Short abstract
Objective
The present study was performed to investigate the relationships between swallowing outcomes and lesion location, bolus characteristics, and age in patients with subcortical stroke.
Patients: Patients with subcortical and insular stroke (mean age, 57.38 ± 12.71 years) were investigated. All patients (n = 21) completed both brain magnetic resonance imaging studies and videofluoroscopic swallowing studies.
Main Outcome Measures
The oral transit duration, pharyngeal transit duration (PTD), laryngeal response duration, and Penetration-Aspiration Scale (PAS) score were applied to examine the efficiency of propulsion and airway protection in three swallowing tasks. Path analyses were performed to assess the relationships between swallowing outcomes and lesion location, age, bolus viscosity, and bolus volume.
Results
Caudate nucleus (CN) lesions were associated with higher PAS scores. Insular lesions were associated with a longer PTD. Advanced age was associated with a longer PTD. Bolus viscosity significantly moderated the association between CN lesions and higher PAS scores.
Conclusions
In the present cohort, CN lesions impacted airway protection and insular lesions impacted pharyngeal transit. An increased bolus viscosity reduced the aspiration severity. These results suggest that lesion location is an important indicator to predict subsequent dysphagia in patients with subcortical stroke.
Keywords: Subcortical stroke, caudate nucleus, insula, pharyngeal transit duration, age, videofluoroscopic swallowing study
Introduction
Post-stroke dysphagia is characterized by a misdirection or delay in the transport of swallowed materials.1–3 Research of neurological swallowing dysfunction has implicated lesions in the anterior insula,4,5 the basal ganglia and internal capsule,1 and the periventricular white matter.6 Subcortical brain lesions may result in increased muscle tone or involuntary movements. Therefore, a plausible hypothesis is that patients with subcortical stroke may develop swallowing deficits secondary to an increase in the pharyngeal transit duration (PTD) and/or laryngeal response duration (LRD). A previous case-control study demonstrated an increased oral transit duration (OTD) and LRD in a small cohort of patients with subcortical stroke.1 Daniels and Foundas7 suggested that damage to subcortical structures, such as the thalamus and basal ganglia, may be more likely to increase the risk of aspiration in patients with stroke. Thus, damage to these subcortical structures is thought to not only delay the swallowing duration but also induce symptoms such as aspiration. However, although the neural network of subcortical structures is characterized by descending inputs from the cortical and subcortical regions to the cranial nerves and integration of inputs in both the cortical-subcortical and subcortical-bulbar regions,8 the neural control of swallowing is unclear. Indeed, the roles of specific subcortical structures are not fully understood. Identification of which subcortical lesions are dysfunctional is expected to provide clearer information regarding the neural network involved in swallowing control and the patient’s need for further evaluation or intervention.9
Evaluation of swallowing dysfunction in patients with subcortical stroke may require the assessment of two parameters: the efficiency of propulsion and the airway protection ability. Videofluoroscopic swallowing study (VFSS) is the recognized gold standard for the diagnosis and management of oropharyngeal dysphagia.10,11 VFSS also detects laryngeal aspiration during swallowing; i.e., the accidental inhalation of swallowed materials into the trachea due to inadequate airway protection.12,13 In addition, temporal measurements using VFSS have been used to evaluate the efficiency of propulsion (increased OTD or/and LRD) and PTD.14–16 Given the fact that these swallowing outcomes using VFSS can be as measures of the key characteristics of dysphagia, we sought to determine what specific subcortical lesions could affect swallowing efficiency and function for airway protection. Therefore, we investigated swallowing dysfunction in patients with stroke presenting with subcortical lesions. Analyses were performed to determine the relationship between lesion location and swallowing outcomes, such as temporal measurements and laryngeal aspiration severity.
