Key Points
Question
For polypoidal choroidal vasculopathy, is monotherapy with intravitreal aflibercept injection (IAI) noninferior to IAI when photodynamic therapy (PDT) is added for eyes that have a suboptimal response?
Findings
In this randomized clinical trial, of 318 older adults, 5.1% and 6.8% participants at 12 weeks (12.1% and 14.3% by 52 weeks) suboptimally responded to IAI alone or IAI plus active PDT, respectively. Monotherapy with IAI was noninferior to IAI plus PDT.
Meaning
Monotherapy with IAI exhibited clinically meaningful Early Treatment Diabetic Retinopathy Study letter gains (+10.7); the benefits of adding PDT cannot be elucidated, as most participants responded to IAI alone.
Abstract
Importance
Polypoidal choroidal vasculopathy (PCV) is common in Asian populations, but an optimal treatment approach remains to be confirmed.
Objective
To evaluate intravitreal aflibercept injection (IAI) in participants with PCV and compare IAI monotherapy with IAI plus rescue photodynamic therapy (PDT).
Design, Setting, and Participants
This 96-week, double-masked, sham-controlled phase 3b/4 randomized clinical trial was conducted at multiple centers in Australia, Germany, Hong Kong, Hungary, Japan, Singapore, South Korea, and Taiwan from May 2014 to August 2016, and included adults 50 years or older with symptomatic macular PCV and a best-corrected visual acuity of 73 to 24 Early Treatment Diabetic Retinopathy Study letters (20/40-20/320 Snellen equivalent).
Interventions
Participants received 2 mg of IAI at weeks 0, 4, and 8. At week 12, participants with a suboptimal response were randomized 1:1 to receive IAI plus sham PDT (IAI monotherapy) or a “rescue” of IAI plus rescue PDT (IAI/PDT). Participants who did not qualify for rescue received IAI every 8 weeks; those qualifying for rescue received IAI every 4 weeks plus sham/active PDT. When the rescue criteria were no longer met, injection intervals were gradually extended to 8 weeks.
Main Outcomes and Measures
Noninferiority of IAI monotherapy to IAI/PDT for mean change in best-corrected visual acuity from baseline to week 52 (95% CI of the difference entirely above −5 letters).
Results
Of the 318 participants, the mean (SD) age was 70.6 (8.2) years, 96 (30.2%) were women, and 152 (47.8%) were Japanese. Monotherapy with IAI was noninferior to IAI/PDT for the primary end point (+10.7 vs +10.8 letters, respectively; 95% CI, −2.9 to 1.6; P = .55), with few participants requiring rescue therapy (19 [12.1%] vs 23 [14.3%], respectively). Participants in both treatment groups had similar reductions in central subfield thickness from baseline to week 52 (−137.7 [IAI monotherapy] vs −143.5 μm [IAI/PDT]). At week 52, 49 (38.9%) and 60 participants (44.8%) had no polypoidal lesions observed on indocyanine green angiography in the IAI monotherapy and IAI/PDT groups, respectively. Furthermore, 116 (81.7%) and 136 (88.9%), respectively, had no polypoidal lesions with leakage. The most frequent ocular adverse events were conjunctival hemorrhage (IAI monotherapy, 8 [5.1%]) and dry eye (IAI/PDT, 9 [5.6%]).
Conclusions and Relevance
Improvement in visual and/or functional outcomes was achieved in more than 85% of participants who were treated with IAI monotherapy, with no signs of leakage from polypoidal lesions in more than 80%. As fewer than 15% met the criteria of a suboptimal response to receive PDT, the potential benefit of adding PDT cannot be determined.
Trial Registration
ClinicalTrials.gov Identifier: NCT02120950
This randomized clinical trial evaluates the use of intravitreal aflibercept injection with and without added photodynamic therapy for patients with polypoidal choridal vasculopathy.
Introduction
Polypoidal choroidal vasculopathy (PCV), a subtype of neovascular age-related macular degeneration (nAMD), presents as serosanguinous exudative maculopathy characterized by retinal pigment epithelial detachment, serous exudation, and hemorrhage in multiple retinal layers.1,2 Polypoidal choroidal vasculopathy is particularly prevalent among Asian populations; studies report that 25% to 50% of Asian patients with nAMD have PCV.1,3 Among white people, PCV prevalence was shown to be 4.0% to 9.8% of the nAMD population.4,5,6 Polypoidal choroidal vasculopathy may be underdiagnosed in people who are not Asian, as indocyanine green angiography (ICGA), an essential test for PCV diagnosis, is neither routinely nor frequently performed in non-Asian countries.
Based on the Anti–Vascular Endothelial Growth Factor (VEGF) Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR), Minimally Classic/Occult Trial the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA), and Vascular Endothelial Growth Factor VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet Age-Related Macular Degeneration (VIEW) studies,7,8,9 the intravitreal injection of the anti-VEGF agents ranibizumab and aflibercept is approved in the United States, Europe, and Japan for treating nAMD,10,11 with monotherapy as current standard care.12 While not approved in these countries, intravitreal bevacizumab is also used off-label to treat nAMD.13,14 However, the optimal treatment algorithms for PCV are unclear. Various treatment options are used, including photodynamic therapy (PDT) with verteporfin,15 anti-VEGF monotherapy, and combination therapy with an anti-VEGF agent and PDT.16 Although PDT was the first widely used PCV treatment, it carries associated risks of recurrent hemorrhages or exudation and late atrophy.15 Also, PDT is dependent on ICGA for guidance, requiring both specific equipment and training, which are not always readily available. Finally, concerns that PDT results in visual loss over time,17 possibly due to choroidal ischemia and retinal atrophy, persist.15 Thus, when treating PCV, a clinical need exists to determine if PDT can be administered only in situations as a rescue therapy when anti-VEGF monotherapy results in suboptimal improvement in vision and/or functional measures.
