Key Points
Question
What is the potential importance of vitreous bands detected with handheld spectral-domain optical coherence tomography among premature infants screened for retinopathy of prematurity?
Findings
In this cohort study of 65 premature infants, 37% had vitreous bands detected with handheld spectral-domain optical coherence tomography. Vitreous bands were associated with epiretinal membranes and cystoid macular edema but not with advanced retinopathy of prematurity.
Meaning
Vitreous bands may represent posterior vitreous abnormalities contributing to the development of cystoid macular edema and epiretinal membrane among premature infants.
Abstract
Importance
Handheld spectral-domain optical coherence tomography (SD-OCT) can provide insights into the complex interactions occurring at the vitreoretinal interface in retinopathy of prematurity (ROP) to enhance our understanding of ROP pathology.
Objective
To characterize vitreous bands in premature infants with use of handheld SD-OCT.
Design, Setting, and Participants
Prospective cohort study conducted from July 7, 2015, to February 28, 2017, at 2 university-based neonatal intensive care units. Seventy-three premature infants who required routine ROP screening examination were recruited. Informed consent was obtained from all legal guardians. Trained graders who were masked to the clinical assessment analyzed each SD-OCT scan of the right eye for vitreoretinal findings. A third trained grader mediated disagreements.
Main Outcomes and Measures
Associations between the presence of vitreous bands in premature infants with ROP diagnoses and the presence of other vitreoretinal SD-OCT findings were investigated.
Results
Of the 73 infants recruited, 6 infants’ parents withdrew their children from the study, and 2 infants were too hemodynamically unstable for imaging, leaving a total of 65 participants. Of these, 32 (49%) were female, 36 (55%) were white, 10 (15%) were Hispanic, 3 (5%) were Native American, 4 (6%) were African American, 4 (7%) were Asian/Pacific Islander, and 8 (12%) were other. The mean (SD) gestational age was 28 (2.7) weeks, the mean (SD) birth weight was 997 g (286 g), and the mean (SD) postmenstrual age at imaging was 34 (3) weeks (mean [SD] total of 3 [2] imaging sessions). Comparing the 24 infants (37%) who had a right eye vitreous band at any time with the 41 (63%) who did not, no difference in mean birth weight, gestational age, postmenstrual age at imaging, sex, or race/ethnicity was identified. No associations with ROP stage (eg, in 6 [25%] infants with vitreous bands vs 4 [9.8%] in those without; P = .23), presence of plus disease (2 [8%] vs 2 [5%]; P = .84), or type 1 ROP (3 [12%] vs 3 [7%]; P = .66) were identified. Vitreous bands were associated with epiretinal membrane detected on SD-OCT (P = .001) with an odds ratio of 9.4 (95% CI, 2.8-31.3) in 15 [62%] infants with vitreous bands vs 6 [15%] in those without. Vitreous bands were also associated with cystoid macular edema (in 15 [62%] infants with vitreous bands vs 1 [27%] in those without; P = .005) with an odds ratio of 4.5 (95% CI, 1.5-13.3).
Conclusions and Relevance
In this study, the development of vitreous bands was associated with both cystoid macular edema and epiretinal membrane. These findings suggest a tractional pathogenesis to these entities among premature infants. This study did not find a direct association between vitreous bands and severe ROP. Additional study is needed to determine whether vitreous bands represent subclinical hyaloidal organization leading to retinal detachment in advanced ROP.
This cohort study uses handheld spectral-domain optical coherence tomography to explore the association between vitreous bands in infants with retinopathy of prematurity and the presence of other vitreoretinal findings.
Introduction
Despite advances in neonatal care, retinopathy of prematurity (ROP) continues to be a major cause of vision loss in the United States.1 Fundus examination with indirect ophthalmoscopy remains the criterion standard for screening and monitoring patients with ROP.2 Even when strict screening protocols are followed, ROP can still progress to retinal detachment. Advanced ROP (stage 4 and 5 disease) is heralded by fibrovascular traction and usually requires surgical intervention.3 Timely identification and treatment of infants with fibrovascular proliferation is essential for halting the devastating vision loss accompanying ROP-associated retinal detachment.
