998; 27: 421–434. [DOI] [PubMed] [Google Scholar]
- 22.Italian Association for the Study of the Pancreas (AISP), Pezzilli R, Zerbi A, et al. Consensus guidelines on severe acute pancreatitis. Dig Liver Dis 2015; 47: 532–543. [DOI] [PubMed] [Google Scholar]
- 23.Yi F, Ge L, Zhao J, et al. Meta-analysis: total parenteral nutrition versus total enteral nutrition in predicted severe acute pancreatitis. Intern Med 2012; 51: 523–530. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Xu Y, Lu T, et al. Meta-analysis of enteral nutrition versus total parenteral nutrition in patients with severe acute pancreatitis. Ann Nutr Metab 2008; 53: 268–275. [DOI] [PubMed] [Google Scholar]
- 25.Al-Omran M, Albalawi ZH, Tashkandi MF, et al. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev 2010; (1):CD002837. doi: 10.1002/14651858.CD002837.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med 1991; 19: 627–641. [DOI] [PubMed] [Google Scholar]
- 27.Al-Omran M, Groof A, Wilke D. Enteral versus parenteral nutrition for acute pancreatitis. Cochrane Database Syst Rev 2003;( 1):CD002837. [DOI] [PubMed] [Google Scholar]
- 28.Ammori BJ, Leeder PC, King RF, et al. Early increase in intestinal permeability in patients with severe acute pancreatitis: correlation with endotoxemia, organ failure, and mortality. J Gastrointest Surg 1999; 3: 252–262. [DOI] [PubMed] [Google Scholar]
- 29.Helton WS. Intravenous nutrition in patients with acute pancreatitis. Clinical Nutrition Parenteral Nutrition Wb Saunders 1990.
- 30.Saadia R, Schein M, Macfarlane C, et al. Gut barrier function and the surgeon. Br J Surg 1990; 77: 487–492. [DOI] [PubMed] [Google Scholar]
- 31.Buchman AL, Moukarzel AA, Bhuta S, et al. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. JPEN J Parenter Enteral Nutr 1995; 19: 453–460. [DOI] [PubMed] [Google Scholar]
- 32.Xu CF, Huang XX, Shen YZ, et al. [The effects of enteral nutrition versus total parenteral nutrition on gut barrier function in severe acute pancreatitis]. Zhonghua Nei Ke Za Zhi 2011; 50: 370–373. (in Chinese) [PubMed] [Google Scholar]