Short abstract
Objective
This study was performed to investigate the correlation among decreased regional cerebral oxygen saturation (rSO2), blood levels of brain injury biomarkers, and postoperative cognitive disorder (POCD) after cardiac surgery with cardiopulmonary bypass (CPB).
Methods
This prospective observational study included 59 patients undergoing coronary artery bypass graft surgery with CPB. All patients underwent neuropsychological tests (Mini Mental State Evaluation, Rey Auditory Verbal Learning Test, digit span test, digit symbol substitution test, and Schulte table) the day before and 10 days after the surgery. The blood levels of two brain injury biomarkers, neuron-specific enolase (NSE) and glial fibrillary acidic protein (GFAP), were measured before and 1 day after the surgery.
Results
The rSO2 decreased during surgery in 21 (35%) patients. POCD was detected in 22 (37%) patients. After the surgery, no significant changes in the GFAP blood level occurred in any patients. No significant correlations were found among the decreased rSO2, increased NSE blood level, and rate of POCD.
Conclusion
These results suggest that a decrease in rSO2 during cardiac surgery is not necessarily related to the development of POCD or an increased blood level of the brain injury biomarker NSE.
Keywords: Regional cerebral oxygen saturation, postoperative cognitive dysfunction, cardiac surgery, glial fibrillary acidic protein, neuron-specific enolase, cardiopulmonary bypass
Introduction
Postoperative cognitive disorder (POCD) is a common complication after cardiac surgery with cardiopulmonary bypass (CPB). It occurs in 30% to 80% of patients during the first postoperative week, and it can persist up to several months postoperatively in 50% of patients.1,2 The differences in the rate of POCD among different studies may be associated with the lack of standardized diagnostic methods for POCD. Various authors use different tests that evaluate different cognitive functions. The diagnostics used for POCD are not routine in most cardiac surgery centers. Cognitive dysfunction is detected in patients without a history of cardiac surgery as well as in intensive care unit (ICU) patients (25%) and in the nonsurgical population of the same age (3%). However, the rate of POCD after cardiac surgery is much higher.1
The etiology of POCD remains unclear and is considered to be a polyetiological complication. Reported predisposing factors are older age, concomitant diseases such as diabetes mellitus, pre-existing neurological diseases, and injuries. The operative factors considered to influence POCD are micro- and macro-embolization (atherosclerotic plaques, air emboli, and thrombi), hypotension, hypoperfusion, and systemic inflammatory reactions. Routinely used monitoring techniques such as pulse oximetry and arterial blood pressure measurement only indirectly indicate tissue and brain perfusion and oxygenation. Monitoring of the regional cerebral oxygen saturation (rSO2) allows for more accurate evaluation of the oxygen balance (delivery and consumption) in the brain tissue.
This study was performed to evaluate the correlation among a decrease in the rSO2 from baseline, the blood levels of brain injury biomarkers, and the development of POCD after cardiac surgery with CPB.
Material and methods
This prospective observational clinical study was conducted at the Clinic of Cardiothoracic and Vascular Surgery in the Hospital of the Lithuanian University of Health Sciences Kauno Klinikos. The study was approved by the Kaunas Regional Bioethics Committee (permission No. BE-2-49). All patients provided written agreement to participate by signing an informed consent form.
Patients undergoing elective coronary artery bypass grafting (CABG) surgery without pre-existing neurological disease and/or lesions were enrolled in the study. We excluded patients who underwent combined surgery (CABG + valve surgery); underwent emergency or redo surgery; were suffering from alcohol abuse; had existing cognitive dysfunction; had a history of psychoneurological disease and/or disorders; were taking medications affecting the central nervous system (CNS); had hearing, vision, or any other disorders that could affect the possibility of physically performing the test; and who were unable to speak Lithuanian.