Handheld spectral-domain optical coherence tomography (SD-OCT), which allows for in vivo detailed imaging of the retinal microarchitecture in the supine position, is gaining popularity for use among the infant population. Several earlier studies have demonstrated occult features of the retina in infants screened for ROP. Examination with SD-OCT can identify epiretinal membrane (ERM), cystoid macular edema (CME), retinoschisis, intraretinal deposits, and macular detachments that are not detected on clinical examination by experienced ROP screeners.4,5,6,7,8,9 At present, there is a paucity of literature regarding vitreous SD-OCT findings in premature infants.
Vitreous bands visualized during vitrectomy procedures in advanced ROP are typically targeted to release tractional retinal detachment.10 Handheld SD-OCT may identify such bands or their precursors. Even for those infants who obtain a good anatomic outcome, vitreous pathologic features related to ROP may linger for many years. Studies have shown that patients with a history of ROP are known to have worse visual outcomes than age-matched infants without ROP.11,12 Occult CME and ERM may play a role in the decreased vision observed in this population.9,12,13 Furthermore, longitudinal data on adults with a history of ROP show that these patients have a high risk for premature cataract formation,14,15 glaucoma,16,17,18,19 retinal tears,20 and retinal detachments,21,22,23 all of which may in part result from vitreous abnormalities. Some studies suggest that these complications occur with greater frequency with increasing severity of ROP,15,19 yet severe complications can occur even in patients with regressed disease that never required treatment.20,24 This study sought to characterize vitreous bands in premature infants by using handheld SD-OCT and to evaluate whether these bands are associated with worsening ROP stage or other vitreoretinal pathologies.
Methods
This prospective, observational study was approved by the institutional review board at Seattle Children’s Hospital and the University of Washington (Seattle, Washington). Informed consent was obtained from the legal guardians of the neonates while in the neonatal intensive care unit (NICU) of 2 university hospitals: Seattle Children’s Hospital and the University of Washington Medical Center. Handheld SD-OCT was performed at the time of each routine ROP screening examination.
Study Participants
Necessity of initial clinical examination was defined as less than 30 weeks’ gestation, less than a 1500-g birth weight, and selected infants with a birth weight between 1500 and 2000 g or gestational age of more than 30 weeks with an unstable clinical course, including those neonates who required cardiorespiratory support. Infants whose medical condition was too unstable to have SD-OCT imaging or examination were excluded from the study. Each infant’s pupil was dilated with an ophthalmic solution containing phenylephrine hydrochloride, 1%, and cyclopentolate, 0.2% (Cyclomydril; Alcon Laboratories).
Imaging Procedure
Imaging was performed using a handheld SD-OCT (Envisu C2300; Leica Microsystems) with the patient in the supine position without anesthesia and most often without an eyelid speculum as described by Maldonado et al.25 All SD-OCT scans were obtained by 1 of 2 trained imagers (A.S. and L.G.). Sucrose solution or a pacifier was occasionally used to soothe the infant during imaging. The SD-OCT scans were obtained each time an enrolled infant required a clinical screening ROP examination. The scans were performed with special attention to the posterior pole, with the goal being to capture the highest-quality fovea and optic nerve images using multiple individual volume scans.
ROP Screening and Data Collection
Infants underwent their usual indirect ophthalmoscopic clinical examination by 1 of 3 pediatric ophthalmologists (including K.T.-H. and M.T.C.) after SD-OCT imaging on the same day that SD-OCT imaging was performed. The ophthalmologist who performed the clinical examination was masked to the SD-OCT findings. All clinical observations, follow-up, and treatment decisions were made independently of SD-OCT findings and according to standard ROP screening and treatment guidelines.2 Each infant’s medical record was reviewed for baseline demographic characteristics (gestational age, birth weight, sex, and race/ethnicity), ROP diagnoses, and any mention of vitreous findings based on the clinical indirect ophthalmoscopic examination.