Despite several case series and few small randomized clinical trials (RCTs) on anti-VEGF use for PCV,18,19,20,21,22,23 large RCTs determining the best management options are limited. The Aflibercept in Polypoidal Choroidal Vasculopathy (PLANET) study aimed to evaluate the efficacy, safety, and tolerability of monotherapy with intravitreal aflibercept injection (IAI) vs IAI plus rescue PDT in the treatment of PCV.
Methods
Study Design
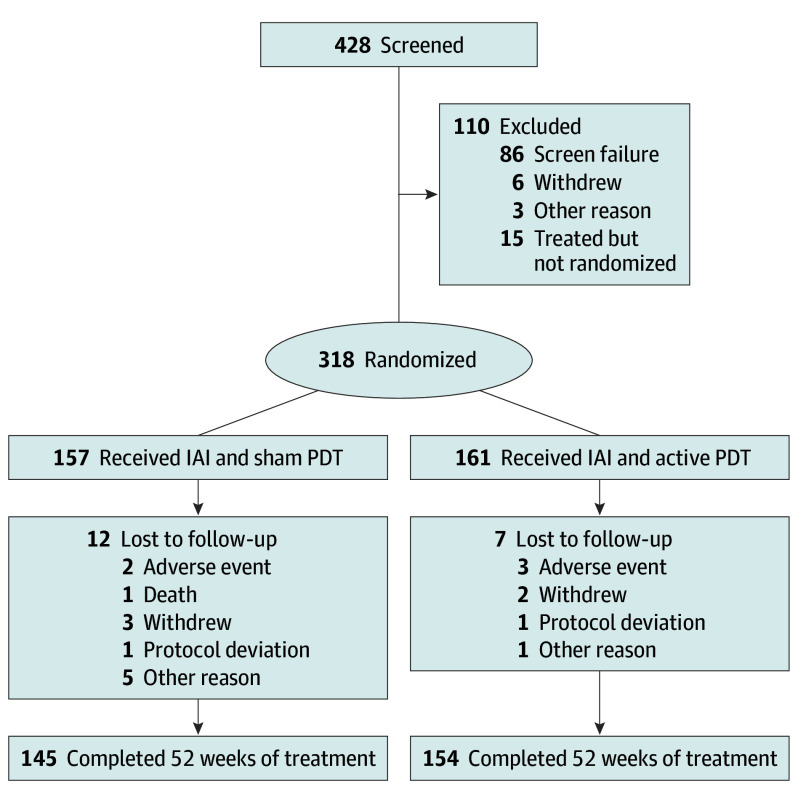
The PLANET clinical trial was a randomized, double-masked, sham-controlled phase 3b/4 study of participants with PCV (Figure 1; eFigure 1 in Supplement 1; protocol in Supplement 2) that was conducted at 62 sites (57 in Asia, 1 in Germany, and 4 in Hungary). Institutional review board/ethics committee approval was obtained, and the study adhered to the tenets of the Declaration of Helsinki. Participants were enrolled after providing written informed consent.
Figure 1. Consolidated Standards of Reporting Trial Diagram of Study Participant Disposition.
IAI indicates intravitreal aflibercept injection; PDT, photodynamic therapy.
Participants and Treatments
Participants (age ≥ 50 years) with a diagnosis of symptomatic macular PCV (greatest linear dimension of lesion, <5400 mm or ~ 9 Macular Photocoagulation Study disc areas) were included; an active PCV diagnosis and study eligibility were investigator determined. Participants must also have had a best-corrected visual acuity (BCVA) of 73 to 24 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (20/40-20/320 Snellen) in the study eye. Full inclusion/exclusion criteria are provided in eTable 1 in Supplement 1. After screening and eligibility confirmation (investigator determined), participants received IAI 2 mg every 4 weeks, at weeks 0, 4, and 8 (run-in phase). At week 12, participants were randomized 1:1 into either (1) an IAI plus sham PDT group (“IAI monotherapy”) or (2) an IAI plus rescue PDT group (“IAI plus active PDT”).
Stratification at randomization was based on the presence/absence of qualification for rescue therapy per rescue therapy criteria at week 12 and by race/ethnicity (Japanese or non-Japanese). To qualify for rescue therapy, participants must have met criteria on BCVA (criterion 1 and either criterion 2 or 3), optical coherence tomography (OCT) (criterion 4), and ICGA (criterion 5) (eFigure 2 in Supplement 1). Best-corrected visual acuity and OCT assessments were performed at each visit; ICGA assessments evaluating criterion 5 were performed only if criteria 1 and 4 plus either criterion 2 or 3 were met.