Methods
Patients
The patients in this study were drawn from a cohort of patients with subcortical stroke who were recruited from consecutive admissions to the Neurorehabilitation Unit of our institution from March 2011 to November 2013. The inclusion criteria were the presence of a subcortical lesion, no previous history of neurological disease, and aspiration or/and disorder of several components of swallowing (respiratory distress; harsh, wet, and or nasal voice; and oral intake ability). The lesion location was confirmed by brain magnetic resonance (MR) imaging. Two radiologists reviewed each MR image at different times. The initial MR image was clinically interpreted by a reviewer within a few hours after the patient underwent the MR imaging study, and another radiologist also reviewed the image in the same manner after several days.17,18 All discrepancies were resolved by consensus through a discussion involving both reviewers. Lesions in the following locations were classified as subcortical and insular: putamen, caudate nucleus (CN), globus pallidus, insula, periventricular white matter, thalamus, external capsule, lateral ventricle, frontal lobe white matter, parietal lobe white matter, and temporal lobe white matter (Table 1). All patients completed VFSS within 14 days after the MR imaging study. The study was approved by the institutional review board of Chonbuk National University Hospital. All participants provided written informed consent prior to inclusion.
Table 1.
Age and lesion site for each patient.
| Patient no. | Age (y) | Subtype | Lesion side | Lesion site |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | CN | GP | I | PVW | T | EC | LV | FW | TW | PW | ||||
| 1 | 56 | Hemorrhage | Left | O | x | O | x | x | x | O | x | x | x | x |
| 2 | 64 | Hemorrhage | Left | O | x | x | x | x | x | x | x | x | x | x |
| 3 | 43 | Hemorrhage | Left | O | x | O | x | x | O | x | x | x | x | O |
| 4 | 44 | Hemorrhage | Left | O | x | O | x | x | O | O | x | O | O | x |
| 5 | 45 | Hemorrhage | Left | O | x | x | x | x | x | x | x | x | x | x |
| 6 | 53 | Infarction | Left | O | O | O | O | x | x | x | x | x | x | O |
| 7 | 77 | Infarction | Left | O | O | x | x | x | x | x | x | x | x | x |
| 8 | 42 | Infarction | Left | O | O | O | x | x | x | x | x | x | x | x |
| 9 | 49 | Infarction | Left | O | x | x | x | x | x | x | O | x | x | x |
| 10 | 49 | Infarction | Left | O | O | O | x | x | x | x | x | x | x | x |
| 11 | 52 | Infarction | Left | O | x | x | x | O | x | x | x | O | x | O |
| 12 | 61 | Infarction | Left | O | x | x | x | O | x | x | x | x | x | x |
| 13 | 55 | Hemorrhage | Right | O | x | O | x | O | x | x | x | x | x | x |
| 14 | 44 | Hemorrhage | Right | O | O | O | x | x | x | x | x | x | x | x |
| 15 | 54 | Infarction | Right | O | x | x | x | x | x | x | x | x | x | x |
| 16 | 69 | Infarction | Right | O | O | O | x | x | x | x | x | x | x | x |
| 17 | 81 | Infarction | Right | O | O | O | O | x | O | x | x | x | x | x |
| 18 | 49 | Infarction | Right | O | x | x | x | O | x | x | x | x | x | x |
| 19 | 63 | Infarction | Right | O | x | x | x | O | x | x | x | x | x | x |
| 20 | 74 | Infarction | Right | O | x | x | x | O | x | x | x | x | x | x |
| 21 | 81 | Infarction | Right | O | O | x | x | O | x | x | x | x | x | x |
x, present; O, absent; P, putamen; CN, caudate nucleus; GP, globus pallidus; I, insula; PVW, periventricular white matter; T, thalamus; EC, external capsule; LV, lateral ventricle; FW, frontal lobe white matter; PW, parietal lobe white matter; TW, temporal lobe white matter.
