Short abstract
Objective
To investigate levels of regulatory B (Breg) cells, plasma cells, and memory B cells in the peripheral blood, and interleukin (IL)-10 in the serum of multiple sclerosis (MS) patients, and to determine the correlation between Breg cell levels and the Expanded Disability Status Scale (EDSS) score.
Methods
Levels of Breg cells, plasma cells, and memory B cells in the peripheral blood of 12 MS patients were measured using flow cytometry. IL-10 serum levels were measured by enzyme-linked immunosorbent assay. The correlation between Breg cell levels and MS EDSS score was measured using Pearson’s correlation coefficient.
Results
Compared with healthy controls, MS patients had decreased levels of CD19+CD24hiCD38hi Breg cells in their peripheral blood and reduced serum levels of IL-10; however, the ratios of CD19+CD27hiCD38hi plasma cells and CD19+CD27+CD24hi memory B cells to total B cells did not differ significantly between healthy controls and MS patients. CD19+CD24hiCD38hi Breg cell levels in the peripheral blood of MS patients were not significantly correlated with MS EDSS score.
Conclusion
Peripheral blood CD19+CD24hiCD38hi Breg cell levels and serum IL-10 levels were reduced in MS patients compared with controls, but Breg cell levels were not correlated with MS EDSS score.
Keywords: Multiple sclerosis, regulatory B (Breg) cells, interleukin-10, Expanded Disability Status Scale, plasma cells, memory B cells
Introduction
Multiple sclerosis (MS) is a chronic disease of the central nervous system (CNS). Its major pathological feature is the presence of multiple demyelinating plaques in CNS white matter with reactive gliosis and axonal injury.1,2 As one of the most common neurological diseases worldwide, MS is the leading cause of non-traumatic neurological disability among young patients. The incidence of MS in China is 0 to 5/10 million, but there has been an upward trend in recent years.3,4
Although the pathogenesis of MS is currently unclear, it is generally considered to be an autoimmune disease, and is associated with viral infection, genetic polymorphisms, autoimmune reactivity, and environmental factors.5 B lymphocytes can produce autoantibodies, so they have long been considered to play a key role in autoimmune diseases. Neta and Salvin previously identified a type of B cell that inhibits T cell immune activity in the delayed-type hypersensitivity guinea pig model.6 This was supported by the work of Blair et al.7 in 2010, which found that immature interleukin (IL)-10-secreting B cells in human peripheral blood have a CD19+CD24hiCD38hi phenotype that suppresses the differentiation of helper T cells. More recently, Mizoguchi showed that B cells negatively regulate the immune system in an inflammatory bowel disease model, and first proposed the term “regulatory B (Breg) cells”.8 These cells have an immunosuppressive function via their release of cytokines such as IL-10, IL-35, and transforming growth factor-β, and through their induction of other regulatory cells. Breg cells are also thought to be affected in a variety of autoimmune diseases.9 For example, they are diminished in quantity or function in patients with systemic lupus erythematosus, rheumatoid arthritis, and immune thrombocytopenia, and in renal transplant recipients.10–13 However, few studies have examined the role of Breg cells in MS patients.
Therefore, in the present study, we investigated multiple B cell subgroups in the peripheral blood of MS patients, including Breg cells, plasma cells, and memory B cells, as well as the role of serum IL-10. We also studied the relationship between Breg cell levels and the Expanded Disability Status Scale (EDSS) score of MS.
Patients and methods
Patients and control
This study was approved by the Bengbu Medical College Ethics Committee. MS patients were diagnosed in the First Affiliated Hospital of Bengbu Medical College according to the 2010 McDonald diagnostic criteria.14 All patients were newly diagnosed and were at the acute stage of MS at the time of the study. Patients had never been treated with steroids, interferon, or other MS drugs. The EDSS score was used to measure disease severity. Patients provided written informed consent to participate in the study.
Sample collection
A total of 5 mL of peripheral venous blood was aseptically collected from each participant into heparin anticoagulation tubes. One mL of the whole blood sample was used for fluorescent antibody staining followed by flow cytometry (see below). The remaining sample was centrifuged at 1847 × g for 10 minutes, and the supernatant was stored at −70°C until IL-10 detection.