Participants in both groups who did not meet rescue treatment criteria received IAI 2 mg every 8 weeks until week 52. Participants who met rescue treatment criteria at week 12 or any subsequent visit received IAI 2 mg every 4 weeks plus active or sham PDT depending on their randomization assignment. Once the visual/anatomic outcomes allowed for it, IAI treatment intervals increased back to 2 mg every 8 weeks. Repeated IAI administrations had minimum intervals of 4 weeks. Active or sham PDT administrations were performed according to approved labeling, which generally allowed repeated PDT after a 12-week minimum in case rescue treatment criteria were still met at that time. The central reading center (CRC) was used for reading imaging data, including OCT, fundus photography, and fluorescein ICGA.
Study Objectives
The primary objectives of the PLANET study were to evaluate the efficacy and safety of IAI monotherapy vs IAI plus active PDT as a rescue treatment in participants with PCV and explore whether IAI monotherapy is noninferior (based on BCVA) to IAI plus active PDT. Secondary objectives included estimating the proportion of participants who received a diagnosis of PCV who required active PDT, and whether (and to what extent) active PDT was beneficial in participants with PCV who experienced a suboptimal response to IAI monotherapy.
End Points
The primary efficacy end point was change from baseline in BCVA (ETDRS letter score) for the study eye at week 52, and the secondary end point was the proportion of participants who avoided a moderate vision loss of 15 or more ETDRS letters from baseline to week 52. Prespecified exploratory end points were also evaluated (eTable 2 in Supplement 1), as were the frequency/severity of ocular/nonocular adverse events (AEs).
Statistical Analysis
Statistical analyses were performed using SAS, version 9.2 (SAS Institute). Descriptive statistics are provided for all variables. For statistical testing, analysis of covariance models were used for continuous variables and Cochran-Mantel-Haenszel models for categorical variables. For the primary analysis, statistical testing was conducted at a significance level of .05 (2-sided); no adjustment was made for multiple comparisons.
The primary efficacy variable analysis was conducted on the full analysis set (FAS). Statistical testing was conducted to prove the noninferiority of IAI monotherapy to IAI plus active PDT; IAI monotherapy was considered noninferior to IAI plus active PDT if the 95% confidence interval of the difference was entirely above −5 letters.
Sample size estimation resulted in 147 evaluable participants per treatment group. With expected dropout rate of 5%, desired randomized sample size was 310 participants (155 per treatment group). It was estimated that approximately 50 participants would qualify for PDT in each treatment group of 155.
An analysis of covariance model, with the baseline measure as a covariate, and treatment group, race/ethnicity, and qualification for rescue therapy at week 12 as fixed factors, was used. The last observation carried forward (LOCF) was used for missing values at 52 weeks.
If IAI monotherapy was noninferior to IAI plus active PDT, confirmatory noninferiority testing was continued for the proportion of participants who avoided a moderate vision loss of 15 or more ETDRS letters from baseline to week 52 (LOCF for missing 52-week ETDRS letter score; FAS). Two-sided 95% Cochran-Mantel-Haenszel intervals, adjusted for race/ethnicity (Japanese vs non-Japanese) and qualification for rescue therapy at week 12 (yes/no), were used. Intravitreal aflibercept monotherapy was considered noninferior to IAI plus active PDT if the confidence interval of the difference was more than −7%. All exploratory efficacy analyses presented were prespecified and conducted on the FAS; the safety analysis set (SAF) included all patients who received any study drug.
Results
Participant Disposition and Baseline Characteristics
In total, 428 participants were screened: 333 entered the treatment stage (SAF), of whom 318 were randomized at week 12 (FAS), and 157 and 161 participants were assigned to IAI monotherapy and IAI plus active PDT, respectively. A total of 145 (92.4%) and 154 (95.7%) participants, respectively, completed 52 weeks of the study (Figure 1).
Most participants (305 of 318 [95.9%]) had a confirmed diagnosis of PCV by CRC. Key baseline demographics and participant characteristics were similar across treatment groups (Table 1). The mean (SD) age at enrollment was 70.6 (8.4) years, and 222 participants (69.8%) were men.
Table 1. Baseline Demographics and Disease Characteristics (Full Analysis Set).
