Short abstract
Objective
This study was performed to determine the prevalence and risk factors associated with nasal methicillin-resistant Staphylococcus aureus (MRSA) colonization upon intensive care unit (ICU) admission and during the ICU stay in mainland China.
Methods
A prospective observational study was performed in a 50-bed general ICU of a 4300-bed teaching hospital in China from 2011 to 2013. Nasal swabs for MRSA detection were obtained upon ICU admission and at discharge for patients having stayed in the ICU for longer than 3 days.
Results
In total, 115 patients (4.1%; 95% confidence interval [CI], 3.4–4.9) were already colonized with MRSA on ICU admission, and another 185 patients (10.7%; 95% CI, 9.3–12.2) acquired MRSA during their ICU stay. Development of an MRSA infection was significantly more likely in patients with than without MRSA colonization on ICU admission (odds ratio [OR], 2.8; 95% CI, 1.1–7.3). Patients who acquired MRSA had significantly prolonged lengths of stay in the ICU (23.3 days) and higher hospital bills (135,171 RMB; about 19,590 USD) than those who tested negative for MRSA.
Conclusion
The MRSA colonization rate among ICU patients in mainland China is high. Patients with MRSA-positive nasal swabs are more likely to develop MRSA infections.
Keywords: Active surveillance culture, methicillin-resistant Staphylococcus aureus, intensive care unit, colonization, nasal swab, risk factors
Background
Methicillin-resistant Staphylococcus aureus (MRSA), which was first reported in 1961 in the UK, is a major cause of healthcare-associated infection throughout the world.1 Patients with healthcare-associated MRSA infections have an increased risk of mortality both prior to and after discharge, require longer hospital stays, and accumulate greater medical costs.2–4 During 2011 in the United States, an estimated 80,461 invasive MRSA infections occurred, 48,353 of which were healthcare-associated community-onset infections and 14,156 of which were hospital-onset infections.5,6 MRSA infections are associated with an estimated 11,285 deaths per year in the United States.5,6 The prevalence of MRSA infection is likely to be much higher in intensive care units (ICUs). In one study, the healthcare-associated MRSA infection rate was 1.64 infections per 1000 patient-days in the ICU and 0.47 infections per 1000 patient-days in non-ICU settings.7 In light of the fact that many patients are already MRSA-positive on ICU admission and that the majority of MRSA infections are acquired while patients are receiving healthcare, it has been suggested that an important measure in mitigating MRSA spread in the ICU is the routine collection of active surveillance cultures (ASCs). However, some studies have found that such measures are ineffective.8,9
MRSA was first reported in mainland China in the 1980s.10 A limited number of studies have demonstrated that 29.2% of all S. aureus infections are MRSA infections, and patients who have been infected with MRSA have longer hospital stays and a higher risk of mortality.11,12 Although several small-scale studies examining the prevalence of MRSA colonization on ICU admission have been published in China, whether MRSA-carrying patients are more likely to develop MRSA infections during their hospitalization remains unknown. A previous study that took place in the same hospital in which our study was conducted showed a high degree of genetic relatedness between their patients and clinical MRSA isolates.13 In the present study, routine MRSA screening was performed in a general ICU to examine the prevalence and risk factors associated with nasal MRSA colonization among ICU patients on admission to the ICU and during their ICU stay.
Methods
Study population
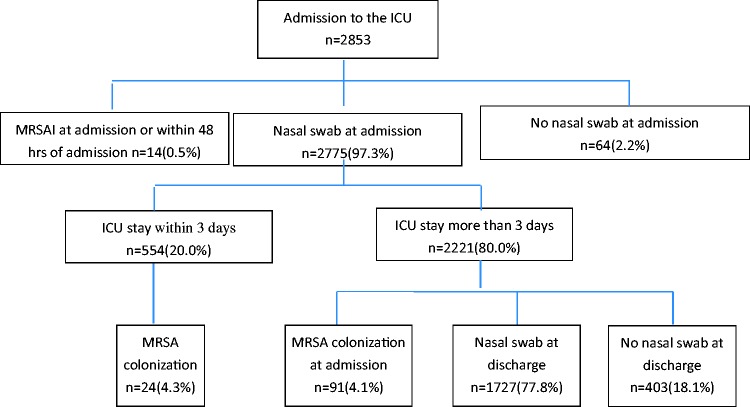
This prospective study was carried out in a general 50-bed ICU at West China Hospital, which is a 4300-bed university hospital in western China. From 1 January 2011 to 31 December 2013, all patients who were admitted to the ICU were enrolled in the study except those with a known MRSA infection on ICU admission. Nasal swabs for MRSA screening were collected from patients within 48 hours of admission; they were also collected at discharge for patients having stayed in the ICU for longer than 3 days. The study population is shown in Figure 1.
