Key Points
Question
Which children receive adjuvant therapy for unilateral retinoblastoma?
Findings
In this single-arm multicenter study in Latin America with 175 children, overall survival was 100% in those with low risk of relapse and no receipt of adjuvant therapy and 95% in those with high risk of relapse and receipt of an intensive chemotherapy regimen. Preoperative chemotherapy was administered in children with buphthalmia.
Meaning
These data suggest that adjuvant chemotherapy may not be needed in children having unilateral retinoblastoma with low risk of relapse; a schema of intense adjuvant chemotherapy may be effective in preventing extraocular relapse.
Abstract
Importance
Multi-institutional collaborative studies that include large patient populations for the management of retinoblastoma with histopathological risk factors could provide important information for patient management.
Objective
To evaluate the implementation of a strategy for the management of nonmetastatic unilateral retinoblastoma in children based on standardized diagnostic and treatment criteria.
Design, Setting, and Participants
This single-arm prospective study applied a strategy based on a single-center experience. The setting was a multicenter study in Latin America (Grupo de America Latina de Oncologia Pediatrica [GALOP]). Participants were children with nonmetastatic unilateral retinoblastoma (staged with the International Retinoblastoma Staging System). The study opened on July 1, 2008, and closed on December 31, 2014. Follow-up was updated until June 30, 2017.
Interventions
Stage 0 patients (without enucleation) were given conservative therapy without a protocol. Stage I patients (with enucleation and no residual tumor) were divided into a high-risk group (retrolaminar invasion and/or scleral invasion) and a low-risk group (all remaining patients). High-risk children received adjuvant chemotherapy with 4 alternating cycles of regimen 1 (cyclophosphamide [65 mg/kg/d] [plus sodium-2-mercaptoethane sulfonate], idarubicin hydrochloride [10 mg/m2/d], and vincristine sulfate [0.05 mg/kg/d]) and 4 cycles of regimen 2 (carboplatin [500 mg/m2/d, days 1 and 2] and etoposide [100 mg/m2/d, days 1-3]). Low-risk children did not receive adjuvant therapy. Children with buphthalmia received neoadjuvant and adjuvant chemotherapy for a total of 8 cycles.
Main Outcomes and Measures
Probability of event-free survival (extraocular relapse and death from any cause were considered events).
Results
Among 187 children registered in the study, 175 were evaluable (92 [52.5%] female; median age, 22 months; age range, 3-100 months). Forty-two were stage 0 children, 84 were stage I low-risk children, and 42 were stage I high-risk children; there were 7 children in the buphthalmia group. With a median follow-up of 46 months, the 3-year probability of event-free survival was 0.97 (95% CI, 0.94-0.99), and the probability of overall survival was 0.98 (95% CI, 0.94-1.00). Stage 0 patients had no events, stage I low-risk patients had 1 event (orbital relapse treated with second-line therapy), stage I high-risk patients had 2 events (1 central nervous system relapse and 1 death from sepsis), and the buphthalmia group had 1 event (orbital relapse, followed by central nervous relapse and death).
Conclusions and Relevance
Adjuvant therapy may be effective for high-risk unilateral retinoblastoma but is toxic, and neoadjuvant chemotherapy for buphthalmus appears feasible.
This single-arm multicenter study evaluates the implementation of a treatment strategy to improve survival among Latin American children with unilateral retinoblastoma based on standardized diagnostic and treatment criteria.