| Characteristic | IAI Plus Sham PDT (n = 157) |
IAI Plus Active PDT (n = 161) |
|---|---|---|
| Sex, No. (%) | ||
| Male | 110 (70.1) | 112 (69.6) |
| Female | 47 (29.9) | 49 (30.4) |
| Race, No. (%) | ||
| Asian | 145 (92.4) | 151 (93.8) |
| Non-Asian | 12 (7.6) | 10 (6.2) |
| Ethnicity, country, No. (%) | ||
| Japanese | 75 (47.8) | 77 (47.8) |
| Non-Japanese | 82 (52.2) | 84 (52.2) |
| Age, mean (SD), y | 70.8 (8.4) | 70.4 (8.0) |
| Disease duration, median, mo | 0.49a | 0.39 |
| Baseline BCVA score, mean (SD) | 57.7 (11.3) | 59.0 (11.5) |
| Total NEI VFQ-25 score at baseline, mean (SD) | 79.0 (11.5) | 75.4 (14.6) |
| Mean CST at baseline, mean (SD) | 347.8b (118.9) | 346.1c (117.5) |
| Type of CNV lesions at screening, No. (%) | ||
| CNV < 50% of lesion | 22 (14.0) | 19 (11.8) |
| CNV > 50% of lesion | ||
| Predominantly classic | 23 (14.6) | 13 (8.1) |
| Minimally classic/occult | 112 (71.3) | 129 (80.1) |
| Total lesion size at baseline, mean (SD), mm2 | 8.13 (7.89) | 7.91 (6.67) |
| CNV size at baseline, mean (SD), mm2 | 6.06 (6.28) | 5.86 (5.06)d |
| Presence of polypoidal lesions, detected with ICGA, No. (%) | 139 (88.5) | 143 (88.8) |
| Presence of branch vessel network, No. (%) | 122 (77.7) | 138 (85.7) |
| Presence of serous/hemorrhagic PED, No. (%) | 78 (49.7) | 70 (43.8) |
| Smoking history, No. (%) | ||
| Never | 64 (40.8) | 73 (45.3) |
| Former | 67 (42.7) | 65 (40.4) |
| Current | 26 (16.6) | 23 (14.3) |
| Qualified for rescue therapy at week 12, No. (%) | ||
| Rescue therapy | 8 (5.1) | 11 (6.8) |
| Nonrescue therapy | 149 (94.9) | 150 (93.2) |
Abbreviations: BCVA, best-corrected visual acuity; CNV, choroidal neovascularization; CRT, central retinal thickness; CST, central subfield thickness; IAI, intravitreal aflibercept injection; ICGA, indocyanine green angiography; NEI VFQ-25, 25-item National Eye Institute Visual Function Questionnaire; PDT, photodynamic therapy; PED, pigment epithelial detachment.
n = 156.
n = 152.
n = 158.
n = 160.
Treatment Experience
Over 52 weeks, the mean number of IAIs for IAI monotherapy and IAI plus active PDT groups was 8.1 and 8.0, respectively; the mean number of PDT administrations (including sham) was 0.2 and 0.2, respectively. At week 12, 5.1% (n = 8) and 6.8% (n = 11) of the study participants required and received rescue therapy in the IAI monotherapy and IAI plus active PDT groups, respectively. By week 52, only 12.1% (n = 19) required and received rescue therapy in the IAI monotherapy group, while 22 (13.7%) of the 23 (14.3%) participants who required rescue therapy in the IAI plus active PDT group received rescue therapy.
Efficacy End Points
Primary and Secondary End Points
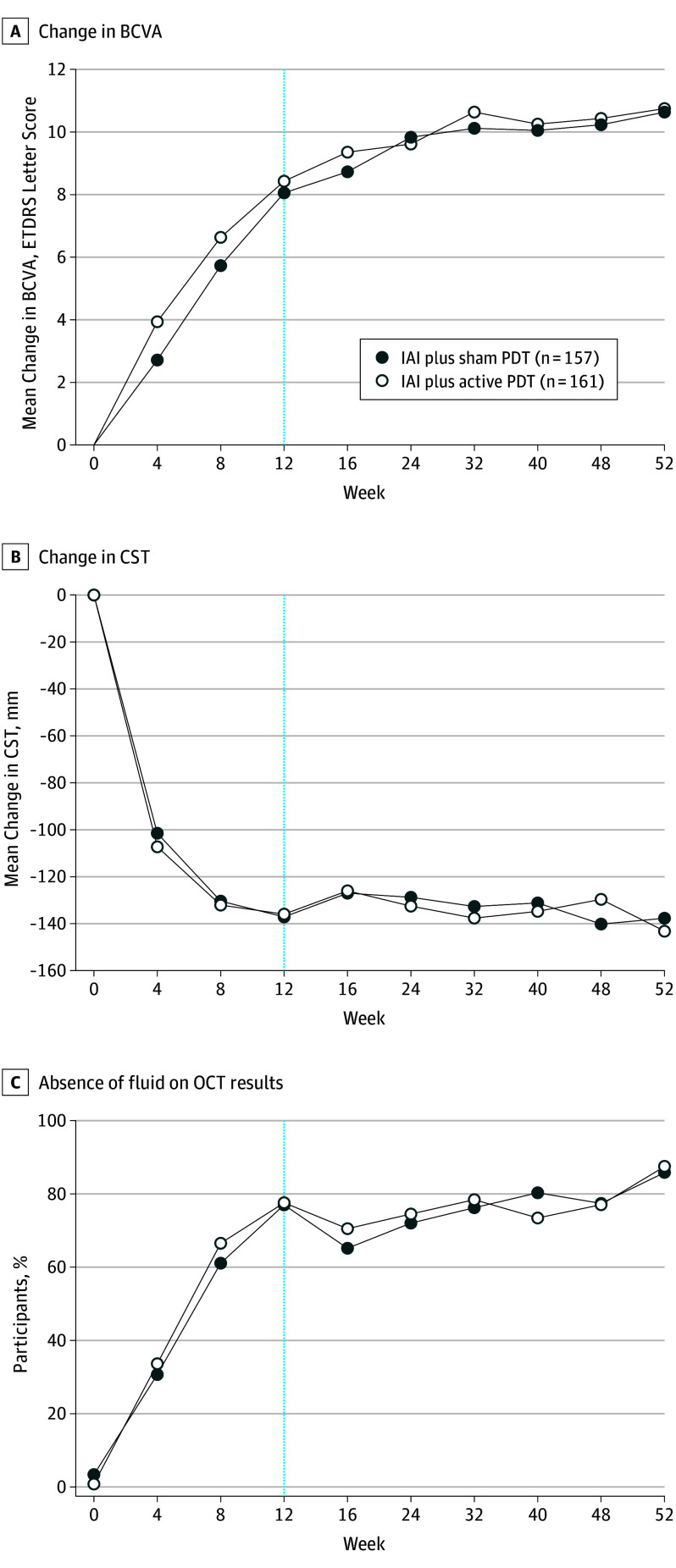
Rapid improvement in BCVA was noted after the first 3 IAI injections in both groups and continued through week 52 (Figure 2A). Intravitreal aflibercept monotherapy was noninferior to IAI plus active PDT, with mean (SD) gain in BCVA from baseline to week 52 of more than 2 lines (10.7 [11.3] vs 10.8 [10.7] letters, respectively; 95% CI, −2.9 to 1.6) (Figure 2A).