Figure 1.
Description of the study population. Data are presented as n (%) of patients. ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; MRSAI, methicillin-resistant Staphylococcus aureus infection.
ICU nurses collected the swab samples after receiving training by microbiologists and infection control practitioners. For patients with multiple ICU admissions during a single hospitalization period, only their first ICU admission was included. Demographic data such as age, sex, reason for ICU admission, existence of underlying diseases, length of stay (LOS) in the ICU, and mortality in the ICU were retrieved from the patients’ medical records.
Microbiological surveillance
Surveillance cultures were plated on ChromID MRSA-Select agar plates (bioMérieux, Marcy-l'Étoile, France) and incubated at 35°C for 18 to 24 hours. Clinical samples from patient infection sites were tested by hospital microbiology laboratory personnel, and MRSA isolates were identified using the VITEK 2 system (bioMérieux). Methicillin resistance was confirmed using cefoxitin (30 mg) disk diffusion susceptibility testing according to the guidelines of the Clinical and Laboratory Standards Institute.12
Definitions of colonization and infection
MRSA colonization was assumed based upon the isolation of MRSA from that patient’s nasal swab. MRSA colonization was reported as ICU-acquired only if the patient had an MRSA-positive nasal swab that was collected at least 48 hours after ICU admission, no history of prior colonization or infection at West China Hospital, and no MRSA-positive swabs or clinical samples within 48 hours of admission. MRSA infection was diagnosed in patients with an MRSA-positive clinical sample. ICU-acquired MRSA infection was defined as the development of MRSA infection at least 48 hours after ICU admission. The incidence of MRSA infection was reported per 100 patients.
Review of Chinese data
The following major electronic databases were systematically searched: China National Knowledge Infrastructure (CNKI) database, Chinese Biomedical Literature database (CBM), Chinese VIP database, Chinese Wanfang database, and Medline database. The search terms were “‘MRSA’ OR ‘methicillin-resistant Staphylococcus aureus,’” “‘active surveillance’ OR ‘nasal colonization,’” and “‘ICU’ OR ‘intensive care unit.’” The search location was restricted to mainland China.
Statistical analysis
Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA, USA) was used to manage the data. Statistical analyses were performed using PASW Statistics, version 18.0 (SPSS Inc., Chicago, IL, USA). Because our continuous data did not follow a normal probability distribution using the Kolmogorov–Smirnov test, they are expressed as medians along with 25th and 75th percentiles and were analyzed using the Mann–Whitney U-test. Categorical data are presented as number and percentage and were analyzed using the chi-square test or Fisher’s exact test. All tests were two-tailed, and a P-value of <0.05 was considered significant.
Because this study was only an epidemiological survey, no intervening measures were enforced on any patients. The ethics committee of West China Hospital approved the study and waived the need for informed consent.
Results
During the study period, 2853 patients were admitted to the ICU. Of those admitted, 14 (0.5%) had an MRSA infection within 48 hours of admission, while 64 (2.2%) did not have a nasal swab collected within 48 hours of ICU admission. During the study period, 554 patients (20.0%) were discharged within 3 days and 2221 patients (77.8%) remained in the ICU for more than 3 days,; of these 2221 patients, 1727 (77.8%) were sampled at discharge according to the study protocol.