Introduction
Compared with the United States and Western Europe, retinoblastoma manifests more commonly as advanced disease in Latin America and other less developed areas.1,2 In Latin America, overt extraocular disease at presentation of retinoblastoma is now less common; however, microscopic extraretinal extension occurs frequently, and patients may need adjuvant therapy to decrease the risk of metastatic relapse.3,4
Experience at a single institution in Buenos Aires, Argentina (Hospital J. P. Garrahan), over 3 prospective protocols suggested that adjuvant treatment of retinoblastoma may not be necessary in patients with choroidal invasion alone or in combination with prelaminar optic nerve invasion.5,6,7 It was also reported that adjuvant therapy that includes moderate-intensity carboplatin and alkylating agent–based combinations may be less effective in a limited cohort of patients with postlaminar optic nerve involvement (PLONI) and/or microscopic scleral invasion, leading to a relapse risk ranging from 25% to 50%.6 Subsequently, it was found that a more intensive regimen reduced the risk of extraocular relapse in these high-risk patients.8,9 Based on these results, we aimed to further validate this strategy in a larger population in a multicenter study using a standardized procedure for staging and histopathological processing of enucleated eyes applying international consensus guidelines.10 However, multicenter investigations in cooperative studies are challenging in less developed countries. With the creation of GALOP (Grupo de America Latina de Oncologia Pediatrica), supported by the Children’s Oncology Group (COG), a platform for performing collaborative clinical studies became available in the region, and this study became feasible.
In addition, some patients with unilateral retinoblastoma are treated with eye-conserving therapy. Controversy exists on the safety of eye-conserving therapy in terms of the risk of extraocular relapse, especially with the use of intra-arterial chemotherapy (IAC).11,12 The objective of the present study was to evaluate the implementation of a strategy for the management of nonmetastatic unilateral retinoblastoma in children based on standardized diagnostic and treatment criteria, including those treated conservatively.
Other aims were the evaluation of avoiding therapy for lower-risk patients and determination if the use of high-intensity adjuvant therapy is associated with a low extraocular relapse rate in high-risk children. Also evaluated was the use of preenucleation chemotherapy for children with buphthalmia as a way of reducing the occurrence of tumor at the resection margin of the optic nerve.
Methods
This investigation was a single-arm prospective study applying a strategy based on a single-center experience. The setting was a multicenter study in Latin America. Participants were children with nonmetastatic unilateral retinoblastoma (staged with the International Retinoblastoma Staging System [IRSS]). The inclusion criteria were (1) previously untreated patients with sporadic unilateral retinoblastoma diagnosed histopathologically or clinically with IRSS stages 0 and I and (2) normal renal, cardiac, and hepatic function and a Lansky Performance Scale score exceeding 50 in those receiving adjuvant therapy.
To be evaluable, patients had to have received all treatment and follow-up at the participating institutions. The study was performed under the tenets of the Declaration of Helsinki,13 and written informed consent was obtained from parents or guardians at the time of ophthalmological diagnosis of unilateral retinoblastoma. Institutional review board approval was obtained originally at the Hospital J. P. Garrahan. Children with stages II to IV were invited to enroll in the COG ARET0321 protocol.
The study initially opened at the Hospital J. P. Garrahan on July 1, 2008. The Chilean cooperative group Programa Infantil Nacional de Drogas Antineoplásicas (PINDA), the national referral center in Uruguay, a second Argentinian institution, and the Hospital Sant Joan de Déu in Barcelona, Spain, joined subsequently. The study closed at all institutions on December 31, 2014. Follow-up was updated until June 30, 2017.
Before launching the study at each GALOP center, a training session was held to ensure agreement of histopathological criteria, which were based on the IRSS.14 A web resource for data capturing was designed: each center was given an access code to upload the data, and a coordinating center in Argentina (Hospital J. P. Garrahan) was appointed. Yearly groupwide meetings were held for discussion of updates. An additional interim meeting in 2012 and a closure meeting in 2016 were held for histopathology and clinical data review. An external data monitoring committee was appointed. Also appointed was a pathology committee with representatives from each group, and selected histopathological slides were collectively reviewed by this committee to assess consistency in staging. All slides of relapsing patients were reviewed by the committee, and 94 randomly selected enucleation specimens were chosen to evaluate consistency of the pathology reports. There was no centralized real-time review of histopathological slides; however, this was done in 1 case where the decision would influence therapy. In 2 other cases, there were discrepancies in the extent of choroidal invasion that did not influence the treatment choice.