Figure 2. Functional and Anatomic Outcomes.
A, Change in best-corrected visual acuity (BCVA) from baseline to week 52 (last observation carried forward [full analysis set]). B, Change in central subfield thickness (CST) from baseline to week 52. Last observation carried forward (fullanalysis set [FAS]). C, Proportion of participants with absence of fluid detected on optical coherence tomography (OCT) over 52 weeks (observed cases [safety analysis set]). ETDRS indicates Early Treatment Diabetic Retinopathy Study; IAI, intravitreal aflibercept; PDT, photodynamic therapy.
The proportion of participants who avoided a moderate vision loss of 15 or more ETDRS letters from baseline to week 52 was similarly high in both groups: 97.5% for IAI monotherapy and 96.9% for IAI plus active PDT (difference, 0.6; 95% CI, −3.1 to 4.3; P = .74) (Table 2). Both the primary and secondary end point results were confirmed via sensitivity analyses that used multiple and worst-case imputations, respectively (eTable 3 in Supplement 1).
Table 2. Additional Outcomes at Week 52.
| Characteristic | IAI Plus Sham PDT (n = 157) |
IAI Plus Active PDT (n = 161) |
P Value |
|---|---|---|---|
| Proportion of participants avoiding a moderate vision loss of ≥15 ETDRS letters at week 52, %a | 97.5 | 96.9 | .74b |
| Difference (95% CI), % | 0.6 (–3.1 to 4.3) | ||
| Proportion of participants with no ICG leakage from polypoidal lesions at week 52, % | 81.7 | 88.9 | NA |
| Mean area of polypoidal lesions,c mm2 | NA | ||
| Baseline | 0.21 | 0.19 | |
| Week 52 | 0.07 | 0.08 | |
| Difference, baseline to week 52, % | –65.9 | –60.9 | |
| Proportion of participants with complete absence of polypoidal lesions on ICG at week 52, %d | 38.9 | 44.8 | NA |
| Difference (95% CI), % | –6.0 (–17.8 to 5.9) | .32b | |
Abbreviations: ETDRS, Early Treatment Diabetic Retinopathy Study; IAI, intravitreal aflibercept injection; ICG, indocyanine green; PDT, photodynamic therapy.
Last observation carried forward full analysis set.
Cochran-Mantel-Haenszel.
Observed cases, full analysis set.
Observed cases, safety analysis set.
Exploratory End Points
There was rapid and marked reduction in central subfield thickness (CST) after the first 3 IAI in both treatment groups that was maintained through week 52 (Figure 2B). The reduction in CST from baseline to week 52 was similar for IAI monotherapy and IAI plus active PDT (−137.7 μm vs −143.5 μm, respectively; difference, 1.1; 95% CI, −9.2 to 11.3; P = .84) (Figure 2B).
Over 52 weeks, the proportion of participants with an absence of fluid detected on OCT (investigator-assessed) increased in both groups. At week 12, 121 (77.1%) (IAI monotherapy) and 125 participants (77.6%) (IAI plus active PDT) had an absence of fluid detected on OCT; at week 52, more than 85.0% of participants in both groups (n = 135 and n = 141, respectively) had absence of fluid detected on OCT (Figure 2C). At week 52, 81.7% (n = 116) who received IAI monotherapy and 136 (88.9%) who received IAI plus active PDT had no evidence of active polypoidal lesions.
Overall, from baseline to week 52, the mean area of polypoidal lesions was reduced by 65.9% in the IAI monotherapy group and 60.9% in the IAI plus active PDT group (Table 2). The rates of complete regression of polypoidal lesions by ICGA were similar at week 52 (38.9% vs 44.8%, respectively; difference, −6.0; 95% CI, −17.8 to 5.9; P = .32) (Table 2).