VFSS procedure
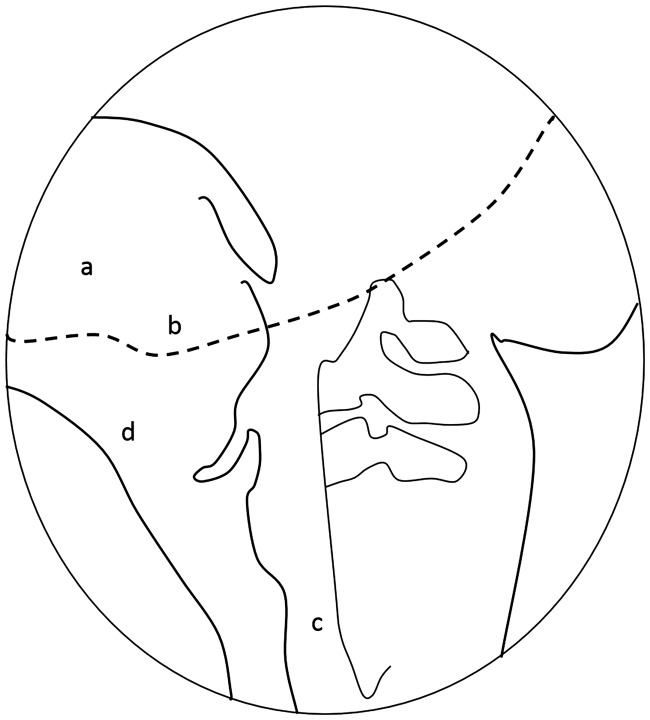
VFSS was performed with a SONIALVISION VERSA 100I/DAR-8000 (SHIMADZU Corp., Kyoto, Japan) in a fluoroscopy suite by a clinician, radiology technician, and speech-language pathologist. The patient was positioned in the examination chair in a lateral position and asked to hold a bolus in their mouth until instructed to swallow. The patient was asked to swallow the following three substances: 2 mL of thin liquid [3.2 centipoise (cP)], 5 mL of thin liquid (3.2 cP), and 5 mL of curd-type yogurt (5500 cP) and a diet adjusted to the patient’s swallowing function (i.e., rice porridge or steamed rice). The viscosity of the three bolus types was measured at the Korea Testing & Research Institute using a Brookfield viscometer (LV3 spindle, 30 r/min, 25ºC ± 1ºC; Brookfield Engineering, Middleboro, MA, USA). The bolus doses were mixed with barium sulfate and water or barium sulfate and yogurt, the maximum contaminant level of which was set at 100 (100% w/v) in water. The anatomical boundaries in the lateral fluoroscopic view were the lips, nasopharynx, cervical spine, and cervical esophagus (Figure 1). Fluoroscopic images were stored frame-by-frame in a computer at a rate of 30 frames per second.
Figure 1.
A lateral videofluoroscopic image with boundaries identifying the edges of the oral transit duration and pharyngeal transit duration and with the hyoid bone and laryngeal muscles for tracking of the laryngeal response duration in videofluoroscopic images. a, Tongue; b, ramus of mandible; c, anterior and posterior walls of cricopharyngeal region.
Outcome measures
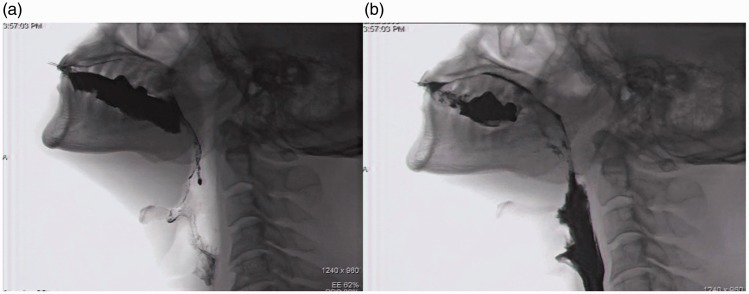
Temporal measurements of oropharyngeal swallowing and scores on the 8-point Penetration-Aspiration Scale (PAS)12 were analyzed using Final Cut Pro X software for Macintosh (Apple Inc., Cupertino, CA, USA) (Figure 2). Temporal measurements were made for the OTD, PTD, and LRD. For each swallowing event, temporal measurements were made of the time from onset to offset. For OTD, onset was defined as the time at which the bolus initiated a posterior movement in the oral cavity, and offset was defined as the time at which the bolus head reached the ramus of the mandible.10 For PTD, onset was defined as the time at which the bolus head reached the ramus of the mandible, and offset was defined as the time at which the tail of the bolus passed through the upper esophageal sphincter.19 For LRD, onset was defined as the time at which the bolus head reached the ramus of the mandible, and offset was defined as the time at which laryngeal elevation occurred as part of pharyngeal swallowing.10,16 These temporal measurements were evaluated by two speech-language pathologists. The main examiner analyzed all VFSS images. The second examiner analyzed a randomly selected 50% sample of the VFSS images to determine reliability.
Figure 2.
Videofluoroscopic sequencing image. a, Onset frame for oral transit duration; b, movement of bolus to esophagus.