All patients underwent an evaluation of the extent of disease, including ocular examination under general anesthesia and head and orbital gadolinium-enhanced magnetic resonance imaging. Lumbar puncture with examination of the cytospin and bone marrow assessment, including bilateral bone marrow aspiration and biopsy, were performed only in patients who were scheduled for adjuvant therapy. The IRSS was used for staging,15 and the International Retinoblastoma Classification was used for eye grouping.16 For the present study, patients were assigned a pTNM stage using the eighth edition of the American Joint Committee on Cancer’s AJCC Cancer Staging Manual.17
Treatment
Upfront enucleation was recommended for all International Classification of Retinoblastoma group E eyes at diagnosis, except for children with buphthalmia, who underwent planned secondary enucleation. Initially enucleated patients with stage I PLONI and/or intrascleral invasion were considered high risk and were scheduled to receive adjuvant chemotherapy with 4 cycles of regimen 1 and 4 alternating cycles of regimen 2 (Table 1). Initially enucleated patients with other histopathological features were considered low risk and were not scheduled to receive adjuvant therapy. In the first 2 years, when the study was performed only at the Hospital J. P. Garrahan, patients with PLONI with less than 1-mm extension from the lamina cribrosa8 did not receive adjuvant therapy. To apply a consistent strategy, such patients were scheduled for adjuvant chemotherapy when the study was proposed to other institutions.
Table 1. Use of Adjuvant Therapy According to Pathology Features in Initially Enucleated Patients With Stage I Unilateral Retinoblastomaa.
| Treatment Group | Pathology Features | Adjuvant Therapy |
|---|---|---|
| Low risk (n = 84) | Intraretinal invasion only, prelaminar optic nerve, isolated choroid | Not scheduled |
| High risk (n = 42) | Postlaminar optic nerve, scleral invasion | Regimen 1 at weeks 0, 6, 12, and 18b |
| Regimen 2 at weeks 3, 9, 15, and 21c |
According to the International Retinoblastoma Staging System.
Four alternating cycles of regimen 1 (cyclophosphamide [65 mg/kg/d] [plus sodium-2-mercaptoethane sulfonate], idarubicin hydrochloride [10 mg/m2/d], and vincristine sulfate [0.05 mg/kg/d]).
Four cycles of regimen 2 (carboplatin [500 mg/m2/d, days 1 and 2] and etoposide [100 mg/m2/d, days 1-3]).
A subgroup of group E patients with glaucoma (intraocular tension >20 mm Hg) and buphthalmia (>30% difference in corneal diameter between each eye) were scheduled to receive neoadjuvant therapy, followed by secondary enucleation after 2 cycles (one course of regimen 1 and one course of regimen 2). Adjuvant therapy with regimen 1 and regimen 2 was given regardless of the histopathological report findings to complete a total of 8 chemotherapy cycles. External beam radiotherapy (45 Gy to the orbit up to the chiasm [to convert gray to rad, multiply by 100]) was recommended at the end of the 8 cycles when viable or necrotic tumor cells were evident at the resection margin.
Several other patients were invited to enroll in the COG ARET0321 protocol. These included 2 initially enucleated patients with invasion to the optic nerve beyond the resection margin and 7 patients with stages III and IV.
All patients with group B or C eyes (n = 21) and selected patients with group D eyes (n = 21) in whom a conservative approach was deemed possible by the local groups were offered conservative therapy. However, conservative treatment was not standardized, and each center used systemic chemotherapy or OAC depending on availability and the preference of the team. Systemic chemotherapy used included carboplatin, etoposide, and vincristine sulfate combinations,18 and OAC regimens were based on melphalan and topotecan hydrochloride.11
Statistical Analysis
The outcome end points for this study were the probability of event-free survival (pEFS) exceeding 0.95% in the low-risk group and exceeding 0.9% in the high-risk group. Fatal toxic effects were considered acceptable if the rate was lower than 3% of patients treated with adjuvant therapy. Extraocular relapse, secondary malignant tumors, and death from any cause were considered events. Survival estimates were calculated according to the Kaplan-Meier method. P values were 2-sided.