Sensitivity Analyses
A sensitivity analysis that included only CRC-confirmed cases of PCV found no difference between the IAI monotherapy (n = 151) and IAI plus PDT (n = 154) groups in mean (SD) change in BCVA (+10.8 [11.4] vs +10.8 [10.5] letters). Among participants who met the rescue therapy criteria in the first year (n = 42), the absolute mean (SD) change in BCVA from baseline to week 52 was +1.9 (8.6) letters in the IAI monotherapy group (n = 19) and +4.2 (13.8) letters in the IAI plus PDT group (n = 23; LOCF); the adjusted least squares LS mean (SE) change was +2.9 (2.7) letters and +3.4 (2.4) letters, respectively. For participants who did not meet the rescue criteria, the absolute mean (SD) change in BCVA from baseline to week 52 was +12.0 (11.1) letters for IAI monotherapy (n = 138) and +11.9 (9.7) letters for IAI plus PDT (n = 138); the adjusted LS mean (SE) change was +11.7 (0.8) letters and +12.2 (0.8) letters, respectively. For participants who did not meet the rescue criteria, polypoidal lesion–related outcomes were similar across both treatment arms; however, among the few participants who met the rescue criteria, polypoidal lesion–related outcomes were poorer with IAI monotherapy vs IAI plus PDT (eTable 4 in Supplement 1).
The results for the mean change in BCVA and CST in participants who met the rescue criteria at the time of randomization (week 12) are provided in eFigure 3 in Supplement 1. A total of 50.0% and 36.4% of participants in the IAI monotherapy (n = 8) and IAI plus PDT groups (n = 11), respectively, gained 10 or more letters from week 12 to week 52 (difference, 18.5; 95% CI, −20.5 to 57.5; P = .35).
As part of regulatory requirements, the data from the patient groups stratified by Japanese or non-Japanese ethnicity were also analyzed. Visual/anatomic outcomes were consistent with the overall study population; however, the mean change in BCVA from baseline to week 52 was slightly higher in non-Japanese vs Japanese participants in both treatment groups (eTable 5 in Supplement 1).
Adverse Events
The incidence of ocular treatment–emergent AEs was 31.2% and 29.2% in the IAI monotherapy and IAI plus active PDT groups, respectively, at week 52 (Table 3). The most common ocular AEs in each group were conjunctival hemorrhage (8 [5.1%]) and dry eye (9 [5.6%]), respectively. There were no ocular serious AEs (SAEs) in the IAI monotherapy group, but there were 5 (3.1%) ocular SAEs with IAI plus active PDT. Of the 23 participants in the IAI plus active PDT arm who qualified for rescue and received active PDT, 1 (4.3%) experienced reduced visual acuity, with subretinal and vitreous hemorrhages in the study eye; this event was reported as a PDT-related SAE. The incidence of nonocular AEs was similar between treatment groups; there were no meaningful differences in the incidence of Antiplatelet Trialists’ Collaboration–defined arterial thromboembolic events between treatments (Table 3).
Table 3. Safety Overview at Week 52.
| Characteristic | IAI Plus Sham PDT (n = 157) |
IAI Plus Active PDT (n = 161) |
Treated But Not Randomizeda (n = 15) |
|---|---|---|---|
| Study participants with AEs, No. (%) | |||
| Any AE | 102 (65.0) | 86 (53.4) | 6 (40.0) |
| Any pretreatment AE | 16 (10.2) | 11 (6.8) | 1 (6.7) |
| Any posttreatment AE | 9 (5.7) | 4 (2.5) | 0 |
| Any TEAE | 96 (61.1) | 83 (51.6) | 6 (40.0) |
| Any ocular TEAE | 49 (31.2) | 47 (29.2) | 3 (20.0) |
| Study eye | 43 (27.4) | 34 (21.1) | 3 (20.0) |
| Fellow eye | 23 (14.6) | 27 (16.8) | 0 |
| Any nonocular TEAE | 74 (47.1) | 64 (39.8) | 5 (33.3) |
| Any SAE | 17 (10.8) | 16 (9.9) | 4 (26.7) |
| Any ocular SAE | 0 | 5 (3.1) | 1 (6.7) |
| Study eye | 0 | 5 (3.1) | 1 (6.7) |
| Fellow eye | 0 | 0 | 0 |
| Any nonocular SAE | 17 (10.8) | 12 (7.5) | 3 (20.0) |
| Any AE leading to discontinuation of the study drug | 3 (1.9) | 3 (1.9) | 2 (13.3) |
| Any AE leading to interruption of the study drug | 3 (1.9) | 1 (0.6) | 0 |
| Any death | 1 (0.6) | 0 | 1 (6.7) |
| Any APTC-classified event | 1 (0.6) | 1 (0.6) | 1 (6.7) |
| Nonfatal stroke | |||
| Cerebral infarction | 0 | 1 (0.6) | 0 |
| Vascular death | |||
| Arrhythmia | 1 (0.6) | 0 | 0 |
| Sudden cardiac death | 0 | 0 | 1 (6.7) |
Abbreviations: AE, adverse event; APTC, Antiplatelet Trialists' Collaboration; IAI, intravitreal aflibercept injection; PDT, photodynamic therapy; SAE, serious AE; TEAE, treatment-emergent AE.
All study participants who were enrolled in the study and received treatment but were not randomized at week 12 because of protocol deviation, adverse event, withdrawal, death, or loss to follow-up.