Statistical analysis
To investigate the relative effects of lesion location, temporal measurements, and PAS scores, path analysis was performed using AMOS (IBM Corp., Armonk, NY, USA). Simultaneous estimation and direct testing was performed for indirect and total effects. A multi-regression method was used to confirm causal relationships between independent variables. The chi-square value of the research model was 15.92 (df = 9), the comparative fit index was 0.98, the goodness of fit index was 0.97, and the critical ratio (CR) of the multivariate normality test was 1.49. Variables X15 and Y5 reduced the total model fit and were therefore removed.
The 0.05 significance level of the research model supported the null hypothesis, and the research was therefore considered valid. Multicollinearity between independent variables was not present as indicated by a correlation coefficient of <0.22. Use of multiple regression analysis was therefore appropriate. Multiple group factor analyses were carried out (p > 0.05) to compare measurements obtained during the 2- and 5-mL thin liquid tasks (difference in volume) and the 5-mL thin liquid and 5-mL curd-type yogurt tasks (difference in viscosity). The present study used swallowing outcomes according to three bolus-swallowing tasks. Statistical analyses were performed using IBM SPSS Statistics, version 20.0 for Windows (IBM Corp.).
Results
Patients
Twenty-one patients were included in this study, Their mean age was 57.38 years [standard deviation (SD) = 12.71 years]. The demographic characteristics of the cohort are shown in Table 2.
Table 2.
Demographic characteristics of patients.
| Characteristics | Patients (n = 21) |
|---|---|
| Age, y | 57.38 (12.71) |
| Sex | |
| Male/female | 13/8 |
| NIHSS score | 8.47 (5.57) |
| Stroke type | |
| Infarction/hemorrhage | 7/14 |
| Lesion side | |
| Left/right | 12/9 |
| Lesion site | |
| Putamen | 21 |
| Caudate nucleus/globus pallidus/thalamus | 8/10/3 |
| Periventricular white matter | 7 |
| Insula | 2 |
| External capsule/lateral ventricle | 2/1 |
| Frontal/temporal/parietal lobe white matter | 2/2/1 |
Data are presented as n or mean (standard deviation). NIHSS, National Institutes of Health Stroke Scale.
Interobserver and intraobserver agreement
Test–retest reliability was assessed using the intraclass correlation coefficient (ICC). The ICC for interobserver agreement was excellent [ICC = 0.95, 95% confidence interval (CI) = 0.95–0.98]. Intraobserver agreement was also excellent (ICC = 0.94, 95% CI = 0.94–0.98).
Temporal measurements: OTD, PTD, and LRD
The mean OTD for the 2- and 5-mL thin liquids and the 5-mL yogurt was 0.87 s (SD = 0.63 s), 0.80 s (SD = 0.69 s), and 0.93 s (SD = 0.91 s), respectively. The mean PTD for the 2- and 5-mL thin liquids and the 5-mL yogurt was 0.83 s (SD = 0.50 s), 0.81 s (SD = 0.57 s), and 0.51 s (SD = 0.68 s), respectively. The mean LRD for the 2- and 5-mL thin liquids and the 5-mL yogurt was 0.40 s (SD = 0.61 s), 0.60 s (SD = 1.04 s), and 0.82 s (SD = 0.39 s), respectively.
Effects of bolus viscosity
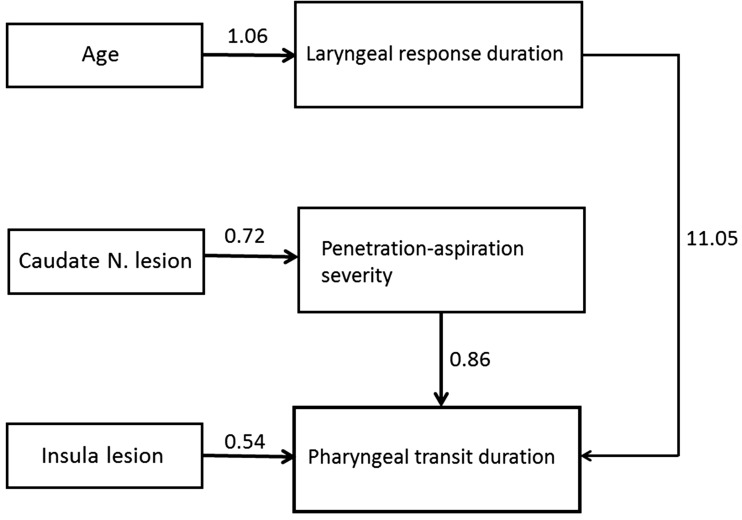
In the 5-mL thin liquid task, CN lesions were significantly associated with PAS scores (ß = 0.72, CR= 3.18, p = 0.001). Age was significantly associated with the LRD (ß = 1.06, CR = 2.62, p = 0.01). The PAS score was significantly associated with the PTD (ß = 0.83, CR = 4.32, p = 0.001). The LRD was significantly associated with the PTD (ß = 11.05, CR = 2.82, p = 0.001). In the 5-mL yogurt task, insular lesions were significantly associated with the PTD (ß = 0.54, CR = 2.23, p = 0.03) (Tables 3 and 4).