Detection of B cell subtypes
Peripheral venous whole blood was collected from subjects, and 0.83% ammonium chloride was used to separate red blood cells. Whole blood was then stained with the following fluorescent antibodies: anti-CD24-FITC, anti-CD19-PE, anti-CD27-PEcy5, and anti-CD38-APC (eBioscience, San Diego, CA, USA). Fluorescently-stained cells were then detected by BD FACSVerse flow cytometry (BD Biosciences, San Jose, CA, USA). Peripheral venous blood B cells (CD19+ lymphocytes) were divided into Breg cells (CD19+CD24hiCD38hi), memory B cells (CD19+CD27+CD24hi), and plasma cells (CD19+CD27hiCD38hi).
Serum IL-10 measurement
Serum IL-10 detection was performed using a commercially available enzyme-linked immunosorbent assay kit according to the manufacturer's instructions (CUSABIO, Wuhan, Hubei, China). Absorbance was measured at 450 nm using a microplate reader (Huawe Delang, Wuxi, Jiangsu, China).
Statistical analyses
Statistical analyses were performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Data are expressed as means ± standard deviation, and t-tests and Mann–Whitney non-parametric tests were used to compare results between groups. Correlations between two variables were analyzed by Pearson’s correlation coefficient. A P value < 0.05 was considered statistically significant.
Results
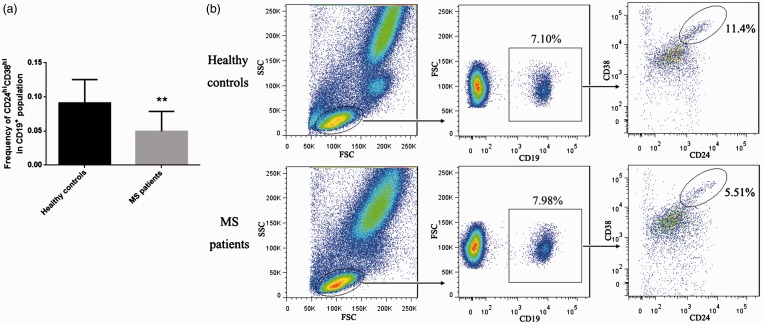
The CD19+CD24hiCD38hi Breg cell to total B cell ratio is decreased in the peripheral blood of MS patients
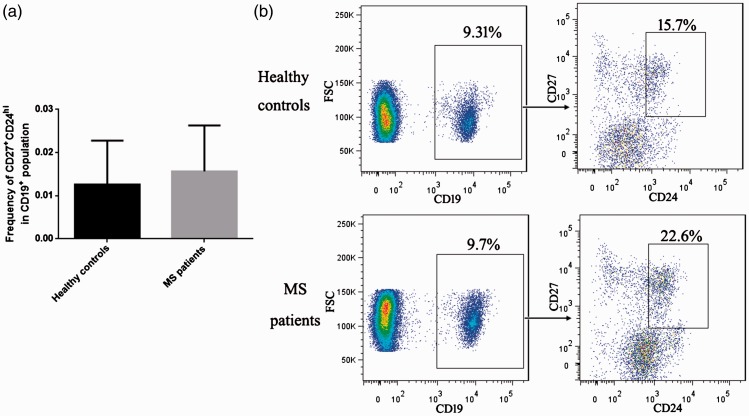
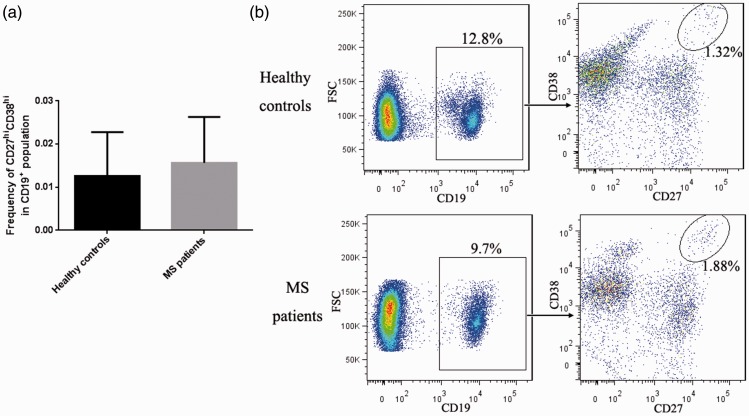