Discussion
PLANET is one of few large-scale RCTs that evaluates anti-VEGF agent use in PCV. Until recently, treatment algorithms for PCV were based on case series and smaller RCTs.18,19,20,21,22,23
In PLANET, IAI monotherapy demonstrated, on average, clinically meaningful BCVA gains in participants with PCV, with most not needing rescue PDT. In fact, only 19 participants (6%) with PCV required rescue PDT therapy at week 12 (after 3 IAI loading doses) and more than 85% did not require rescue treatment up to week 52, suggesting that IAI monotherapy can achieve clinically meaningful responses averaging more than 2 lines of BCVA gain for most people with PCV. Because only 19 (6%) at randomization and 42 (13%) by 52 weeks met the predefined suboptimal response criteria warranting PDT, the primary analysis largely compares 2 groups in which most eyes received the same treatment and, as such, following randomization with well-balanced groups, does not show differences (ie, noninferiority). As most participants did not have a suboptimal response to warrant adding PDT, the benefits of adding PDT cannot be elucidated.
Based on current evidence, rates of complete regression of polypoidal lesions with IAI treatment are highly variable. In this study, complete closure rates of polypoidal lesions, an outcome reported in other studies, were 38.9% for IAI monotherapy and 44.8% for IAI plus rescue PDT. A small, prospective study with IAI monotherapy reported closure rates of 75.0%,22 and a 1-year study of IAI plus PDT reported rates of 78.0%.24 In PLANET, because the closure of polypoidal lesions was determined by CRC using ICGA alone, this method may overestimate polypoidal lesion presence, because minute hypofluorescent dots may be interpreted as residual polypoidal disease.
EVEREST II compared ranibizumab monotherapy with ranibizumab plus adjunctive PDT administered at baseline, reporting rates of complete polypoidal lesion regression of 33.8% vs 69.7%, respectively.25 Best-corrected visual acuity gains from baseline to week 52 for the ranibizumab monotherapy and ranibizumab plus PDT groups were 5.1 vs 8.3 letters (P = .01), respectively, demonstrating that, on average, combining ranibizumab and PDT achieved significantly greater BCVA gains than ranibizumab monotherapy.25
When interpreting the EVEREST II and PLANET results, besides differences in anti-VEGF agents, one must consider distinct methodological differences—in particular, the role of PDT (adjunct vs rescue in EVEREST II and PLANET, respectively), timing of PDT administration (from baseline vs deferred after 3 months, respectively), and anti-VEGF dosing (pro re nata vs fixed). These design differences notwithstanding, in this study, after 3 initial monthly IAI (3 months), 121 participants (77.1%) in the IAI monotherapy and 125 participants (77.6%) in IAI plus active PDT groups had an absence of fluid on OCT results. In EVEREST II, at the same time (3 months), 39.3% and 73.6% of participants in the monotherapy and combination groups, respectively, had no disease activity. This may suggest differences in the treatment effect of intravitreal aflibercept vs ranibizumab for individuals with PCV.
Limitations
The study limitations should be discussed. While there appear to be no meaningful differences between IAI monotherapy and IAI with rescue PDT treatments in PLANET, the proportion of participants who qualified and received rescue PDT therapy was small (<15% qualified for rescue up to week 52), which limited formal statistical conclusions. In addition, the rescue criteria for PLANET may not be reflective of all clinical practice (eg, in cases in which PDT may be used earlier on in treatment). Regarding rescue treatment, the study was not designed to determine if an increased frequency of IAI from an 8-week to 4-week interval would provide additional benefits compared with fixed, every-8-week injections. Finally, PCV diagnosis and rescue criteria evaluation were performed primarily by investigators and not the reading center. While this can produce greater variability in the study population, it can also provide a better representation of real-world populations that are seen in clinical practice. The reading center’s gradings were compared with investigators’ assessments, and PCV diagnosis was confirmed by the reading center in more than 95% of participants (data not shown).
The results of the PLANET study at 1 year show that IAI monotherapy is a suitable treatment option to safeguard most people with PCV from deteriorating to a level at which rescue treatment would be required; however, it is not known how adjunctive combination treatment with PDT at the initiation of IAI treatment would compare with IAI given alone. Considering the potential risks of PDT, including cumulative damage to normal choroidal vasculature and retinal pigment epithelium following repeated administration,15 deferring PDT in patients in whom fluid is detected on OCT but with good visual acuity, as in the PLANET protocol, appears to be a reasonable approach.
Conclusions
In conclusion, IAI monotherapy, as administered in PLANET, demonstrates, on average, substantial visual acuity gains and anatomic benefits in patients with PCV, without meeting criteria to add PDT as a rescue therapy in most individuals. In the PLANET study, monotherapy with IAI was associated with decreased ICG angiographic leakage or the presence of polypoidal lesions in most participants and was well tolerated. The addition of PDT to IAI did not demonstrate additional benefits in visual outcomes; however, as only a few participants required and received PDT, the benefit of adding PDT to IAI for PCV cannot be elucidated from this trial.