Therefore, 2775 patients (97.3%) were screened for MRSA on ICU admission. Their mean age was 56.6 ± 19.3 years, and the male:female ratio was 1.8:1.0 (64.8%:35.2%). A total of 115 patients (4.1%; 95% confidence interval [CI], 3.4–4.9) had nasal MRSA colonization. There were no significant differences in demographic characteristics or underlying diseases between patients with and without MRSA colonization. The ICU LOS, hospital expenses, and mortality rates were not significantly different between patients with and without MRSA colonization (Table 1). A total of 47 patients (1.7%; 95% CI, 1.2–2.2) acquired MRSA infections in the ICU during the study period. Patients with MRSA colonization on admission were more likely to develop an MRSA infection than those without (4.3% vs. 1.6%, respectively; odds ratio [OR], 2.8; 95% CI, 1.1–7.3). The MRSA infection rate in 2011, 2012, and 2013 was 2.2% (95% CI, 1.2–3.2), 2.0% (95% CI, 1.2–2.9), and 0.8% (95% CI, 0.2–1.4), respectively (linear-by-linear association, 5.04; P = 0.02).
Table 1.
Characteristics and outcomes of MRSA-positive and -negative patients on ICU admission.
| Characteristics | MRSA-negative (n = 2660) | MRSA-positive (n = 115) | Z/χ 2 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Sex | ||||
| Male | 1728 (65.0) | 71 (61.7) | ||
| Female | 932 (35.5) | 44 (38.3) | 0.50 | 0.48 |
| Age, years | ||||
| Mean ± standard deviation | 56.6 ± 19.2 | 56.2 ± 20.3 | 2.21 | 0.14 |
| Median (range) | 58.3 (0–98) | 56.5 (13–89) | ||
| Pre-ICU LOS, days | ||||
| Mean | 6.7 | 6.2 | 0.67 | |
| Underlying disease | ||||
| COPD | 346 (13.0) | 21 (18.3) | 2.65 | 0.10 |
| Coma | 24 (0.9) | 3 (2.6) | 0.10 | |
| Heart failure | 146 (5.5) | 9 (7.8) | 1.14 | 0.29 |
| Renal failure | 238 (8.9) | 13 (11.3) | 0.74 | 0.39 |
| Diabetes mellitus | 150 (5.6) | 6 (5.2) | 0.04 | 0.89 |
| Hypertension | 248 (9.3) | 17 (14.8) | 3.80 | 0.05 |
| Respiratory failure | 552 (20.8) | 27 (23.5) | 0.50 | 0.48 |
| Cancer | 338 (12.7) | 13 (11.3) | 0.20 | 0.69 |
| Blood disease | 41 (1.5) | 2 (1.7) | 0.69 | |
| Prior invasive procedure | ||||
| Mechanical ventilation | 672 (25.3) | 32 (27.8) | 0.38 | 0.54 |
| Central venous catheterization | 194 (7.3) | 11 (9.6) | 0.83 | 0.36 |
| Urinary catheterization | 547 (20.6) | 23 (20.0) | 0.02 | 0.88 |
| Dialysis | 42 (1.6) | 3 (2.6) | 0.43 | |
| Surgery | 698 (26.2) | 27 (23.5) | 0.44 | 0.51 |
| Outcome | ||||
| MRSA infection | 42 (1.6) | 5 (4.3) | 0.04 | |
| Length of stay in ICU, days | ||||
| Median (range) | 8.9 (1–646) | 9.8 (1–102) | 0.07 | 0.79 |
| Mean ± standard deviation | 14.6 ± 22.2 | 15.2 ± 17.7 | 0.26 | 0.79 |
| Mortality (%) | 376 (14.1) | 16 (13.9) | 0.00 | 0.95 |
| Hospital charges, median (range) | 67,306 (736–1,075,038) | 56,519 (1586–525,564) | 0.63 | 0.43 |
Data are presented as n (%) unless otherwise indicated. MRSA, methicillin-resistant Staphylococcus aureus; ICU, intensive care unit; LOS, length of stay; COPD, chronic obstructive pulmonary disease.