Twelve patients (seven men and five women, mean age: 37.6 ± 10.7 years) were included in the study, and 12 healthy adult volunteers (seven men and five women, mean age: 20 ± 0.9 years) were enrolled as control subjects. Compared with the controls, MS patients had significantly lower ratios of CD19+CD24hiCD38hi Breg cells to total B cells in their peripheral blood (Figure 1; P<0.01). Because memory B cells and plasma cells are also associated with the pathogenesis of autoimmune diseases,15,16 we examined the ratios of CD19+CD27+CD24hi memory B cells and CD19+CD27hiCD38hi plasma cells to total B cell levels in peripheral blood. However, as shown in Figures 2 and 3, these ratios did not differ significantly between MS patients and healthy controls.
Figure 1.
The ratio of CD19+CD24hiCD38hi Breg cells to total B cells in peripheral blood was decreased in MS patients. (a) Bar chart comparing MS patients with healthy controls, P<0.01. (b) Representative flow cytometry analysis.
Figure 2.
The ratio of CD19+CD27+CD24hi memory B cells to total B cells in peripheral blood was unchanged in patients with MS. (a) Bar chart comparing MS patients with healthy controls. (b) Representative flow cytometry analysis.
Figure 3.
The ratio of CD19+CD27hiCD38hi plasma cells to total B cells in peripheral blood was unchanged in patients with MS. (a) Bar chart comparing MS patients with healthy controls. (b) Representative flow cytometry analysis.
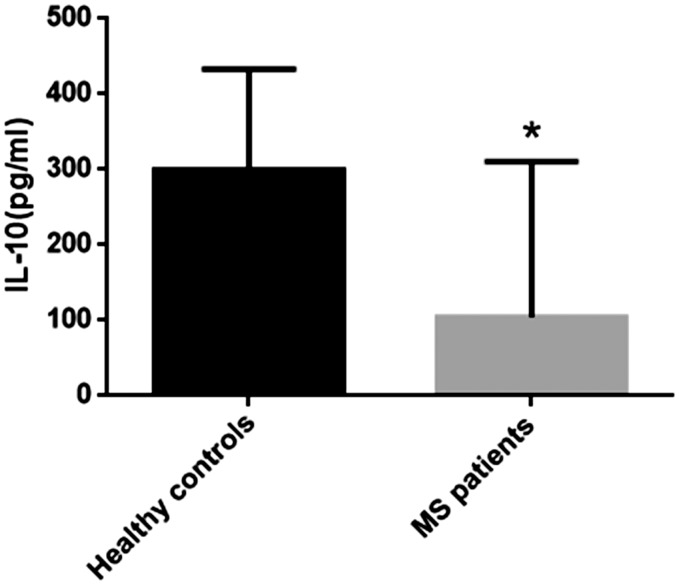
IL-10 levels are decreased in the peripheral blood of MS patients
IL-10 plays an important role in regulating the immune response, and the immunosuppressive function of Breg cells is mainly performed by secreting IL-10.9 Therefore, we next measured serum IL-10 levels, and found that MS patients had significantly lower serum IL-10 levels than healthy controls (Figure 4; P<0.05).
Figure 4.
IL-10 serum levels were decreased in MS patients as shown by ELISA. P<0.05, compared with controls.