Perioperative period
Midazolam or diazepam was prescribed for premedication. All patients received standard general anesthesia: 1–2 μg/kg of fentanyl, 0.05 mg/kg of midazolam, 1.0–2.5 mg/kg of propofol, and 0.08–0.1 mg/kg of pipecuronium for induction and 3 mg/kg/h of fentanyl, 1–4 mg/h of midazolam, and 1.5%–2.0% MAC of sevoflurane for maintenance. Standard monitoring of vital functions was performed: continuous electrocardiography, heart rate, invasive arterial blood pressure, pulse oximetry, esophageal temperature, and end-tidal carbon dioxide in the exhaled gas mixture.
Operating technique and CPB
All patients underwent median sternotomy. The aorta was cross-clamped, and anterograde cold crystalloid cardioplegia was applied for myocardial protection. The CPB perfusion pump was primed with 1.5 L of crystalloid solution containing 10,000 units of heparin. A roller-pump with a membrane oxygenator (Dideco D703; Dideco S.p.A., Mirandola, Italy) and a venous reservoir were used. The nonpulsatile pump flow rate was maintained between 2.4 and 2.6 L/min/m2. During CABG, proximal anastomoses were constructed after removal of the aortic cross-clamp using a side-bite clamp. Certain aspects of patient management, such as the target mean arterial pressure and the rate of patient rewarming, were determined by standard practice and not dictated by the study protocol.
ICU
The patients received standard postoperative care and monitoring in the ICU and on the postoperative ward.
Evaluation of cognitive function
Cognitive function was evaluated on the eve of surgery and 7 to 10 days after surgery, before discharge from the hospital. The following tests were performed: Mini Mental State Evaluation; Rey Auditory Verbal Learning Test; number sequence matching task (Trail-Making Test, Parts A and B); two subtests of the Wechsler Adult Intelligence Scale (i.e., the digit span test and digit symbol substitution test) that were adapted to the Lithuanian language according to the Wechsler method by Goštautas, Dembinskas, and Pilkauskienė in 1979; and the Schulte table for attention and concentration.
Relative changes between the preoperative and postoperative test scores were calculated and expressed as Z scores. The change in a Z score relative to its associated error was used to estimate deterioration of mental abilities and to detect POCD. POCD was diagnosed when the sum of all Z scores was >2 or at least two Z scores for separate tests were >2.3,4
Blood tests of brain injury biomarkers
Blood samples were collected for measurement of glial fibrillary acidic protein (GFAP) and neuron-specific enolase (NSE) before surgery, immediately before anesthetic induction (baseline level), and 24 hours after surgery (in the ICU). The levels of biomarkers were measured by standard sandwich enzyme-linked immunosorbent assay in accordance with the manufacturer’s instructions (BioVendor, Brno, Czech Republic). The absorbance was measured using a Stat-Fax 4200 microplate reader (Awareness Technology, Palm City, FL, USA) at a 430-nm wavelength. Each plasma sample was double-blind, resulting in an average concentration.
Cerebral oximetry
An INVOS™ oximeter (Somanetics, Troy, MI, USA) was used to monitor rSO2. A decrease in the rSO2 of ≥20% from baseline or an rSO2 of ≤45% was defined as desaturation. The baseline rSO2 value was established after patient arrival to the operating room at a 21% fraction of inspired oxygen (ambient air).
Statistical analyses
All statistical analyses were performed with IBM SPSS Statistics, version 20.0 (IBM Corp., Armonk, NY, USA). Quantitative variables are described as mean and standard deviation. The Kolmogorov–Smirnov test revealed that the variables did not meet the criteria for a normal distribution; therefore, nonparametric methods were used for further analysis. The Mann–Whitney U test was used to compare quantitative variables. The Pearson chi-squared test (χ2) was used to determine qualitative variables. Spearman’s correlation coefficient was calculated to determine the relationship between POCD and a decreased rSO2 and between POCD and changes in the blood levels of brain injury biomarkers. The data were considered statistically significant at p < 0.05.