Image Analysis
Two trained graders (A.S. and E.M.Z.) evaluated each SD-OCT scan of the right eye using the imaging system’s software (Envivovue; Leica Microsystems) while masked to the clinical assessment. The graders had access to all complete volume scans obtained on each day the participant had an ROP screening clinical examination. The highest-quality foveal and optic nerve volume scans were graded for both retinal and vitreous findings. Additional volume scans were reviewed for any unusual findings not present in the first 2 volume scans. Retinal findings included CME and ERM. A finding of CME was defined as intraretinal hyporeflective spaces that distorted the normal architecture of the retina and were seen in more than 1 frame. A finding of ERM was defined as a distinct linear hyperreflective band at the vitreoretinal interface, with some separation from the retinal surface outside the hyperreflective artifact often seen overlying the retinal vessels. Vitreous findings included punctate hyperreflective opacities and vitreous bands. Punctate hyperreflective opacities were identified as multiple discreet hyperreflective opacities in the vitreous visible above background. Vitreous bands were coalescing linear opacities hovering over the retina that were visible in at least 3 consecutive frames with intensity above the background. Vitreous bands were divided into tractional and nontractional bands. Tractional vitreous bands were described as straight bands at a steep angle that made a connection to the retinal surface. Nontractional bands did not appear to have a connection to the retina in the frames captured by the scan and hovered parallel over the retina. Disagreement between the 2 readers in any of the above analysis was mediated by a third trained reader (M.T.C.).
Statistical Analysis
Only findings of examinations of the right eye were included in the analysis. Infants who had ever developed vitreous bands in their right eye were compared with infants who had not developed vitreous bands for presence or absence of clinically determined ROP, type 1 ROP,26 worst ROP stage, worst plus disease status, vitreous haze or hemorrhage, birth weight, gestational age, and other SD-OCT findings (development of hyperreflective vitreous opacities, CME, or ERM in that eye at any time). Separate analyses were performed for the above comparisons using the subcategories of tractional vitreous band and nontractional vitreous band.
The Shapiro-Wilk test of normality showed that birth weight and gestational age at birth were not normally distributed; thus, data analysis comparing the groups was performed using the nonparametric Mann-Whitney test for continuous variables. All categorical data were analyzed using either the χ2 test or the Fisher exact test. The unweighted Cohen κ statistic was applied to assess the strength of interobserver agreement. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc). A 2-sided value of P < .05 was considered statistically significant.
Results
Subject Characteristics
Seventy-three premature infants were recruited for the study, of whom 8 were excluded. Six of these infants were withdrawn from the study by their guardians, who perceived the examination as too stressful for the infant. Two infants missed their imaging sessions because of hemodynamic instability. The remaining 65 infants were included in the analysis, of whom 33 of 65 (51%) were male; the infants had a mean (SD) birth weight of 997 (286) g and a mean (SD) gestational age of 28 (2.7) weeks. The mean (SD) postmenstrual age at imaging was 34 (3) weeks. Of 65 infants, 36 (55%) were white, 10 (15%) were Hispanic, 3 (5%) were Native American, 4 (6%) were African American, 1 (2%) was Pacific Islander, 3 (5%) were Asian, and 8 (12%) were other. Among the 65 infants for whom images were obtained, there were 209 total ROP screening examinations and imaging sessions involving the right eye with a mean (SD) of 3 (2) ROP screenings and imaging sessions per participant.
Clinical Examination Findings
On clinical examination, 22 of the 65 infants (34%) received a diagnosis of ROP, and only 6 of 65 (9%) received a diagnosis of type 1 ROP requiring treatment. All of these infants were treated with laser photocoagulation. The worst ROP stage observed on clinical examination was stage 3 ROP, which was diagnosed in 19 of 209 clinical examinations (9%). The most frequent stage observed was stage 0, which was identified in 117 of 209 clinical examinations (56%). The clinician made note of “vitreous findings” in only 6 of 209 clinical examinations (3%). The findings described were vitreous haze in 5 examinations (2%) and frank vitreous hemorrhage in 1 examination (1%).
Vitreous Findings by Handheld SD-OCT
The 2 trained graders agreed on 188 of 209 SD-OCT scans (90%). The Cohen κ score was 0.8 (95% CI, 0.74-0.94). Of the 21 cases in which the scores provided by the trained graders disagreed, 10 (48%) of the disagreements were owing to differing opinions on the presence of ERM vs a normal hyperreflective nerve fiber layer, and 7 (33%) were attributable to disagreement on the presence of small intraretinal cysts vs normal reflective variability in darker scans. The readers disagreed regarding vitreous bands only 4 times (19%) with disagreement secondary to a question of hyperreflective artifact. The 2 readers agreed in all cases involving punctate hyperreflective opacities.