Table 3.
Results of path analysis in research model.
| Path analysis |
Dependent variables |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relationship | PAS score |
Pharyngeal transit duration |
Laryngeal response duration |
|||||||||
| Independent variables | ß | CR | p | ß | CR | p | ß | CR | p | |||
| Caudate nucleus lesion | Thin liquid | 2 mL | → | 0.25 | 0.86 | 0.39 | −0.36 | −1.15 | 0.25 | 0.49 | 1.05 | 0.30 |
| 5 mL | → | 0.72 | 3.18 | 0.001† | −0.01 | −0.29 | 0.77 | −0.75 | −1.96 | 0.06 | ||
| Yogurt | 5 mL | → | 0.03 | 0.17 | 0.86 | −0.56 | −1.69 | 0.09 | −0.28 | −0.59 | 0.55 | |
| Insular lesion | Thin liquid | 2 mL | → | 0.29 | 1.18 | 0.24 | −0.05 | −0.23 | 0.81 | −0.13 | 1.02 | 0.30 |
| 5 mL | → | −0.15 | −0.74 | 0.46 | 0.01 | −0.03 | 0.98 | 0.41 | 1.62 | 0.10 | ||
| Yogurt | 5 mL | → | 0.10 | 0.50 | 0.62 | 0.54 | 2.23 | 0.03* | 0.57 | 1.82 | 0.07 | |
| Age | Thin liquid | 2 mL | → | 1.61 | 0.49 | 0.63 | −0.04 | −0.08 | 0.93 | |||
| 5 mL | → | −0.19 | −0.50 | 0.62 | 1.06 | 2.62 | 0.009† | |||||
| Yogurt | 5 mL | → | 0.55 | 1.60 | 0.12 | 0.04 | 0.07 | 0.94 | ||||
| PAS score | Thin liquid | 2 mL | → | 0.14 | 0.81 | 0.42 | ||||||
| 5 mL | → | 0.86 | 4.32 | 0.00† | ||||||||
| Yogurt | 5 mL | → | −0.23 | −1.04 | 0.30 | |||||||
| Laryngeal response duration | Thin liquid | 2 mL | → | −0.92 | −0.71 | 0.48 | −0.11 | −0.12 | 0.90 | |||
| 5 mL | → | 6.33 | 1.428 | 0.158 | 11.05 | 2.82 | 0.005† | |||||
| Yogurt | 5 mL | → | −1.07 | −1.06 | 0.29 | −0.01 | −0.07 | 0.94 | ||||
CR, critical ratio; PAS, Penetration-Aspiration Scale. *p < 0.05, †p < 0.01
Table 4.
Relationship between variables in path analysis.
| Bolus type |
Independent variables | Relationship | Dependent variables | ß | CR | p | |
|---|---|---|---|---|---|---|---|
| Viscosity | Volume | ||||||
| 3.2 cP | 5 mL | Caudate nucleus lesion | → | PAS score | 0.72 | 3.18 | 0.001 |
| Age | → | LRD | 1.06 | 2.62 | 0.009 | ||
| PAS score | → | PTD | 0.86 | 4.32 | <0.001 | ||
| LRD | → | PTD | 11.05 | 2.82 | 0.005 | ||
| 5500 cP | 5 mL | Insular lesion | → | PTD | 0.54 | 2.23 | 0.03 |
CR, critical ratio; LRD, laryngeal response duration; PTD, pharyngeal transit duration; PAS, Penetration-Aspiration Scale. *p < 0.05, †p < 0.01.