A total of 2221 of 2775 patients stayed in the ICU for more than 3 days, among whom 91 had MRSA colonization on admission. Among the remaining 2130 patients, 1727 (81.1%) were sampled at discharge and 185 (10.7%; 95% CI, 9.3–12.2) had acquired MRSA colonization during their ICU stay. The incidence of MRSA colonization while in the ICU was much higher than that on admission. Risk factors for acquiring MRSA colonization in the ICU included having chronic obstructive pulmonary disease, being on mechanical ventilation, and receiving dialysis (P < 0.05) (Table 2). The mean ICU LOS for patients with MRSA colonization was 23.3 days, which was significantly longer than that for patients without MRSA colonization (15.1 days, P = 0.00). The mean overall expense for MRSA-colonized patients was also significantly higher than that for patients without (135,171.0 vs. 107,209.3 Yuan, respectively; P = 0.00). However, the mortality rates between the two groups were not significantly different. Among the 185 patients with MRSA colonization at discharge, 20 (10.8%; 95% CI, 6.3–15.3) had acquired an MRSA infection during their ICU stay, which was significantly more than those without MRSA colonization at discharge (0.9%; 95% CI, 0.4–1.4; P = 0.00).
Table 2.
Characteristics and outcomes of MRSA-positive and -negative patients at ICU discharge.
| Characteristics | MRSA-negative (n = 1542) | MRSA-positive (n = 185) | Z/χ 2 | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Sex | ||||
| Male | 1022 (66.3) | 110 (59.5) | 3.40 | 0.07 |
| Female | 520 (33.7) | 75 (40.5) | ||
| Age, years | ||||
| Mean ± standard deviation | 57.1 ± 19.1 | 58.5 ± 18.5 | 0.84 | 0.36 |
| Median (range) | 59 (4–98) | 61 (15–90) | ||
| Pre-ICU LOS, days | ||||
| Mean | 6.8±14.1 | 6.8 ± 13.7 | 0.00 | 0.99 |
| Underlying disease | ||||
| COPD | 244 (15.8) | 42 (22.7) | 5.66 | 0.02 |
| Coma | 15 (1.0) | 2 (1.1) | 0.02 | 0.89 |
| Heart failure | 95 (6.2) | 10 (5.4) | 0.16 | 0.69 |
| Renal failure | 146 (9.5) | 19 (10.3) | 0.12 | 0.73 |
| Diabetes mellitus | 97 (6.3) | 16 (8.6) | 1.50 | 0.22 |
| Hypertension | 183 (11.9) | 22 (11.9) | 0.00 | 0.99 |
| Respiratory failure | 389 (25.2) | 44 (23.8) | 0.18 | 0.67 |
| Cancer | 190 (12.3) | 10 (5.4) | 7.72 | 0.01 |
| Blood disease | 17 (1.1) | 2 (1.1) | 0.00 | 0.98 |
| Prior invasive procedure | ||||
| Mechanical ventilation | 922 (59.8) | 151 (81.6) | 33.45 | 0.00 |
| Central venous catheterization | 637 (41.3) | 76 (41.1) | 0.01 | 0.95 |
| Urinary catheterization | 1392 (90.3) | 175 (94.6) | 3.67 | 0.06 |
| Dialysis | 121 (7.8) | 29 (15.7) | 12.76 | 0.00 |
| Surgery | 395 (25.6) | 27 (14.6) | 10.87 | 0.00 |
| Outcome | ||||
| MRSA infection | 14 (0.9) | 20 (10.8) | 89.93 | 0.00 |
| Length of stay in ICU | ||||
| Median (range), days | 10 (4–159) | 15 (4–128) | ||
| Mean ± standard deviation | 15.1 ± 15.9 | 23.3 ± 23.3 | 39.03 | 0.00 |
| Mortality (%) | 214 (13.9) | 26 (14.1) | 0.01 | 0.95 |
| Hospital charges, mean | 107,209.3 ± 97,731.9 | 135,171.0 ± 137,902.0 | 12.06 | 0.00 |
| Hospital charges, median (range) | 76,005.7 (4345.0–816,052.2) | 86,326.9 (15,896.6–818,153.7) |
Data are presented as n (%) unless otherwise indicated. MRSA, methicillin-resistant Staphylococcus aureus; ICU, intensive care unit; LOS, length of stay; COPD, chronic obstructive pulmonary disease.