Findings of either vitreous bands or punctate hyperreflective vitreous opacities (Figure 1) occurred in 86 of 209 imaging sessions (41%) among 46 of 65 participants (71%). Among the 65 study participants, 24 (37%) had evidence of a vitreous band at least once during all imaging sessions involving the right eye. The more frequent finding was a nontractional band (Figure 2), which occurred in 32 of 209 imaging sessions (15%) among 22 of 65 participants (34%). A vitreous tractional band (Figure 3) was observed in 8 of 209 imaging sessions (4%) among 5 of 65 enrolled infants (8%). The punctate hyperreflective vitreous opacities occurred in 46 of 209 imaging sessions (22%) obtained for 19 of 65 study participants (29%). The vitreous bands (including both subtypes) and the punctate hyperreflective vitreous opacities occurred together in 9 of 209 imaging sessions (4%) in 7 of 65 study participants (11%) (P = .26).
Figure 1. Handheld Spectral-Domain Optical Coherence Tomography of Hyperreflective Vitreous Opacities.
Image obtained from a study participant with stage 1 retinopathy of prematurity showing multiple discrete, punctate hyperreflective opacities hovering above the retina. Punctate hyperreflective opacities were present in 19 of 65 scans (29%) obtained from premature infants screened for retinopathy of prematurity.
Figure 2. Handheld Spectral-Domain Optical Coherence Tomography of Nontractional Vitreous Band.
Image obtained from a study participant with stage 0 retinopathy of prematurity, vascularized to zone 3. A hyperreflective band (arrowheads) appeared to be hovering parallel to the retina in the right eye of 22 of 65 subjects (34%) screened for retinopathy of prematurity.
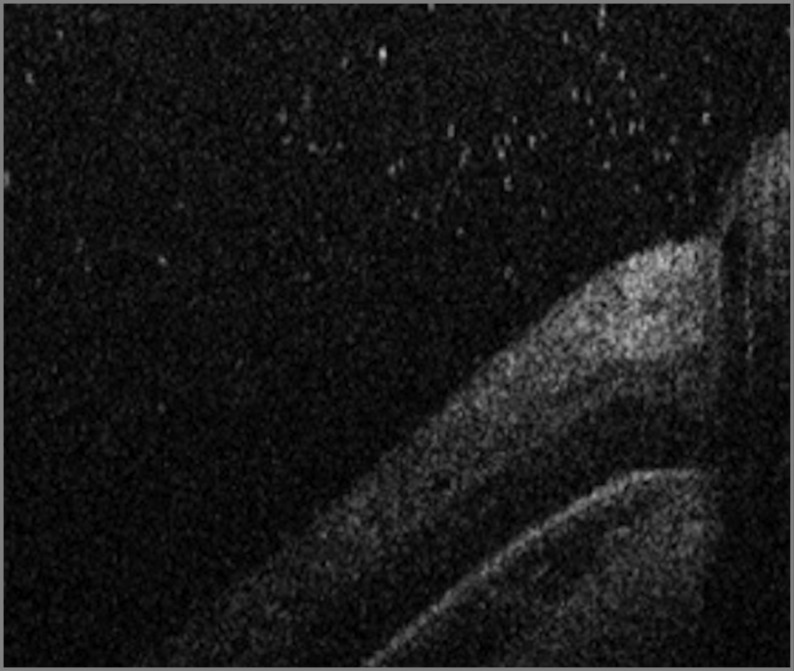
Figure 3. Handheld Spectral-Domain Optical Coherence Tomography Image of a Vitreous Tractional Band.
Image obtained from an infant with stage 3 retinopathy of prematurity. A hyperreflective band (arrowheads) approaches at a steep angle, connecting with the retina near the fovea. This type of band was seen in 5 of 65 participants (8%). Note the numerous punctate hyperreflective vitreous opacities.
The presence of a vitreous band was associated with the presence of an ERM observed on SD-OCT (in 15 [62%] infants with vitreous bands vs 6 [15%] in those without; P = .001) (Table). A neonate was 9 times more likely to have a vitreous band when an ERM was also present than when an ERM was not present (odds ratio, 9.4; 95% CI, 2.8-31.3). In the infants who developed an ERM, this finding was seen most commonly (7 of 19 infants; 37%) between 32 and 34 weeks’ postmenstrual age. Cystoid macular edema was also associated with vitreous bands (in 15 [62%] infants with vitreous bands vs 11 [27%] in those without; P = .005) (Table). When CME was present on the SD-OCT image, a vitreous band was 5 times more likely to be observed (odds ratio, 4.6; 95% CI, 1.5-13.3). For those neonates who developed CME, this finding was most commonly present (9 of 23 infants; 39%) at 33 to 35 weeks postmenstrual age.