Results
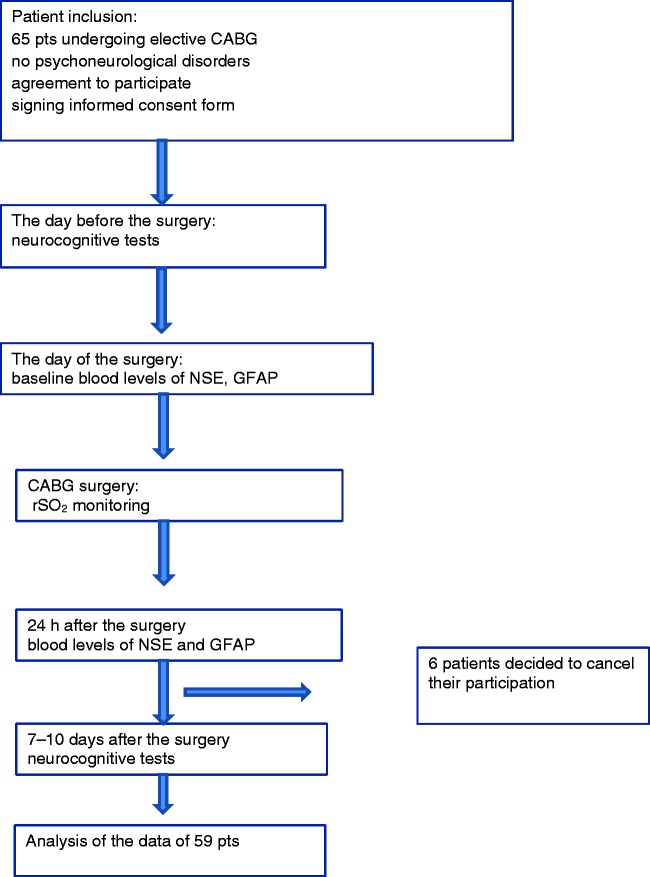
In total, 65 patients were initially enrolled in the study. Six patients were excluded after the surgery because they refused to perform the postoperative cognitive function tests and decided to withdraw from the study. Therefore, the data of 59 patients were analyzed (Figure 1).
Figure 1.
Flow chart of the study protocol. CABG, coronary artery bypass grafting; NSE, neuron-specific enolase; GFAP, glial fibrillary acidic protein; rSO2, regional cerebral oxygen saturation.
POCD was detected in 22 (37%) patients. During the surgery, a decrease in rSO2 was detected in 21 (35%) patients. The shortest episode of decreased rSO2 lasted 0.2 minutes, and the longest lasted 168 minutes. The disturbances of rSO2 were effectively corrected, and the average total time of a decreased rSO2 was 13.8 minutes. In two patients, no measures were effective, and the decreased rSO2 lasted for 66 and 168 minutes, respectively. Neither of these patients developed POCD. The patients were divided into two groups according to the disturbances of rSO2: patients in Group I had no episodes of decreased rSO2 (≥20%) (n = 38), and patients in Group II had episodes of decreased rSO2 (≥20%) (n = 21). The two groups showed no differences in demographics (age, sex,) (Table 1) or clinical data (concomitant diseases, left ventricular ejection fraction, American Society of Anesthesiologists class) (Table 2). The rate of POCD did not differ between the groups.
Table 1.
Demographic data of the study population.
| Characteristic | All patients(n = 59) | Group I(n = 38) | Group II(n = 21) | p value(Groups I and II) |
|---|---|---|---|---|
| Mean age, years | 67.1 ± 8.7 | 67.7 ± 8.3 | 64.9 ± 8.6 | 0.2 |
| Sex, male/female | 35/24 (59/41) | 25/13 (66/34) | 10/11 (48/52) | 0.2 |
| Class ASA III patients | 50 (85) | 32 (84) | 18 (86) | 0.6 |
| Class ASA IV patients | 9 (15) | 6 (16) | 3 (14) | 0.6 |
| Diabetes mellitus | 15 (25) | 11 (29) | 4 (19) | 0.3 |
| Hb | 135 ± 16 | 135 ± 14 | 134 ± 18 | 0.7 |
| Hct | 40.5 ± 4.2 | 40 ± 4 | 40 ± 5 | 0.6 |
Data in the table are presented as mean ± standard deviation, n, or n (%). Hb, hemoglobin; Hct, hematocrit; ASA, American Society of Anesthesiologists; Group I, patients who had no decrease in regional cerebral oxygen saturation; Group II, patients who had a ≥20% decrease in regional cerebral oxygen saturation.