Results
Among 187 children registered in the study, 175 were evaluable (92 [52.5%] female; median age, 22 months; age range, 3-100 months). Forty-two were stage 0 children, 84 were stage I low-risk children, and 42 were stage I high-risk children; there were 7 children in the buphthalmia group. Nonevaluable patients included 5 with metachronic bilateral retinoblastoma, 5 with referral to a nonparticipating institution for follow-up, 1 with incorrect staging, and 1 with incorrect diagnosis. One hundred forty-three included patients were from Argentina, 18 were from Spain, 12 were from Chile, and 2 were from Uruguay.
Initially Enucleated Low-Risk Patients
An event occurred in this group (isolated orbital relapse [n = 84]), but the probability of overall survival (pOS) was 1.00 because this child received rescue therapy, including chemotherapy with the same regimen used for adjuvant therapy and orbital radiotherapy (45 Gy). The pEFS was 0.99 (95% CI, 0.92-1.00). The distribution of patients according to histopathological features is summarized in Table 2.
Table 2. Outcome According to Pathology Features in Initially Enucleated Patients With Stage I Unilateral Retinoblastoma.
| Treatment Group | Pathology Features | Events |
|---|---|---|
| Low risk (n = 84) | No optic nerve, choroidal, or scleral invasion (n = 18) | Orbital relapse treated with second-line therapy |
| Isolated prelaminar optic nerve invasion (n = 12) | No events | |
| Isolated focal choroidal invasion (n = 9) | No events | |
| Combined prelaminar and focal choroidal invasion (n = 25) | No events | |
| Isolated postlaminar optic nerve involvement <1 mm (n = 7) | No events | |
| Isolated massive choroidal involvement (n = 13) | No events | |
| High risk (n = 42) | Isolated postlaminar optic nerve involvement or combined with focal choroidal invasion (n = 15) | 1 CNS relapse, 1 death owing to toxic effects |
| Postlaminar optic nerve involvement with massive choroidal invasion (n = 13) | No events | |
| Postlaminar optic nerve involvement with massive choroidal invasion and microscopic scleral invasion (n = 7) | No events | |
| Microscopic scleral invasion and less than postlaminar optic nerve invasion (n = 7) | No events |
Abbreviation: CNS, central nervous system.
Initially Enucleated High-Risk Patients
Overall, there were 2 events in this group (1 central nervous system [CNS] relapse at 11 months after diagnosis and 1 toxic death from neutropenic sepsis [n = 42]), described below), both occurring in patients with PLONI. The child who experienced CNS relapse achieved a second remission with further chemotherapy but ultimately had a subsequent relapse and died. Three-year pEFS and pOS were both 0.95 (95% CI, 0.82-0.98). Details of histopathological staging and outcome are listed in Table 2, and the distribution after reclassification according to the pTNM system is summarized in Table 3.
Table 3. Use of Adjuvant Therapy and Outcome According to pTNM Staging in 175 Patients With Unilateral Retinoblastoma.
| pTNM | No. | Adjuvant Therapy | Events | pEFS | pOS |
|---|---|---|---|---|---|
| pT1-pT2 | 77 | No (n = 74) | 1 Orbital relapse | 0.97 | 0.99 |
| Yes (n = 3)a | 1 Orbital relapse | ||||
| pT3a | 17 | No (n = 15) | No events | 1.00 | 1.00 |
| Yes (n = 2)a,b | |||||
| pT3b | 38 | No (n = 7) | No events | 0.95 | 0.95 |
| Yes (n = 31) | 1 CNS relapse, 1 death owing to toxic reaction | ||||
| pT3c | 15 | No (n = 1) | No events | 1.00 | 1.00 |
| Yes (n = 14) | |||||
| pT3d | 3 | Yes (n = 3) | No events | 1.00 | 1.00 |
| pTx | 25 | No (n = 25) | No events | 1.00 | 1.00 |
Abbreviations: CNS, central nervous system; pEFS, probability of event-free survival; pOS, probability of overall survival.
Adjuvant chemotherapy was given irrespective of pathology findings because of prior chemotherapy exposure for the treatment of buphthalmia.
Adjuvant therapy was given based on the preference of the team.