Table. Demographic Characteristics, Clinical Examination Findings, and Imaging Characteristics of 65 Premature Infants Screened for ROP.
| Characteristic | No. (%) of Infants | |
|---|---|---|
| No Vitreous Band (n = 41) | Vitreous Band (n = 24)a | |
| Demographic characteristic | ||
| Male | 22 (55) | 11 (46) |
| Birth weight, mean (SD), g | 974 (281) | 1039 (297) |
| Gestational age, mean (SD), wk | 28.2 (3.0) | 27.6 (2.0) |
| Postmenstrual age at imaging, mean (SD), wk | 34.3 (3.2) | 34.3 (2.5) |
| Race/ethnicity | ||
| White | 11 (46) | 25 (61) |
| Hispanic | 4 (17) | 6 (15) |
| Native American | 1 (4) | 2 (5) |
| African American | 1 (4) | 0 |
| Pacific Islander | 1 (4) | 0 |
| Asian | 2 (8) | 1 (2) |
| Otherb | 4 (17) | 4 (10) |
| Indirect ophthalmic examination findings | ||
| ROP stage 0 | 30 (73) | 13 (54) |
| ROP stage 1 | 4 (10) | 4 (17) |
| ROP stage 2 | 3 (7) | 1 (4) |
| ROP stage 3 | 4 (10) | 6 (25) |
| Plus disease | 2 (5) | 2 (8) |
| Type 1 ROP | 3 (7) | 3 (12) |
| Vitreous haze | 5 (21) | 1 (4) |
| SD-OCT findings | ||
| Punctate hyperreflective vitreous opacities | 10 (24) | 9 (38) |
| Cystoid macular edema | 11 (27) | 15 (62) |
| Epiretinal membrane | 6 (15) | 15 (62) |
Abbreviations: ROP, retinopathy of prematurity; SD-OCT, spectral-domain optical coherence tomography.
Infant was categorized as having a vitreous band if he or she ever had a vitreous band, including tractional and nontractional vitreous bands, detected in the right eye using handheld SD-OCT at any point throughout ROP screening.
Patients who did not have a race/ethnicity identified in their medical record.
Twenty-four of 65 study participants (37%) had a vitreous band observed in the right eye at least once during the imaging sessions. Comparing the infants who had a vitreous band observed in the right eye at any time with those who did not, no difference in mean birth weight, gestational age, postmenstrual age at imaging, sex, or race/ethnicity was identified (Table). The vitreous bands were distributed evenly with respect to postmenstrual age at imaging and did not appear to be associated with particular ages. Among those infants with vitreous bands, concurrent clinical examination of the right eye revealed ROP stage 0 for 13 of 24 infants (54%), stage 1 for 4 of 24 infants (17%), stage 2 for 1 of 24 infants (4%), and stage 3 for 6 of 24 infants (25%). No associations between the presence of a vitreous band and markers of ROP severity, including ROP stage 3 (eg, in 6 [25%] infants with vitreous bands vs 4 [10%] in those without; P = .23), plus disease (2 [8%] vs 2 [5%]; P = .84), and type 1 ROP (3 [12%] vs 3 [7%]; P = .66), were identified. Among 10 of 209 imaging sessions (5%) in which a vitreous finding was described by the clinician (6 of 65 infants [9%]), only 1 of 65 infants (2%) had coexisting vitreous bands seen by SD-OCT (P = .43) (Table).
Discussion
Retinopathy of prematurity is a complex vasoproliferative disease affecting both the retina and the vitreous. With the increasing use of handheld SD-OCT among the infant population, we are now able to appreciate subclinical findings that affect the retinal microarchitecture in premature infants.4,5,6,7,8,9 To our knowledge, this is the first comprehensive report on vitreous findings documented by handheld SD-OCT among infants screened for ROP.