Relationship among lesion location, PAS score, PTD, and bolus viscosity and volume
A significant moderating effect on the association between CN lesions and higher PAS scores was observed for bolus viscosity (CR = −2.48, over 95% CI). A significant moderating effect was also found for the relationship between insular lesions and the PTD (CR = 0.25, over 95% CI). The results of the path analysis are presented in Figure 3.
Figure 3.
Schematic diagram of path analyses for swallowing outcomes, age, and lesion location. The numbers are the standardized coefficients.
Discussion
The present study used VFSS data to determine associations between lesion location and specific swallowing problems in patients with subcortical stroke, particularly the efficiency of swallowing function and airway protection. The results of the present study suggest that some subcortical lesions were associated with swallowing outcomes. Specifically, CN lesions were associated with increased PAS scores in the 5-mL thin liquid swallowing task. This suggests that patients with stroke-associated CN lesions are vulnerable to aspiration. However, because all 21 patients in the present study had lesions in the putamen, a plausible hypothesis is that the observed insufficiency in airway protection was attributable to impaired striatum control. The CN and putamen are cytoarchitecturally similar structures with small neuronal cell bodies and are collectively referred to as the striatum.20 Previous MR imaging studies have demonstrated activation of both the CN and the putamen during swallowing.21 Thus, given the simultaneous activation of these structures, airway protection during swallowing appears to be under the control of the neurological unit that comprises the CN and the putamen. Moreover, another finding of the present study was the moderating effect of bolus viscosity on the relationship between CN lesions and the PAS score. Increasing viscosity was associated with a decrease in the PAS score. Previous studies of patients with dysphagia have demonstrated that increasing the viscosity of a thin liquid bolus results in increased swallowing safety.22–24 Newman et al.25 also reported a significant decrease in the PAS score with an increase in viscosity from thin liquid to 1750 cP. The present findings thus support the results of previous research and suggest that increased viscosity could improve the airway protection aspect of swallowing function.
Insular lesions were associated with an increased PTD in the 5-mL curd-type yogurt swallowing task. This result should be interpreted with caution because of the small number of patients with insular lesions. Previous functional MR imaging studies in healthy subjects indicated activation of the insula during swallowing.20,26 Another study showed connections between the insula and the primary and supplementary motor cortices and revealed that these brain regions facilitate coordinated interaction between the oral and pharyngeal phases of swallowing.27,28 Other research has shown that patients with insula lesions exhibit pharyngeal swallowing problems.4,29 Therefore, it is possible that damage to the insula might delay transport during pharyngeal swallowing, but a large cohort study is need to provide more definitive results.
Age was significantly associated with the LRD. This result is unsurprising because several studies of healthy older adults have demonstrated an increase in the duration of pharyngeal triggering and oral and pharyngeal swallowing.14,22,30 Advanced age is associated with sensorimotor impairment, which frequently manifests as inefficient swallowing characterized by an increased LRD.31 Even in the presence of normal swallowing duration, older patients may be required to invest more effort in swallowing than younger adults, who recruit their neural networks more efficiently. Reduced swallowing efficiency by aging has been under discussion for a long time, and the present finding thus supports the hypothesis that patients with subcortical stroke display a tendency to develop inefficient pharyngeal swallowing.
Finally, the PTD was associated with an increased PAS score in the 5-mL thin liquid swallowing task. Previous studies have also shown that an increased PTD is associated with aspiration32,33 and that among patients with dysphagia, the PTD is longer in those with than without aspiration.32 Im et al.34 indicated that when the PTD was >0.71 s in patients with hemisphere stroke, laryngeal penetration occurred. Laryngeal aspiration/penetration is responsible for dysfunction of both pharyngeal and laryngeal movements.35,36 Because it is generally accepted that the mechanism of these movements involves transmission via the brain stem,21,37,38 damage to the sensory motor pathway that passes through the subcortical area from the cortical region to the brain stem may affect reduced swallowing efficiency and airway protection. Thus, damage to subcortical structures is thought to increase pharyngeal transit resulting from reduced sensory input, and aspiration may reflect an increased PTD.