Children With Buphthalmus
Of the 7 children in this group, there was 1 event. A patient whose histopathological examination showed focal choroidal and prelaminar invasion had an orbital relapse, which was treated with chemotherapy and radiotherapy (45 Gy to the orbit). A second complete remission was achieved; however, CNS relapse occurred after 8 months, and the child died. The remaining 6 patients survived disease free for a median of 36 months. Two cycles of neoadjuvant chemotherapy were given before enucleation in 6 patients. The remaining patient received 3 cycles because of a conflict in scheduling of enucleation. In all cases, glaucoma resolved after the first cycle. Histopathological examination revealed tumor necrosis ranging from 10% to 90% and PLONI in 1 case and massive choroidal invasion in 2 cases. Another child had necrotic cells in the optic nerve resection margin, so orbital radiotherapy was used.
Toxic Effects of Adjuvant Therapy
Platelet and red blood cell transfusions were necessary in all but 7 patients receiving adjuvant therapy. At least one hospital admission (median, 2; range, 1-7) because of fever and neutropenia was recorded in all but 5 patients. All patients given adjuvant chemotherapy received granulocyte growth factor support, and an implantable intravenous catheter was inserted in all cases. Serial yearly echocardiograms were performed in all patients, and no occurrence of cardiac toxic effects was reported. One patient died during a neutropenic episode of sepsis caused by multiresistant Acinetobacter.
Modifications Based on Toxic Effects
One patient received only 5 cycles of adjuvant therapy, with the family declining further therapy. As per study guidelines, the dose was reduced by 25% in another patient because of prolonged and severe hematopoietic toxic effects. Neither patient experienced an event.
Patients Not Receiving Treatment According to Study Guidelines
In the 3 patients in this group, 1 patient received only 6 cycles of carboplatin, etoposide, and vincristine18 as adjuvant therapy, with poor compliance because of socioeconomic issues. Another family opted for adjuvant therapy with study-based chemotherapy for their high-risk child who had isolated massive choroidal invasion but was categorized as low risk by our criteria. The third child with microscopic intrascleral invasion did not receive adjuvant therapy because of family request based on socioeconomic issues. All 3 patients are included in the analysis of their respective subgroups, and all were alive at the time of article creation and disease free.
Patients Given Conservative Therapy
Of the 42 patients in this group, 23 patients received primary systemic chemotherapy, and 19 received primary IAC. The probability of eye preservation without the use of external beam radiotherapy at 3 years was 0.42 (95% CI, 0.21-0.61) for the former and 0.61 (95% CI, 0.29-0.82) for the latter (P = .20). No event was recorded in any patient treated conservatively.
Outcome
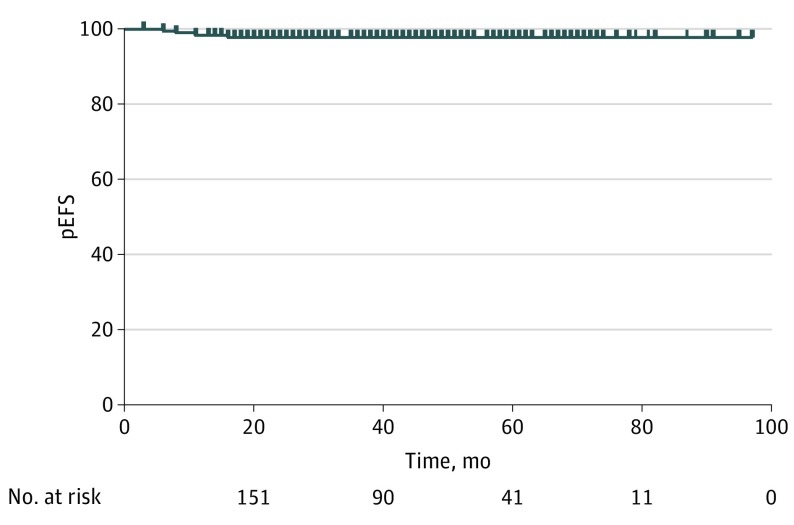
One patient in the low-risk group was lost to follow-up and censored at the day of last contact 3 months after enucleation. The remaining patients had at least 18 months of follow-up. The median follow-up was 46 months (range, 3-99 months). For the whole cohort, the 3-year pEFS was 0.97 (95% CI, 0.94-0.99), and the pOS was 0.98 (95% CI, 0.94-1.00) (Figure).