Discussion
This study revealed that 4.1% of patients admitted to West China Hospital’s ICU were already colonized with MRSA on admission. Our review of existing data from China revealed nine papers that were published in Chinese and one paper describing a study that was conducted in mainland China but published in the English version of a Chinese journal.14–23 The total number of patients included in the 10 articles was 3798, and the percentage of patients already colonized with MRSA on ICU admission ranged from 3.0% to 11.0% (median, 7.9%; 25%–75% interquartile range, 4.2–9.1) (Table 3). Similarly, studies in other countries reported that 4.9% to 13.1% of ICU patients are already colonized with MRSA on admission.7,24–26 The variation in the prevalence of MRSA colonization is likely due to regional differences, as previous studies have demonstrated. In Asia, Izumikawa et al.26 reported that 7.5% of patients in an ICU in Japan had MRSA colonization on admission, while another study involving 342 ICU patients in Korea found that the prevalence of MRSA colonization on admission was 27.5%.27 Even in North America, studies have reported remarkable differences. For instance, Ridenour et al.25 discovered a relatively high proportion of MRSA-colonized patients on ICU admission (11.0%), while Davis et al.28 demonstrated a relatively low proportion (3.4%).
Table 3.
Characteristics of selected Chinese studies.
| First Author | Journal | Publication Year | Vol (Issue):Pages | Location | Unit | MRSA colonization on admission |
MRSA colonization at discharge |
Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||||||
| Zhou Chun-mei | Chin J Nosocomiol | 2011 | 21(12):2592–2594 | Shanghai | ICU | 211 | 9 | 4.3 | 211 | 26 | 12.3 | [14] |
| Fan Shan-hong | Chin J Infect Control | 2015 | 14(3):174–177 | Xi'an | ICU/RICU/NSICU | 197 | 18 | 9.1 | 179 | 4 | 2.2 | [15] |
| Gu Ke-ju | Chin J Infect Control | 2016 | 15(6):401–404 | Shanghai | ICU | 240 | 22 | 9.2 | 218 | 34 | 15.6 | [16] |
| Zou Xiaoni | Modern Hospital Jul | 2013 | 13(7):65–67 | Guangdong | NICU, neonatal intensive care unit | 429 | 28 | 6.5 | [17] | |||
| Li Qiang | Chin Med J (Engl) | 2014 | 127(10):1804–1807 | Beijing | ICU | 350 | 31 | 8.9 | [18] | |||
| Xie Yi | J Trop Med | 2014 | 14(2):223–225 | Guangdong | ICU | 79 | 3 | 3.8 | [19] | |||
| Zhang Yan-qing | Chin J Infect Control | 2014 | 13(11):650–653 | Fujian | SICU | 100 | 11 | 11.0 | [20] | |||
| Zheng Xuan'er | Chin J Obstet Gynecol Pediatr | 2014 | 10(3):328–332 | Guangdong | NICU, neonatal intensive care unit | 581 | 41 | 7.1 | [21] | |||
| Meng Li-hui | Chin J Nosocomiol | 2015 | 25(21):4965–4967 | Beijing | ICU | 238 | 21 | 8.8 | [22] | |||
| Gu Ji-na | Chin J Nosocomiol | 2015 | 25(11):2481–2483 | Zhejiang | ICU | 1373 | 41 | 3.0 | [23] | |||
MRSA, methicillin-resistant Staphylococcus aureus; ICU, intensive care unit; RICU, respiratory intensive care unit; NSICU, neurological surgical intensive care unit; SICU, surgical intensive care unit.
Differences in sample size proportions are another possible reason for the variations in prevalence. Honda et al.24 proposed that such differences may significantly influence results. In their study, the nasal swab sampling percentage was as high as 98.9%, while other studies had a lower nasal swab percentage. Another possible reason for the differences in colonization data is sampling site selection. Previous studies have shown that the sensitivity of MRSA surveillance cultures was significantly higher in throat and tracheal aspirates (82%) than in anterior nares (47%).29 A study in Taiwan revealed that the MRSA screening sensitivity also depended on the anatomic location of sampling, number of sites cultured, culture methods, and distribution of strain types.30
Our study showed that 10.7% of patients became colonized with MRSA during their ICU stay. This result is similar to those found in previous studies in other countries (4.9%–15.0%).31–33 However, after we searched the Chinese-language literature database, only 3 studies provided discharge data, in which 64 ICU patients became MRSA-colonized at percentages ranging from 2.2% to 15.6%.14–16 Our results are slightly lower than those of two previous studies published in China,14,16 both of which had a small sample size and unclear MRSA acquisition rates. Our large sample size and the high incidence of nasal-swab culture collection over a 3-year study period are major strengths of our study. As many previous studies have demonstrated, our study also showed that patients were more likely to be colonized with MRSA if they underwent mechanical ventilation, received dialysis, or had chronic obstructive pulmonary disease.