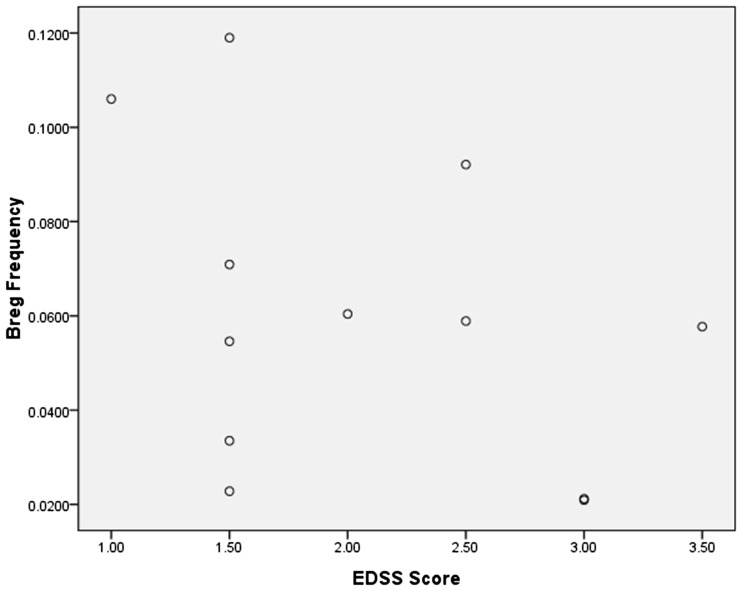
Relationship between EDSS score and CD19+CD24hiCD38hi Breg cell levels in MS patients
The mean EDSS score of the 12 patients was 2.08. Although CD19+CD24hiCD38hi Breg cell levels were reduced in MS patients compared with healthy controls, we did not find a significant correlation between Breg cell levels and MS severity, as measured by the EDSS score (Figure 5; r=−0.391).
Figure 5.
The EDSS score was not correlated with CD19+CD24hiCD38hi Breg cell levels in MS patients. The Pearson correlation coefficient revealed no significant correlation (r=−0.391).
Discussion
B cells were first proposed to have inhibitory functions in the 1970s based on observations that guinea pig spleens depleted of B cells failed to inhibit the adoptive transfer of delayed-type hypersensitivity.6 Since then, B cells have been shown to suppress the immune response by secreting IL-10 in models of colitis, experimental allergic encephalomyelitis (EAE), and arthritis.17–19 During the past 10 years, many advances have been made in immunosuppressive, or Breg cells. For example, several IL-10-producing Breg cells have been identified in mice, including CD5+CDldhi B (B10) cells and CD138+ plasma cells.20,21 In humans, CD19+CD24hiCD38hi cells were shown to secrete IL-10 as an immunosuppressive function.11 Moreover, levels of CD19+CD24hiCD38hi Breg cells were decreased in patients with anti-neutrophil cytoplasmic antibody-related vasculitis and active lesion rheumatoid arthritis,22 as well as in those with non-splenectomy idiopathic thrombocytopenia;23 these latter patients demonstrated a rise in Breg cell numbers with increasing platelet levels.
In the present study, we found that the ratio of CD19+CD24hiCD38hi Breg cells to total B cells in the peripheral blood of MS patients was decreased compared with healthy controls. Previous studies showed that B cell-deficient mice did not recover from EAE, which is an animal model of human MS.24,25 Consistent with this finding, the deletion of B cells using anti-CD20 antibody treatment prior to EAE induction resulted in worsening disease symptoms and the increased infiltration of T cells in the CNS.26 Accordingly, Breg cell treatment of EAE reduced CNS demyelination and inflammatory cell infiltration.27 These findings suggested that Breg cells play an important role in a variety of autoimmune diseases, including MS. However, it is noteworthy that CD19+CD24hiCD38hi Breg cell levels were not correlated with MS disease severity in our study. Because MS is a multifactorial chronic neurological disease, the involvement of each patient in our study was short, and the study included only a small sample. EDSS scores (<4) represented mild to moderate disease severity, but the patients were likely to be biased, which may partially explain the lack of correlation between the EDSS score and Breg cell level.