The present study has several limitations. First, the cohort was small, which limits the generalizability of the results to other patients with subcortical stroke. Second, the time interval between MR imaging and VFSS varied. This may have impacted the results because swallowing outcomes are influenced by the stroke prognosis. Similarly, the analyses did not take stroke severity into account. Therefore, patient heterogeneity may have impacted the results. Future studies should control for stroke severity and the interval between MR imaging and VFSS. Third, the difference in the bolus volume evaluated via VFSS (2 mL versus 5 mL) was small. This may have led to the observed lack of association for this variable. A previous study of patients with left basal ganglion/internal capsule lesions compared bolus volumes of 1 mL versus 10 mL and found an effect.1 Future research should therefore investigate larger differences in bolus volumes.
Conclusions
The present data suggest that in patients with subcortical stroke, CN lesions may impact airway protection while insular lesions may impact pharyngeal transit. CN and insular lesions may therefore predict aspiration and problems with pharyngeal swallowing, respectively. In addition, increasing the bolus viscosity may reduce the aspiration severity. A future study of the relationship between swallowing outcomes and lesion location may lead to the development of a sensitive index for predicting dysphagia associated with subcortical lesions.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No: HI15C1529).
References
- 1.Logemann JA, Shanahan T, Rademaker AW, et al. Oropharyngeal swallowing after stroke in the left basal ganglion/internal capsule. Dysphagia 1993; 8: 230–234. [DOI] [PubMed] [Google Scholar]
- 2.Power ML, Hamdy S, Goulermas JY, et al. Predicting aspiration after hemispheric stroke from timing measures of oropharyngeal bolus flow and laryngeal closure. Dysphagia 2009; 24: 257–264. [DOI] [PubMed] [Google Scholar]
- 3.Robbins J, Levine RL, Maser A, et al. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil 1993; 74: 1295–1300. [DOI] [PubMed] [Google Scholar]
- 4.Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia 1997; 12: 146–156. [DOI] [PubMed] [Google Scholar]
- 5.Stickler D, Gilmore R, Rosenbek JC, et al. Dysphagia with bilateral lesions of the insular cortex. Dysphagia 2003; 18: 179–181. [DOI] [PubMed] [Google Scholar]
- 6.Cola MG, Daniels SK, Corey DM, et al. Relevance of subcortical stroke in dysphagia. Stroke 2010; 41: 482–486. [DOI] [PubMed] [Google Scholar]
- 7.Daniels SK, Foundas AL. Lesion localization in acute stroke. J Neuroimaging 1999; 9: 91–98. [DOI] [PubMed] [Google Scholar]
- 8.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia 1993; 8: 195–202. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Fernandez M, Kleinman JT, Ky PK, et al. Supratentorial Regions of Acute Ischemia Associated With Clinically Important Swallowing Disorders. Stroke 2008; 39: 3022–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logemann JA, Pauloski BR, Rademaker AW, et al. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res 2000; 43: 1264–1274. [DOI] [PubMed] [Google Scholar]
- 11.Logemann JA, Pauloski BR, Rademaker AW, et al. Oropharyngeal swallow in younger and older women: videofluoroscopic analysis. J Speech Lang Hear Res 2002; 45: 434–445. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia 1996; 11: 93–98. [DOI] [PubMed] [Google Scholar]
- 13.Perlman AL, Booth BM, Grayhack JP. Videofluoroscopic predictors of aspiration in patients with oropharyngeal dysphagia. Dysphagia 1994; 9: 90–95. [DOI] [PubMed] [Google Scholar]
- 14.Im I, Kim Y, Oommen E, et al. The effects of bolus consistency in pharyngeal transit duration during normal swallowing. Ann Rehabil Med 2012; 36: 220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Im I. The Effects of bolus viscosity and volume on temporal measurements and aspiration of oropharyngeal swallowing in supratentorial stroke patients. J Speech Hear Disord 2017; 26: 133–141. [Google Scholar]