Table 2.
Intraoperative patient data.
| Characteristic | All patients(n = 59) | Group I(n = 38) | Group II (n = 21) | p value(Groups I and II) |
|---|---|---|---|---|
| Averaged duration of cardiopulmonary bypass, min | 87 ± 20 | 86 ± 17 | 88 ± 25 | 0.7 |
| Averaged aortic cross-clamping duration, min | 44 ± 14 | 42 ± 11 | 47 ± 17 | 0.2 |
| Hb, g/L | 99.8 ± 12 | 100 ± 13 | 97 ± 10 | 0.3 |
| Hct, % | 29.9 ± 3.7 | 30 ± 4 | 29 ± 3 | 0.5 |
Data in the table are presented as mean ± standard deviation. Hb, hemoglobin; Hct, hematocrit; Group I, patients who had no decrease in regional cerebral oxygen saturation; Group II, patients who had a ≥20% decrease in regional cerebral oxygen saturation.
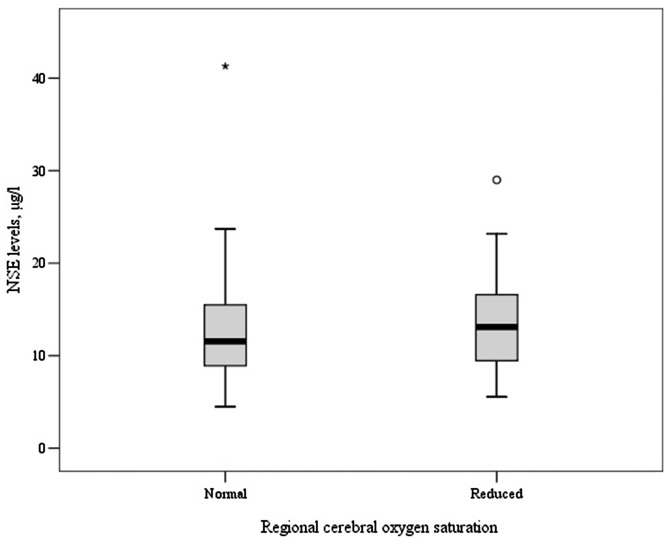
No changes in the blood level of GFAP were observed in any patients after the surgery compared with baseline. The blood level of NSE increased in 29 (49%) patients after the surgery compared with baseline. There was no correlation between the increase in the NSE blood level and POCD. These data are presented in Table 2. The blood level of NSE did not differ significantly between patients with and without POCD (Figure 2).
Figure 2.
Association between rSO2 and increase in NSE. rSO2, regional cerebral oxygen saturation; NSE, neuron-specific enolase.
Discussion
Episodes of decreased rSO2 are detected during both cardiac and noncardiac surgery. A decreased rSO2 may be caused by ventilation or oxygenation disorders, changes in hemodynamics, changes in the hemoglobin concentration, or even the patient’s position during surgery (e.g., anti-Trendelenburg position in shoulder joint surgery or laparoscopic surgery).5 The rate of desaturation during the surgery is independent of the patient’s preoperative condition, but the baseline rSO2 is lower in patients with diabetes, arterial hypertension, or an elevated cholesterol level.6 In the present study, the incidence of a decreased rSO2 was similar to that reported by other authors (35%).7,8 The incidence of POCD was similar between patients with a decreased rSO2 (Group II) and without (Group I). In all patients, the decreased rSO2 was corrected by the usual algorithm, and the average total desaturation time was 2.6 minutes. In two patients (9.5%), the methods used to correct the desaturation were not effective, and it lasted 66 and 168 minutes, respectively. In most patients, however, the duration of decreased rSO2 was short (because it was corrected), which may have led to the lack of significant differences in POCD between the two groups. In a randomized multicenter study including 201 patients, Deschamps et al.7 showed that correction of rSO2 impairment reduced the rate of repetitive impairment during the surgery and the rate of desaturation in the ICU after surgery. Our study also suggests that desaturation during the surgery is not the only cause of POCD and that early correction of rSO2 disorders allows for improvement in the postoperative neurological outcome.