The regulating role of B cells is generally thought to be achieved by IL-10 secretion.9 IL-10 is an important immune regulator that limits the tissue-damaging inflammatory response, and is essential for maintaining the immune balance.28 Mice lacking IL-10 signals are highly susceptible to enteritis because of the lack of a normal immune response to symbiotic bacteria.29 Moreover, IL-10 levels are decreased in patients with inflammatory bowel disease,30 and IL-10 mRNA expression is reduced in the skin of patients with psoriasis.31 Further, serum levels of IL-10 are reduced in patients with type 2 diabetes, and may be considered a risk factor,32 while IL-10 levels are reduced in the CNS of the rat EAE model.33 Peripheral blood IL-10 mRNA levels were previously shown to be reduced in MS patients,34 which is consistent with our current findings. Together, these results indicate that circulating levels of IL-10 combined with the quantity of CD19+CD24hiCD38hi Breg cells could be used as an important index for the diagnosis of MS.
Memory B cells rapidly differentiate into effector cells, so their role in autoimmune diseases has attracted much attention. It has been suggested that the deletion of memory B cells may prevent the progression of MS.35 Indeed, children with MS have considerably higher levels of memory B cells compared with healthy children. Additionally, Schwarz et al.36 found that CD19+CD27+ memory B cells were decreased in the peripheral blood of MS patients but increased in their CNS. Moreover, increased numbers of memory B cells are associated with neurodegenerative changes in recurrent MS.37 However, in our study, CD19+CD27+CD24hi memory B cell levels in the peripheral blood of MS patients did not differ significantly from those of control subjects, although this could reflect the early MS stages of the patients. Nevertheless, it is noteworthy that the proportion of plasma cells in MS patients did not change significantly compared with healthy controls in our study, indicating that the role of antibodies may not be dominant in MS pathogenesis.
In conclusion, we found that MS patients had decreased ratios of CD19+CD24hiCD38hi Breg cells to total B cells and decreased IL-10 levels in their peripheral blood compared with healthy controls, although CD19+CD27hiCD38hi plasma cell and CD19+CD27+CD24hi memory B cell ratios were unchanged. However, we found no significant correlation between Breg cell levels and the MS EDSS score. Our findings help us to better understand the pathogenesis of MS, and provide a basis for exploring new diagnostic methods.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the Scientific Research Innovation Team Project of Anhui Colleges and Universities (2016-40).
References
- 1.Luo C, Jian C, Liao Y, et al. The role of microglia in multiple sclerosis. Neuropsychiatr Dis Treat 2017; 13: 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dargahi N, Katsara M, Tselios T, et al. Multiple sclerosis: immunopathology and treatment update. Brain Sci 2017; 7 pii: E78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 2010; 9: A387–A394. [DOI] [PubMed] [Google Scholar]
- 4.Liu X, Cui Y, Han J. Estimating epidemiological data of Multiple sclerosis using hospitalized data in Shandong Province, China. Orphanet J Rare Dis 2016; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jörg S, Grohme DA, Erzler M, et al. Environmental factors in autoimmune diseases and their role in multiple sclerosis. Cell Mol Life Sci 2016; 73: 4611–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neta R, Salvin SB. Specific suppression of delayed hypersensitivity: the possible presence of a suppressor B cell in the regulation of delayed hypersensitivity. J Immunol 1974; 113: 1716–1725. [PubMed] [Google Scholar]
- 7.Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010; 32: 129–140. [DOI] [PubMed] [Google Scholar]
- 8.Mizoguchi E, Mizoguchi A, Preffer FI, et al. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol 2000; 12: 597–605. [DOI] [PubMed] [Google Scholar]
- 9.Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015; 42: 607–612. [DOI] [PubMed] [Google Scholar]
- 10.Menon M, Blair PA, Isenberg DA, et al. A regulatory feedback between plasmacytoid dendritic cells and regulatory B cells is aberrant in systemic lupus erythematosus. Immunity 2016; 44: 683–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17differentiation. Sci Transl Med 2013; 5: 173ra23. [DOI] [PubMed] [Google Scholar]