Our study confirmed that patients already colonized with MRSA on admission were more likely to acquire MRSA infection (2.3-fold higher risk; 95% CI, 1.1–7.3) than those who had not been colonized, although our percentages were lower than those reported in previous studies (5.9-, 9.5- and 107.7-fold).28,34,35 The different types of ICUs and different prevalences of MRSA in different areas may account for these variations in study results. In our study, 1.7% of patients developed MRSA infections during their ICU stay, which is similar to the findings reported by Milstone et al.34 (1.8%). This suggests a low incidence of MRSA infection in our ICU.
Our study also revealed that the healthcare associated MRSA infection rate decreased significantly from 2.2% (95% CI, 1.2–3.2) in 2011 to 0.8% (95% CI, 0.2–1.4) in 2013, which is also consistent with a few studies that have shown correlations between the presence of an active surveillance program and reduced MRSA infection rates.36 It appears that ASC programs and their data are helpful in reducing ICU MRSA infection rates. However, the voluntary isolation of MRSA-positive patients by clinicians and the hospital’s promotion of hand hygiene during the study period may have also contributed to the low MRSA infection rate.
We recognize that our study has several limitations. First, we used ChromID MRSA-Select agar plates to detect MRSA, but previous studies37,38 have reported that these agar plates may have low sensitivity, and both nonselective enrichment broth and semiselective broth containing cefoxitin and aztreonam can improve its sensitivity.39 In contrast, polymerase chain reaction has greater sensitivity for MRSA identification, but because of hospital capacity limitations, we did not use this technique for MRSA screening.40,41 Second, we used only a single sampling site, the nares, which has a lower incidence of MRSA recovery than throat swabs and tracheal aspirates. Sampling multiple sites for culture has also been shown to increase MRSA-detection sensitivity.30 Third, this was an observational study, and we did not compulsorily isolate those colonized with MRSA nor did we decolonize patients due to insufficient capacity, which may partially explain our MRSA colonization prevalence of 10% at discharge.
In conclusion, our study has demonstrated that the prevalence of MRSA colonization was 4.1% on ICU admission and that 10.7% of patients acquired MRSA during their ICU stay. We have also confirmed that patients who already had MRSA colonization on admission were significantly more likely to develop MRSA infections during their ICU stays. ASCs and their resulting data are helpful in reducing MRSA infections.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (project no. 81222025).
References
- 1. Davis KA, Stewart JJ, Crouch HK, et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004; 39: 776–782. PMID: 15472807. [DOI] [PubMed] [Google Scholar]
- 2.Nelson RE, Stevens VW, Jones M, et al. Health care-associated methicillin-resistant Staphylococcus aureus infections increases the risk of postdischarge mortality. Am J Infect Control 2015; 43: 38–43. [DOI] [PubMed] [Google Scholar]
- 3.Primo MGB, Guilarde AO, Martelli CMT, et al. Healthcare-associated Staphylococcus aureus bloodstream infection: length of stay, attributable mortality, and additional direct costs. Braz J Infect Dis 2012; 16: 503–509. [DOI] [PubMed] [Google Scholar]
- 4.Su CH, Chang SC, Yan JJet al. Excess mortality and long-term disability from healthcare-associated staphylococcus aureus infections: a population-based matched cohort study. PLoS One 2013; 8: e71055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dantes R, Mu Y, Belflower R, et al. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 2013; 173: 1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Antibiotic resistance threats in the United States, 2013. https://www.cdc.gov/drugresistance/threat-report-2013/index.html