- 16.Kim Y, McCullough GH. Stage transition duration in patients poststroke. Dysphagia 2007; 22: 299–305. [DOI] [PubMed] [Google Scholar]
- 17.Ebisu T, Tanaka C, Umeda M, et al. Hemorrhagic and nonhemorrhagic stroke: diagnosis with diffusion-weighted and T2-weighted echo-planar MR imaging. Radiology 1997; 203: 823–828. [DOI] [PubMed] [Google Scholar]
- 18.Lutsep HL, Albers GW, DeCrespigny A, et al. Clinical utility of diffusion‐weighted magnetic resonance imaging in the assessment of ischemic stroke. Ann Neurol 1997; 41; 574–580. [DOI] [PubMed] [Google Scholar]
- 19.Robbins J, Hamilton JW, Lof GL, et al. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 1992; 103: 823–829. [DOI] [PubMed] [Google Scholar]
- 20.McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience 1989; 29: 503–537. [DOI] [PubMed] [Google Scholar]
- 21.Hamdy S, Mikulis DJ, Crawley A, et al. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiology 1999; 277: 219–225. [DOI] [PubMed] [Google Scholar]
- 22.Ballou Stahlman L, Mertz Garcia J, Hakel M, et al. Comparison ratings of pureed versus molded fruits: preliminary results. Dysphagia 2000; 15: 2–5. [DOI] [PubMed] [Google Scholar]
- 23.Bisch EM, Logemann JA, Rademaker AW, et al. Pharyngeal effects of bolus volume, viscosity, and temperature in patients with dysphagia resulting from neurologic impairment and in normal subjects. J Speech Hear Res 1994; 37: 1041–1059. [DOI] [PubMed] [Google Scholar]
- 24.Butler SG, Stuart A, Castell D, et al. Effects of age, gender, bolus condition, viscosity, and volume on pharyngeal and upper esophageal sphincter pressure and temporal measurements during swallowing. J Speech Lang Hear Res 2009; 52: 240–253. [DOI] [PubMed] [Google Scholar]
- 25.Newman R, Vilardell N, Clave P, et al. Effect of Bolus Viscosity on the Safety and Efficacy of Swallowing and the Kinematics of the Swallow Response in Patients with Oropharyngeal Dysphagia: White Paper by the European Society for Swallowing Disorders (ESSD). Dysphagia 2016; 31: 232–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki M, Asada Y, Ito J, et al. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 2003; 18: 71–77. [DOI] [PubMed] [Google Scholar]
- 27.Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia 1997; 12: 146–156. [DOI] [PubMed] [Google Scholar]
- 28.Wan P, Chen X, Zhu L, et al. Dysphagia post subcortical and supratentorial stroke. J Stroke Cerebrovasc Dis 2016; 25: 74–82. [DOI] [PubMed] [Google Scholar]
- 29.Stickler D, Gilmore R, Rosenbek JC, et al. Dysphagia with bilateral lesions of the insular cortex. Dysphagia 2003; 18: 179–181. [DOI] [PubMed] [Google Scholar]
- 30.Cook IJ, Dodds WJ, Dantas RO, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia 1989; 4: 8–15. [DOI] [PubMed] [Google Scholar]
- 31.Gleeson DC. Oropharyngeal swallowing and aging: a review. J Commun Disord 1999; 32: 95–96. [DOI] [PubMed] [Google Scholar]
- 32.Choi KH, Ryu JS, Kim MY, et al. Kinematic analysis of dysphagia: significant parameters of aspiration related to bolus viscosity. Dysphagia 2011; 264: 392–398. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus CL, Logemann JA, Rademaker AW, et al. Effects of bolus volume, viscosity, and repeated swallows in nonstroke subjects and stroke patients. Arch Phys Med Rehabil 1993; 74: 1066–1070. [DOI] [PubMed] [Google Scholar]
- 34.Im I, Kim HH, Kim HG, et al. Relationship between temporal measurements of pharyngeal swallowing and penetration-aspiration in unilateral stroke patients. Commun Sci Disord 2017; 22: 570–577. [Google Scholar]
- 35.Shaker R, Dodds WJ, Dantas RO, et al. Coordination of deglutitive glottic closure with oropharyngeal swallowing. Gastroenterology 1990; 98: 1478–1484. [DOI] [PubMed] [Google Scholar]
- 36.Johnson ER, McKenzie SW, Rosenquist CJ, et al. Dysphagia following stroke: quantitative evaluation of pharyngeal transit times. Arch Phys Med Rehabil 1992; 73: 419–423. [PubMed] [Google Scholar]
- 37.Ertekin C. Voluntary versus spontaneous swallowing in man. Dysphagia 2011; 26: 183–192. [DOI] [PubMed] [Google Scholar]
- 38.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 2001; 81: 929–969. [DOI] [PubMed] [Google Scholar]