Lee et al4 described “vitreous material shadowing” that was seen in 7 of 207 handheld SD-OCT images from premature infants screened for ROP but did not describe the source of the shadowing nor correlate the shadows to ROP stage. It is possible that earlier studies overlooked subtle vitreous findings noted in this study. In this study, vitreous bands and punctate hyperreflective vitreous opacities occurred in 40 of 209 (19%) and 46 of 209 (22%) of the imaging sessions reviewed, respectively, but they may not have a related cause because they infrequently occurred together (9 of 209 imaging sessions [4%]; P = .26).
Our study showed an association between the development of a vitreous band and both CME and ERM. These findings support a tractional pathogenesis to these entities in premature infants. Vinekar et al6 previously suggested that CME of prematurity may be related to the mechanical traction exerted on the macula. Other studies have associated CME with worsening ROP severity.6,27
Among infants with ROP who are at risk for retinal detachment, there are known tractional vectors in the vitreous. These bands are targeted during vitrectomy for stage 4 ROP.28 In histologic studies of membranes removed during vitreoretinal surgery to treat ROP-related retinal detachment, researchers found that the internal limiting membrane was fragmented by retinal glial cells that migrated into the vitreous.29 Vitreous bands observed on handheld SD-OCT may represent the hyaloidal organization of vitreous membranes formed by retinal glial cells, which rarely leads to retinal detachment in advanced ROP. According to our data, there was no significant association between the development of a vitreous band and worsening ROP stage. However, because tractional bands are clinically observed in advanced stages of peripheral ROP,10 it is possible that the limited peripheral scanning ability of the SD-OCT may have resulted in missing a large proportion of these tractional bands. Nonetheless, the few central tractional bands identified in this study may suggest diffuse vitreous changes predisposing these infants to advanced ROP.
We hypothesize that our understanding of diabetic vitreoretinopathy can serve as a parallel disease model for similar vitreous changes and interactions at the vitreoretinal interface among infants with ROP. Earlier studies on diabetic retinopathy have detailed the development of a vitreoschisis cavity, which serves as a scaffold for migrating retinal glial and inflammatory cells leading to nontractional bands. These changes, in turn, create a thickened and taut posterior vitreous cortex that eventually contributes to both CME and ERM.30 Although chronically elevated vascular endothelial growth factor levels may further contribute to prolonged CME in diabetic retinopathy, CME in premature infants appears to be self-limited.6 The role of subsequent vitreous changes in CME resolution in premature infants is unknown. Longer follow-up is needed.
Limitations
Our study was limited by its observational study design and short study follow-up. Nonetheless, we had a high interobserver agreement for SD-OCT findings.
Conclusions
To our knowledge, this was the first study to identify an association between vitreous bands and retinal pathologic features including CME and ERM, providing important clues toward understanding the pathophysiology of ROP and visual impairment in premature infants. Whether any of these findings lead to poor visual or refractive outcomes deserves additional research.
References
- 1.Indications R; Early Treatment for Retinopathy of Prematurity Cooperative Group . Revised indications for the treatment of retinopathy of prematurity: results of the Early Treatment for Retinopathy of Prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684-1694. [DOI] [PubMed] [Google Scholar]
- 2.Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189-195. [DOI] [PubMed] [Google Scholar]
- 3.Hubbard GB., III Surgical management of retinopathy of prematurity. Curr Opin Ophthalmol. 2008;19(5):384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AC, Maldonado RS, Sarin N, et al. Macular features from spectral-domain optical coherence tomography as an adjunct to indirect ophthalmoscopy in retinopathy of prematurity. Retina. 2011;31(8):1470-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubis AM, Subramaniam CD, Godara P, Carroll J, Costakos DM. Subclinical macular findings in infants screened for retinopathy of prematurity with spectral-domain optical coherence tomography. Ophthalmology. 2013;120(8):1665-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinekar A, Avadhani K, Sivakumar M, et al. Understanding clinically undetected macular changes in early retinopathy of prematurity on spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(8):5183-5188. [DOI] [PubMed] [Google Scholar]