- 12.Tebbe B, Wilde B, Ye Z, et al. Renal transplant recipients treated with calcineurin-inhibitors lack circulating immature transitional CD19+CD24hiCD38hi regulatory B-lymphocytes. PLoS One 2016; 11: e0153170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012; 120: 3318–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hummel HM, Brück W, Dreha-Kulaczewski S, et al. Pediatric onset multiple sclerosis: McDonald criteria 2010 and the contribution of spinal cord MRI. Mult Scler 2013; 19: 1330–1335. [DOI] [PubMed] [Google Scholar]
- 15.Tarlinton DM, Hodgkin PD. Targeting plasma cells in autoimmune diseases. J Exp Med 2004; 199: 1451–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker D, Marta M, Pryce G, et al. Memory B cells are major targets for effective immunotherapy in relapsing multiple sclerosis. EBioMedicine 2017; 16: 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fillatreau S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002; 3: 944–950. [DOI] [PubMed] [Google Scholar]
- 18.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol 2012; 30: 221–241. [DOI] [PubMed] [Google Scholar]
- 19.Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 2002; 16: 219–230. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizaki A, Miyagaki T, DiLillo DJ, et al. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 2012; 491: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014; 507: 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aybar LT, McGregor JG, Hogan SL, et al. Reduced CD5(+) CD24(hi) CD38(hi) and interleukin-10(+) regulatory B cells in active anti-neutrophil cytoplasmic autoantibody-associated vasculitis permit increased circulating autoantibodies. Clin Exp Immunol 2015; 180: 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012; 120: 3318–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolf SD, Dittel BN, Hardardottir F, et al. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med 1996; 184: 2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dittel BN, Urbania TH, Janeway CA., Jr. Relapsing and remitting experimental autoimmune encephalomyelitis in B cell deficient mice. J Autoimmun 2000; 14: 311–318. [DOI] [PubMed] [Google Scholar]
- 26.Anthony DC, Dickens AM, Seneca N, et al. Anti-CD20 inhibits T cell-mediated pathology and microgliosis in the rat brain. Ann Clin Transl Neurol 2014; 1: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Lapato A, Bodhankar S, et al. Treatment with IL-10 producing B cells in combination with E2 ameliorates EAE severity and decreases CNS inflammation in B cell-deficient mice. Metab Brain Dis 2015; 30: 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanjabi S, Zenewicz LA, Kamanaka M, et al. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol 2009; 9: 447–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998; 66: 5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Feng BS, Yang SB, et al. Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel disease. J Biol Chem 2012; 287: 3591–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Robaee AA, Al-Zolibani AA, Al-Shobili HA, et al. IL-10 implications in psoriasis. Int J Health Sci (Qassim) 2008; 2: 53–58. [PMC free article] [PubMed] [Google Scholar]
- 32.Yaghini N, Mahmoodi M, Asadikaram GR, et al. Serum levels of interleukin 10 (IL-10) in patients with type 2 diabetes. Iran Red Crescent Med J 2011; 13: 752. [PMC free article] [PubMed] [Google Scholar]
- 33.Diab A1, Zhu J, Xiao BG, et al. High IL-6 and low IL-10 in the central nervous system are associated with protracted relapsing EAE in DA rats. J Neuropathol Exp Neurol 1997; 56: 641–650. [PubMed] [Google Scholar]
- 34.Huang WX, Huang P, Link H, et al. Cytokine analysis in multiple sclerosis by competitive RT - PCR: A decreased expression of IL-10 and an increased expression of TNF-alpha in chronic progression. Mult Scler 1999; 5: 342–348. [DOI] [PubMed] [Google Scholar]
- 35.Thompson SA, Jones JL, Cox AL, et al. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol 2010; 30: 99–105. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz A, Balint B, Korporal-Kuhnke M, et al. B-cell populations discriminate between pediatric- and adult-onset multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 4: e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Comabella M, Sastre-Garriga J, Montalban X. Precision medicine in multiple sclerosis: biomarkers for diagnosis, prognosis, and treatment response. Curr Opin Neurol 2016; 29: 254–262. [DOI] [PubMed] [Google Scholar]