Cognitive decline is often detected in the early postoperative period and shows substantial recovery within 1 to 3 months.2 Deschamps et al.7 found that desaturation may also be detected postoperatively in the ICU. These episodes of rSO2 may influence the development of POCD. In this study, we focused on early POCD. However, our study was limited by the absence of rSO2 monitoring after the surgery in the ICU.7
GFAP is found in astrocytes, which are basic neuroglial cells. We found no postoperative increases in the GFAP blood level in any patient. The concentration of GFAP increases in patients with brain damage (intracranial hemorrhage, traumatic brain injury) and is correlated with the area of injury, clinical severity, and functional outcome.9 Previously reported data show the use of GFAP to differentiate ischemic injury and intracranial hemorrhage.10,11 In the present study, we found no changes in the blood levels of GFAP after the surgery compared with baseline. Additionally, although some studies have shown high sensitivity and specificity of GFAP in brain damage, other data show that an increase in the GFAP level depends on the nature of the damage; an increase in GFAP is more typical of a “mass” effect induced by hemorrhage. Rapid pressure-induced death of brain cells occurs over time. In patients with diffuse brain damage, the growth rate of GFAP is delayed.12
NSE is found in neurons, neuroectoderm cells, and erythrocytes. The serum NSE level is a diagnostic and prognostic measure that is correlated with the extent of brain damage. The normal NSE concentration in the healthy population ranges from 2 to 20 μg/L.12 It becomes elevated in patients with malignant neuroendocrine tumors, small cell lung carcinoma, neuroblastoma, ischemic and/or hemorrhagic stroke, meningoencephalitis, or CNS trauma. In adult perioperative patients with no known CNS lesions, an increase in NSE may be explained by subclinical damage to brain cells undergoing reversible changes (diffuse microembolism and increased blood–brain barrier permeability).13 NSE measurement can be used to effectively predict neurological outcomes after cardiac arrest and in patients with ischemic stroke.12 However, its use in cardiac surgery is controversial. A correlation of NSE with POCD was observed in some studies,12,14–16 but not in others.9,12,17 The NSE concentration in the cerebrospinal fluid after aortic aneurysm repair surgery was increased regardless of the presence or absence of neurological symptoms.12
In the present study, an increase in the blood level of the brain cell injury biomarker NSE was found in 29 (49%) patients. This increase was observed at the same rate in both patients who did and did not have episodes of decreased rSO2 during the surgery. The increase in NSE was not correlated with cognitive impairment after the surgery. NSE is also found in platelets and erythrocytes.9 During CPB, hemolysis is inevitable to a lesser or greater degree because of the interaction of blood cells with artificial surfaces. This could explain the increase in NSE after cardiac surgery with CPB. Although studies of NSE in patients with ischemic and hemorrhagic stroke indicate a correlation between an increased NSE concentration and the extent of the brain damage (stroke area), studies of trauma patients have shown that the NSE blood level increases regardless of the presence of traumatic brain injury.9
Conversely, glial cells (astrocytes) are less susceptible to ischemia and the neurons themselves are more susceptible to ischemia, which could explain why the NSE concentration changed and there was no increase in the GFAP concentration.18 Mondello et al.19 investigated brain biomarkers after brain injury and found that neuronal damage was more pronounced in diffuse brain damage and that glial cells were more vulnerable in cases of mass lesions.
Our study indicates that desaturation during surgery is a fairly frequent disorder. The study had an observational design, and the anesthesiologists took measures to correct cerebral desaturation in accordance with a routinely used protocol in our clinic. The results prove the effectiveness of such a protocol because we found no increase in the blood levels of brain injury markers or correlation with POCD.