Figure. pEFS for the Whole Population Included in the Study.
pEFS indicates probability of event-free survival.
Discussion
We implemented a multicenter study in Latin America (plus 1 European-affiliated institution in Barcelona) with a standardized management of unilateral retinoblastoma in the context of a recently created GALOP cooperative group in the region. In performing such a study in our region, we faced numerous organizational and financial challenges. Despite that the study was based on a published strategy,7 approval at some institutions was a lengthy process, delaying the enrollment of patients and reducing the target for patient accrual. Nevertheless, the study achieved its outcome measures and set the basis for the ongoing second retinoblastoma GALOP protocol. Major challenges for a subsequent protocol are the need to include more institutions to further increase the patient number and ideally to incorporate a centralized imaging and pathology review.
Based on the previous experience,7 only children with PLONI and those with microscopic scleral invasion were given adjuvant therapy to reduce the likelihood of extraocular relapse. All the rest were not given adjuvant therapy because their estimated relapse rate was lower than 4% and overall survival was higher than 98%; many children with extraocular relapse may be successfully treated with rescue therapy.5,6,7 However, the initial single-institution experience included a limited number of patients, and the histopathological guidelines used were not internationally validated. In a larger cohort with a validated histopathological evaluation, the present study confirmed the safety of withdrawing adjuvant therapy in low-risk patients, as defined by our criteria, because none of these patients died. This included 13 patients with isolated massive choroidal invasion and 25 patients with focal choroidal invasion plus prelaminar optic nerve involvement, who would be considered high risk and given adjuvant therapy in other studies. In fact, the extraocular relapse rate in this subgroup was 0% because the sole patient in the low-risk group who relapsed had only intraretinal invasion.
For the treatment of high-risk patients, the adjuvant chemotherapy regimen used in this protocol achieved a pOS of 0.95. Carboplatin-based regimens are the preferred chemotherapy schemas used for adjuvant therapy for the treatment of retinoblastoma and histopathological risk factors.19 However, different research groups define high-risk patients diversely and use regimens with variable dose intensity. The contemporary COG ARET0332 protocol and other researchers use a lower dose and fewer cycles.20,21 Other groups add alkylating agents to these regimens and, less frequently, anthracyclines.20,21,22 In our previous single-center experience,7 when moderate-dose regimens were used, 8 patients with combined PLONI and massive choroid or scleral invasion had only a 0.75 probability of disease-free survival (pDFS), which improved to 1.00 after introduction of the regimen used in this protocol. However, because of the few events in all studies and the diverse pathological criteria to assign risk, it is not possible to unequivocally compare the efficacy of the different available regimens. Specifically, for the patients with PLONI in a prospective study21 from France, none of 12 patients had an extraocular relapse after receiving 4 cycles of a less intensive combination of carboplatin, etoposide, cyclophosphamide, and vincristine. In a retrospective study23 of patients with PLONI, 5 of 71 (7%) had an extraocular relapse after receiving a regimen similar to that in the COG ARET0332 protocol. In the present series, only 1 of 35 patients with PLONI had an extraocular relapse, but an additional child died of toxic effects. The presence of concomitant histopathological risk factors may potentially add higher risk to some subgroups.24 Even more controversial is the treatment of patients with other less common histopathological features, such as scleral invasion. In our study, there was no extraocular relapse among the 18 patients with microscopic scleral invasion. All received adjuvant chemotherapy without orbital radiotherapy, providing more evidence that orbital radiotherapy may not be necessary when an intensive regimen is used in these children.25
To reduce the number of children with microscopic residue in the resection margin of the optic nerve (which was found to be significantly more likely in patients with buphthalmia26), another objective of this study was to assess a previously published strategy of neoadjuvant therapy in these patients.27 As opposed to this approach, other research groups do not give neoadjuvant therapy because of concern about altering the histopathology of the enucleated eye, such that adjuvant therapy would not be tailored precisely.28 In our protocol, the use of this strategy assumed that histopathological interpretation after neoadjuvant therapy was not possible, so there was no treatment deescalation and all patients received adjuvant chemotherapy as high-risk patients regardless of the histopathology of secondarily enucleated eyes. An option for this approach would be to initially enucleate these patients. In the previous single-institution investigations from Argentina, these patients were initially enucleated: up to 16.6% of cases resulted in tumor at the resection margin in the optic nerve, and their pDFS was 0.7 with the use of adjuvant chemotherapy and radiotherapy.6 All of these patients experienced endocrinological and cosmetic sequelae. Among the limited number of patients included in the present study, only one needed orbital radiotherapy, minimizing late effects.29 In future studies, more patients should be included to obtain definitive conclusions.