- 7.Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011; 364: 1419–1430. [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Kralovic SM, Evans ME, et al. Impact of active screening for methicillin-resistant Staphylococcus aureus (MRSA) and decolonization on MRSA infections, mortality and medical cost: a quasi-experimental study in surgical intensive care unit. Crit. Care 2015; 19: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huskins WC, Huckabee CM, O'Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med 2011; 364: 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Liu Y, Jiang X, et al. Rapid Change of Methicillin-Resistant Staphylococcus aureus Clones in a Chinese Tertiary Care Hospital over a 15-Year Period. Antimicrob Agents Chemother 2010; 54: 1842–1847. PMID: 20176895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia XQ, Pang F, Chen JZ, et al. Prevalence and clinical distribution of multidrug-resistant bacteria (3537 isolates) in a tertiary Chinese hospital (January 2012-December 2013). Pathol Biol (Paris) 2015; 63: 21–23. [DOI] [PubMed] [Google Scholar]
- 12.Yao Z, Peng Y, Chen X, et al. Healthcare Associated Infections of Methicillin-Resistant Staphylococcus aureus: A Case-Control-Control Study. PLoS One 2015; 10: e0140604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo L, Xie Y, He C, et al. Molecular epidemiological analysis of methicillin-resistant Staphylococcus aureus isolates from a medical intensive care unit: a comparison of nasal and clinical isolates. Am J Med Sci 2013; 345: 361–365. [DOI] [PubMed] [Google Scholar]
- 14.Zhou C, Hu B, Gao X, et al. Active surveillance of multidrug-resistant organisms in SICU in a general hospital in 2008 and 2011. Chin J Nosocomiol 2011; 21: 2592–2594. [Google Scholar]
- 15.Fan S, Li Y, Ge W, et al. Risk factors for colonization/infection of methicillin-resistant Staphylococcus aureus in intensive care unit patients. Chin J Infect Control 2015; 14: 174–177. doi: 10.3969/j.issn.1671-9638.2015.03.006 [Google Scholar]
- 16.Gu K, Shen Y. Implementation of active screening for preventing and controlling the spread of multidrug-resistant organisms in intensive care unit. Chin J Infect Control 2016; 15: 401–404. doi: 10.3969/j.issn.1671-9638.2016.06.009 [Google Scholar]
- 17.Zou X, Mu X, Li J, et al. Epidemiology characterization and active surveillance culture of com- munity - associated multi resistant organism among young children. Mod Hosp 2013; 13: 65–67. [Google Scholar]
- 18.Li Q, Zhuang T, Lin Y, et al. Risk factors affecting nasal colonization of methicillin-resistant Staphylococcus aureus when admitted in intensive care unit. Chin Med J (Engl) 2014; 127: 1804–1807. [PubMed] [Google Scholar]
- 19.Xie Y, Huang S, Zeng J. Cost-benefit analysis of active surveillance culture of multi-drug-resistant organism. J trop Med 2014; 14: 223–225. [Google Scholar]
- 20.Zhang Y, Guo Y, Gan M, et al. Active screening and risk factors for colonization of multidrug-resistant organisms in a surgical intensive care unit. Chin J Infect Control 2014; 13: 650–653. [Google Scholar]
- 21.Zheng X, Yang J, Lai W, et al. Effects of integrated intervention for the prevention and control of nosocomial infection in neonatal intensive care unit. Chin J Obstet Gynecol Pediatr 2014; 10: 328–332. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhfylcyxzz201403014 [Google Scholar]
- 22.Meng L, Xiong Y, Wang J, et al. Active screening of multidrug-resistant organisms in ICU of pediatric cardiac surgery department and effect of intervention. Chin J Nosocomiol 2015; 25: 4965–4967. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhyygrxzz201521057 [Google Scholar]
- 23.Gu J, Chen L, Liu P, et al. Impact of active screening and prevention measures on multidrug-resistant organisms infections in ICU patients. Chin J Nosocomiol 2015; 25: 2481–2483. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zhyygrxzz201511029 [Google Scholar]