Conclusion
Our data suggest that short-term episodes of decreased rSO2 during surgery are not correlated with either the incidence of cognitive impairment after the surgery or the blood levels of brain injury biomarkers.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a grant from the Research Council of Lithuania (grant number MIP-022/2014).
References
- 1.Gao L, Taha R, Gauvin D, et al. Postoperative cognitive dysfunction after cardiac surgery. Chest 2005; 128: 3664–3670. [DOI] [PubMed] [Google Scholar]
- 2.Bruggemans EF. Cognitive dysfunction after cardiac surgery: Pathophysiological mechanisms and preventive strategies. Neth Heart J 2013; 21: 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frerichs RJ, Tuokko HA. A comparison of methods for measuring cognitive change in older adults. Arch Clin Neuropsychol 2005; 20: 321–333. [DOI] [PubMed] [Google Scholar]
- 4.Lewis MS, Maruff P, Silbert BS, Evered LA, Scott DA. The influence of different error estimates in the detection of post-operative cognitive dysfunction using reliable change indices with correction for practice effects. Arch Clin Neuropsychol 2006; 21: 421–427. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen HB. Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front Physiol 2014; 5: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baikoussis NG, Karanikolas M, Siminelakis S, et al. , Baseline cerebral oximetry values in cardiac and vascular surgery patients: a prospective observational study. J Cardiothorac Surg 2010; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschamps A, Hall R, Grocott H, et al. Cerebral Oximetry monitoring to maintain normal cerebral oxygen saturation during high-risk cardiac surgery: a randomized controlled feasibility trial. Anesthesiology 2016; 124: 826–836. [DOI] [PubMed] [Google Scholar]
- 8.Colak Z, Borojevic M, Bogovic A. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: a randomized, prospective study. Eur J Cardiothorac Surg 2015; 47: 447–454. [DOI] [PubMed] [Google Scholar]
- 9.Seco M, Edelman JJ, Wilson MK, et al. Serum biomarkers of neurologic injury in cardiac operations. Ann Thorac Surg 2012; 94: 1026–1033. [DOI] [PubMed] [Google Scholar]
- 10.Foerch C, Niessner M, Back T, et al. Diagnostic accuracy of plasma glial fibrillary acidic protein for differentiating intracerebral hemorrhage and cerebral ischemia in patients with symptoms of acute stroke. Clin Chem 2012; 58: 237–245. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci 2015. 38: 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cata JP, Abdelmalak B, Farag E. Neurological biomarkers in the perioperative period. Br J Anaesth 2011; 107: 844–858. [DOI] [PubMed] [Google Scholar]
- 13.Reinsfelt B, Ricksten SE, Zetterberg H, et al. Cerebrospinal fluid markers of brain injury, inflammation, and blood-brain barrier dysfunction in cardiac surgery. Ann Thorac Surg 2012; 94: 549–555. [DOI] [PubMed] [Google Scholar]
- 14.Ramlawi B, Rudolph JL, Mieno S, et al. Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann Surg 2006; 244: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann M, Ebert AD, Tober D, et al. A contrastive analysis of release patterns of biochemical markers of brain damage after coronary artery bypass grafting and valve replacement and their association with the neurobehavioral outcome after cardiac surgery. Eur J Cardiothorac Surg 1999; 16: 513–518. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen LS, Christiansen M, Eliasen K, et al. Biochemical markers for brain damage after cardiac surgery – time profile and correlation with cognitive dysfunction. Acta Anaesthesiol Scand 2002; 46: 547–551. [DOI] [PubMed] [Google Scholar]
- 17.Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One 2013; 8: e79624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parpura V, Heneka MT, Montana V, et al. Glial cells in (patho)physiology. J Neurochem 2012; 121: 4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondello S, Papa L, Buki A, et al. Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit Care 2011; 15: R156. [DOI] [PMC free article] [PubMed] [Google Scholar]