Conservative therapy was attempted in 42 of 175 (24.0%) of our patients in the present study compared with 17 of 114 (14.9%) in our previous single-institution experience.7 There was no case of extraocular relapse or death from any cause in our population receiving any conservative therapy in either study.
Limitations
Our study had a major limitation. It lacked randomized comparisons with other treatment regimens across different groups.
Conclusions
A multicenter study for unilateral retinoblastoma was performed in Latin America. Overall survival was 98% when limiting the use of adjuvant therapy to high-risk patients, without metastasis-related death in patients treated only by enucleation. The adjuvant therapy regimen may be effective for unilateral retinoblastoma but is toxic, so future studies might be of value that include a dose reduction. Preenucleation chemotherapy may have helped to reduce the occurrence of tumor at the resection margin of the optic nerve. No children receiving conservative therapy had a metastatic relapse.
References
- 1.Dean M, Bendfeldt G, Lou H, et al. Increased incidence and disparity of diagnosis of retinoblastoma patients in Guatemala. Cancer Lett. 2014;351(1):59-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canturk S, Qaddoumi I, Khetan V, et al. Survival of retinoblastoma in less-developed countries: impact of socioeconomic and health-related indicators. Br J Ophthalmol. 2010;94(11):1432-1436. [DOI] [PubMed] [Google Scholar]
- 3.Leal-Leal C, Flores-Rojo M, Medina-Sansón A, et al. A multicentre report from the Mexican Retinoblastoma Group. Br J Ophthalmol. 2004;88(8):1074-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoneli CB, Steinhorst F, de Cássia Braga Ribeiro K, et al. Extraocular retinoblastoma: a 13-year experience. Cancer. 2003;98(6):1292-1298. [DOI] [PubMed] [Google Scholar]
- 5.Schvartzman E, Chantada G, Fandiño A, de Dávila MT, Raslawski E, Manzitti J. Results of a stage-based protocol for the treatment of retinoblastoma. J Clin Oncol. 1996;14(5):1532-1536. [DOI] [PubMed] [Google Scholar]
- 6.Chantada G, Fandiño A, Dávila MT, et al. Results of a prospective study for the treatment of retinoblastoma. Cancer. 2004;100(4):834-842. [DOI] [PubMed] [Google Scholar]
- 7.Chantada GL, Fandiño AC, Guitter MR, et al. Results of a prospective study for the treatment of unilateral retinoblastoma. Pediatr Blood Cancer. 2010;55(1):60-66. [DOI] [PubMed] [Google Scholar]
- 8.Chantada GL, Casco F, Fandiño AC, et al. Outcome of patients with retinoblastoma and postlaminar optic nerve invasion. Ophthalmology. 2007;114(11):2083-2089. [DOI] [PubMed] [Google Scholar]
- 9.Antoneli CB, Ribeiro KB, Rodriguez-Galindo C, et al. The addition of ifosfamide/etoposide to cisplatin/teniposide improves the survival of children with retinoblastoma and orbital involvement. J Pediatr Hematol Oncol. 2007;29(10):700-704. [DOI] [PubMed] [Google Scholar]
- 10.Chantada GL, Doz F, Orjuela M, et al. ; International Retinoblastoma Staging Working Group . World disparities in risk definition and management of retinoblastoma: a report from the International Retinoblastoma Staging Working Group. Pediatr Blood Cancer. 2008;50(3):692-694. [DOI] [PubMed] [Google Scholar]
- 11.Gobin YP, Dunkel IJ, Marr BP, Brodie SE, Abramson DH. Intra-arterial chemotherapy for the management of retinoblastoma: four-year experience. Arch Ophthalmol. 2011;129(6):732-737. [DOI] [PubMed] [Google Scholar]