eFigure 1. Study Design
eFigure 2. Criteria for Rescue Treatment
eFigure 3. Mean Change from Randomization (Week 12) to Week 52 in (A) BCVA and (B) CST in Subjects Qualifying for Rescue Treatment at Randomization
eTable 1. Inclusion/Exclusion Criteria
eTable 2. Exploratory Endpoints for the PLANET Study
eTable 3. Sensitivity Analyses for (A) Change in BCVA from Baseline to Week 52 and (B) Proportion of Subjects Avoiding ≥15 ETDRS Letters Loss at Week 52
eTable 4. Change in Polypoidal Lesion Characteristics (as Assessed by ICGA) According to Rescue Treatment Criteria at Week 52 (FAS, LOCF)
eTable 5. Overview of Japanese Subject Population at Week 52
Trial Protocol
References
- 1.Wong RL, Lai TY. Polypoidal choroidal vasculopathy: an update on therapeutic approaches. J Ophthalmic Vis Res. 2013;8(4):359-371. [PMC free article] [PubMed] [Google Scholar]
- 2.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina. 1990;10(1):1-8. [PubMed] [Google Scholar]
- 3.Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol. 2008;52(1):57-62. [DOI] [PubMed] [Google Scholar]
- 4.Ladas ID, Rouvas AA, Moschos MM, Synodinos EE, Karagiannis DA, Koutsandrea CN. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye (Lond). 2004;18(5):455-459. [DOI] [PubMed] [Google Scholar]
- 5.Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol. 2000;238(9):752-759. [DOI] [PubMed] [Google Scholar]
- 6.Scassellati-Sforzolini B, Mariotti C, Bryan R, Yannuzzi LA, Giuliani M, Giovannini A. Polypoidal choroidal vasculopathy in Italy. Retina. 2001;21(2):121-125. [DOI] [PubMed] [Google Scholar]
- 7.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T; ANCHOR Study Group . Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e5. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. [DOI] [PubMed] [Google Scholar]
- 9.Heier JS, Brown DM, Chong V, et al. ; VIEW 1 and VIEW 2 Study Groups . Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537-2548. [DOI] [PubMed] [Google Scholar]
- 10.Bayer . Eylea [prescribing information]. http://www.eylea.co.uk/static/documents/Eylea_Vials_PI_UK_007_2_Clean.pdf. Accessed February 12, 2018.
- 11.Genentech . Lucentis [prescribing information]. https://www.gene.com/download/pdf/lucentis_prescribing.pdf. Accessed February 12, 2018.
- 12.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. [DOI] [PubMed] [Google Scholar]
- 13.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarthy U, Harding SP, Rogers CA, et al. ; IVAN Study Investigators . Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399-1411. [DOI] [PubMed] [Google Scholar]
- 15.Wong CW, Cheung CM, Mathur R, et al. Three-year results of polypoidal choroidal vasculopathy treated with photodynamic therapy: retrospective study and systematic review. Retina. 2015;35(8):1577-1593. [DOI] [PubMed] [Google Scholar]
- 16.Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453-1464. [DOI] [PubMed] [Google Scholar]
- 17.Lee WK, Kim KS, Kim W, Lee SB, Jeon S. Responses to photodynamic therapy in patients with polypoidal choroidal vasculopathy consisting of polyps resembling grape clusters. Am J Ophthalmol. 2012;154(2):355-365.e1. [DOI] [PubMed] [Google Scholar]
- 18.Hosokawa M, Shiraga F, Yamashita A, et al. Six-month results of intravitreal aflibercept injections for patients with polypoidal choroidal vasculopathy. Br J Ophthalmol. 2015;99(8):1087-1091. [DOI] [PubMed] [Google Scholar]
- 19.Cho HJ, Kim KM, Kim HS, et al. Intravitreal aflibercept and ranibizumab injections for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2016;165:1-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee JE, Shin JP, Kim HW, et al. ; VAULT study group . Efficacy of fixed-dosing aflibercept for treating polypoidal choroidal vasculopathy: 1-year results of the VAULT study. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ijiri S, Sugiyama K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naïve polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):351-357. [DOI] [PubMed] [Google Scholar]
- 22.Inoue M, Arakawa A, Yamane S, Kadonosono K. Short-term efficacy of intravitreal aflibercept in treatment-naive patients with polypoidal choroidal vasculopathy. Retina. 2014;34(11):2178-2184. [DOI] [PubMed] [Google Scholar]
- 23.Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159(5):853-60.e1. [DOI] [PubMed] [Google Scholar]
- 24.Matsumiya W, Honda S, Otsuka K, et al. One-year outcome of combination therapy with intravitreal aflibercept and verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):541-548. [DOI] [PubMed] [Google Scholar]
- 25.Koh A, Lai TYY, Takahashi K, et al. ; EVEREST II study group . Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol. 2017;135(11):1206-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Design
eFigure 2. Criteria for Rescue Treatment
eFigure 3. Mean Change from Randomization (Week 12) to Week 52 in (A) BCVA and (B) CST in Subjects Qualifying for Rescue Treatment at Randomization
eTable 1. Inclusion/Exclusion Criteria
eTable 2. Exploratory Endpoints for the PLANET Study
eTable 3. Sensitivity Analyses for (A) Change in BCVA from Baseline to Week 52 and (B) Proportion of Subjects Avoiding ≥15 ETDRS Letters Loss at Week 52
eTable 4. Change in Polypoidal Lesion Characteristics (as Assessed by ICGA) According to Rescue Treatment Criteria at Week 52 (FAS, LOCF)
eTable 5. Overview of Japanese Subject Population at Week 52
Trial Protocol