- 7.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muni RH, Kohly RP, Charonis AC, Lee TC. Retinoschisis detected with handheld spectral-domain optical coherence tomography in neonates with advanced retinopathy of prematurity. Arch Ophthalmol. 2010;128(1):57-62. [DOI] [PubMed] [Google Scholar]
- 9.Bondalapati S, Milam RW Jr, Ulrich JN, Cabrera MT. The characteristics and short-term refractive error outcomes of cystoid macular edema in premature neonates as detected by spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2015;46(8):806-812. [DOI] [PubMed] [Google Scholar]
- 10.Roohipoor R, Karkhaneh R, Riazi-Esfahani M, Ghasemi F, Nili-Ahmadabadi M. Surgical management in advanced stages of retinopathy of prematurity; our experience. J Ophthalmic Vis Res. 2009;4(3):185-190. [PMC free article] [PubMed] [Google Scholar]
- 11.Wu WC, Lin RI, Shih CP, et al. Visual acuity, optical components, and macular abnormalities in patients with a history of retinopathy of prematurity. Ophthalmology. 2012;119(9):1907-1916. [DOI] [PubMed] [Google Scholar]
- 12.Vinekar A, Mangalesh S, Jayadev C, et al. Macular edema in Asian Indian premature infants with retinopathy of prematurity. Indian J Ophthalmol. 2015;63(5):432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothman AL, Tran-Viet D, Vajzovic L, et al. Functional outcomes of young infants with and without macular edema. Retina. 2015;35(10):2018-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krolicki TJ, Tasman W. Cataract extraction in adults with retinopathy of prematurity. Arch Ophthalmol. 1995;113(2):173-177. [DOI] [PubMed] [Google Scholar]
- 15.Tasman W. Late complications of retrolental fibroplasia. Ophthalmology. 1979;86(10):1724-1740. [DOI] [PubMed] [Google Scholar]
- 16.Kushner BJ. Ciliary block glaucoma in retinopathy of prematurity. Arch Ophthalmol. 1982;100(7):1078-1079. [DOI] [PubMed] [Google Scholar]
- 17.Smith J, Shivitz I. Angle-closure glaucoma in adults with cicatricial retinopathy of prematurity. Arch Ophthalmol. 1984;102(3):371-372. [DOI] [PubMed] [Google Scholar]
- 18.Kushner BJ, Sondheimer S. Medical treatment of glaucoma associated with cicatricial retinopathy of prematurity. Am J Ophthalmol. 1982;94(3):313-317. [DOI] [PubMed] [Google Scholar]
- 19.Pollard ZF. Lensectomy for secondary angle-closure glaucoma in advanced cicatricial retrolental fibroplasia. Ophthalmology. 1984;91(4):395-398. [DOI] [PubMed] [Google Scholar]
- 20.Witmer R. Giant retinal tears in retrolental fibroplasia and Marfan’s syndrome. Mod Probl Ophthalmol. 1979;20:279-281. [PubMed] [Google Scholar]
- 21.Sneed SR, Pulido JS, Blodi CF, Clarkson JG, Flynn HW Jr, Mieler WF. Surgical management of late-onset retinal detachments associated with regressed retinopathy of prematurity. Ophthalmology. 1990;97(2):179-183. [DOI] [PubMed] [Google Scholar]
- 22.Harris GS. Retinopathy of prematurity and retinal detachment. Can J Ophthalmol. 1976;11(1):21-25. [PubMed] [Google Scholar]
- 23.Faris BM, Brockhurst RJ. Retrolental fibroplasia in the cicatrical stage. Arch Ophthalmol. 1969;82(1):60-65. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser RS, Trese MT, Williams GA, Cox MS Jr. Adult retinopathy of prematurity. Ophthalmology. 2001;108(9):1647-1653. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51(5):2678-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Good WV; Early Treatment for Retinopathy of Prematurity Cooperative Group . Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc. 2004;102:233-248. [PMC free article] [PubMed] [Google Scholar]
- 27.Maldonado RS, O’Connell R, Ascher SB, et al. Spectral-domain optical coherence tomographic assessment of severity of cystoid macular edema in retinopathy of prematurity. Arch Ophthalmol. 2012;130(5):569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu WC, Lai CC, Lin RI, et al. Modified 23-gauge vitrectomy system for stage 4 retinopathy of prematurity. Arch Ophthalmol. 2011;129(10):1326-1331. [DOI] [PubMed] [Google Scholar]
- 29.deJuan E Jr, Machemer R. Retinopathy of prematurity: surgical technique. Retina. 1987;7(2):63-69. [PubMed] [Google Scholar]
- 30.Gale J, Aiello L, Sebag J. Diabetic vitreopathy In: Sebag J. Vitreous: In Health and Disease. New York, NY: Springer;2014:57-79. [Google Scholar]