- 12.Levin MH, Gombos DS, O’Brien JM. Intra-arterial chemotherapy for advanced retinoblastoma: is the time right for a prospective clinical trial? Arch Ophthalmol. 2011;129(11):1487-1489. [DOI] [PubMed] [Google Scholar]
- 13.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 14.Sastre X, Chantada GL, Doz F, et al. ; International Retinoblastoma Staging Working Group . Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Arch Pathol Lab Med. 2009;133(8):1199-1202. [DOI] [PubMed] [Google Scholar]
- 15.Chantada G, Doz F, Antoneli CB, et al. A proposal for an International Retinoblastoma Staging System. Pediatr Blood Cancer. 2006;47(6):801-805. [DOI] [PubMed] [Google Scholar]
- 16.Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1):41-53, viii. [DOI] [PubMed] [Google Scholar]
- 17.Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. 8th ed New York, NY: Springer; 2017:819-831. [Google Scholar]
- 18.Chantada GL, Fandiño AC, Schvartzman E, Raslawski E, Schaiquevich P, Manzitti J. Impact of chemoreduction for conservative therapy for retinoblastoma in Argentina. Pediatr Blood Cancer. 2014;61(5):821-826. [DOI] [PubMed] [Google Scholar]
- 19.Kaliki S, Shields CL, Rojanaporn D, et al. High-risk retinoblastoma based on International Classification of Retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997-1003. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan EM, Wilson MW, Billups CA, et al. Pathologic risk–based adjuvant chemotherapy for unilateral retinoblastoma following enucleation. J Pediatr Hematol Oncol. 2014;36(6):e335-e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aerts I, Sastre-Garau X, Savignoni A, et al. Results of a multicenter prospective study on the postoperative treatment of unilateral retinoblastoma after primary enucleation. J Clin Oncol. 2013;31(11):1458-1463. [DOI] [PubMed] [Google Scholar]
- 22.Lumbroso-Le Rouic L, Savignoni A, Levy-Gabriel C, et al. Treatment of retinoblastoma: the Institut Curie experience on a series of 730 patients (1995 to 2009). J Fr Ophtalmol. 2015;38(6):535-541. [DOI] [PubMed] [Google Scholar]
- 23.Kaliki S, Tahiliani P, Mishra DK, Srinivasan V, Ali MH, Reddy VA. Optic nerve infiltration by retinoblastoma: predictive clinical features and outcome. Retina. 2016;36(6):1177-1183. [DOI] [PubMed] [Google Scholar]
- 24.Chantada GL, Dunkel IJ, de Dávila MT, Abramson DH. Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy? Br J Ophthalmol. 2004;88(8):1069-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuenca A, Giron F, Castro D, et al. Microscopic scleral invasion in retinoblastoma: clinicopathological features and outcome. Arch Ophthalmol. 2009;127(8):1006-1010. [DOI] [PubMed] [Google Scholar]
- 26.Chantada GL, Gonzalez A, Fandiño A, et al. Some clinical findings at presentation can predict high-risk pathology features in unilateral retinoblastoma. J Pediatr Hematol Oncol. 2009;31(5):325-329. [DOI] [PubMed] [Google Scholar]
- 27.Bellaton E, Bertozzi AI, Behar C, et al. Neoadjuvant chemotherapy for extensive unilateral retinoblastoma. Br J Ophthalmol. 2003;87(3):327-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Dimaras H, Massey C, et al. Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol. 2011;29(7):845-851. [DOI] [PubMed] [Google Scholar]
- 29.Friedman DN, Sklar CA, Oeffinger KC, et al. Long-term medical outcomes in survivors of extra-ocular retinoblastoma: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Pediatr Blood Cancer. 2013;60(4):694-699. [DOI] [PubMed] [Google Scholar]