- 24.Honda H, Krauss MJ, Coopersmith CM, et al. Staphylococcus aureus nasal colonization and subsequent infection in intensive care unit patients: does methicillin resistance matter? Infect Control Hosp Epidemiol 2010; 31: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridenour GA, Wong ES, Call MA, et al. Duration of colonization with methicillin-resistant Staphylococcus aureus among patients in the intensive care unit: implications for intervention. Infect Control Hosp Epidemiol 2006; 27: 271–278. [DOI] [PubMed] [Google Scholar]
- 26.Izumikawa K, Yamamoto Y, Yanagihara K, et al. Active surveillance of methicillin-resistant Staphylococcus aureus with the BD GeneOhm MRSA™ assay in a respiratory ward in Nagasaki, Japan. Jpn J Infect Dis 2012; 65: 33–36. [PubMed] [Google Scholar]
- 27.Huh HJ, Kim ES, Chae SL. Methicillin-resistant Staphylococcus aureus in nasal surveillance swabs at an intensive care unit: an evaluation of the LightCycler MRSA advanced test. Ann Lab Med 2012; 32: 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KA, Stewart JJ, Crouch HK, et al. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004; 39: 776–782. [DOI] [PubMed] [Google Scholar]
- 29.Jang HC, Choi OJ, Kim GS, et al. Active surveillance of the trachea or throat for MRSA is more sensitive than nasal surveillance and a better predictor of MRSA infections among patients in intensive care. PLoS One 2014; 9: e99192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauderdale TL, Wang JT, Lee WS, et al. Carriage rates of methicillin-resistant Staphylococcus aureus (MRSA) depend on anatomic location, the number of sites cultured, culture methods, and the distribution of clonotypes. Eur J Clin Microbiol Infect Dis 2010; 29: 1553–1559. [DOI] [PubMed] [Google Scholar]
- 31.Kurup A, Chlebicka N, Tan KY, et al. Active surveillance testing and decontamination strategies in intensive care units to reduce methicillin-resistant Staphylococcus aureus infections. Am J Infect Control 2010; 38: 361–367. [DOI] [PubMed] [Google Scholar]
- 32.Lepelletier D. [Meticillin-resistant Staphylococcus aureus: incidence, risk factors and interest of systematic screening for colonization in intensive-care unit]. Ann Fr Anesth Reanim 2006; 25: 626–632[in French, English Abstract]. [DOI] [PubMed] [Google Scholar]
- 33.Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001; 22: 687–692. [DOI] [PubMed] [Google Scholar]
- 34.Milstone AM, Goldner BW, Ross T, et al. Methicillin-resistant Staphylococcus aureus colonization and risk of subsequent infection in critically ill children: importance of preventing nosocomial methicillin-resistant Staphylococcus aureus transmission. Clin Infect Dis 2011; 53: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stano P, Avolio M, De Rosa Ret al. An antibiotic care bundle approach based on results of rapid molecular screening for nasal carriage of methicillin-resistant Staphylococcus aureus in the intensive care unit. In Vivo 2012; 26: 469–472. [PubMed] [Google Scholar]
- 36.Simmons S. Effects of selective patient screening for MRSA on overall MRSA hospital-acquired infection rates. Crit Care Nurs Q 2011; 34: 18–24. [DOI] [PubMed] [Google Scholar]
- 37.Perry JD, Davies A, Butterworth LA, et al. Development and Evaluation of a Chromogenic Agar Medium for Methicillin-Resistant Staphylococcus aureus. J Clin Microbiol 2004; 42: 4519–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compernolle V, Verschraegen G, Claeys G. Combined use of Pastorex Staph-Plus and either of two new chromogenic agars, MRSA ID and CHROMagar MRSA, for detection of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2007; 45: 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Böcher S, Middendorf B, Westh H, et al. Semi-selective broth improves screening for methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2010; 65: 717–720. [DOI] [PubMed] [Google Scholar]
- 40.Al-Talib H, Yean CY, Al-khateeb A, et al. Comparative evaluation of five culture media with triplex PCR assay for detection of methicillin-resistant Staphylococcus aureus. Curr Microbiol 2010; 61: 1–6. [DOI] [PubMed] [Google Scholar]
- 41.Seki M, Takahashi H, Yamamoto N, et al. Polymerase chain reaction-based active surveillance of MRSA in emergency department patients. Infect Drug Resist 2015; 8: 113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]