Key Points
Question
What is the association between macular vessel density as measured by optical coherence tomography angiography and visual acuity in patients with diabetic retinopathy and poorly controlled type 1 diabetes?
Findings
In a cohort study of 22 eyes of 22 patients with type 1 diabetes and diabetic retinopathy without macular edema, 41% had decreased vision. Vessel density was lower in eyes with diabetic retinopathy and decreased vision than in eyes with diabetic retinopathy and normal vision; loss of vessel density was greater in the deep capillary complex, particularly the deep capillary plexus, than in the superficial vascular plexus.
Meaning
These findings suggest that decreased vision in patients with diabetic retinopathy may be associated with the degree of capillary loss in the deep capillary complex.
Abstract
Importance
Capillary dropout is a hallmark of diabetic retinopathy, but its role in visual loss remains unclear.
Objective
To examine how macular vessel density is correlated with visual acuity (VA) in patients younger than 40 years who have type 1 diabetes without macular edema but who have diabetic retinopathy requiring panretinal photocoagulation.
Design, Settings, and Participants
Retrospective cohort study of VA and optical coherence tomography angiography data collected from consecutive patients during a single visit to Lariboisière Hospital, a tertiary referral center in Paris, France. The cohort included 22 eyes of 22 patients with type 1 diabetes without macular edema but with bilateral rapidly progressive diabetic retinopathy that was treated with panretinal photocoagulation between August 15, 2015, and December 30, 2016. Eyes were classified into 2 groups by VA: normal (logMAR, 0; Snellen equivalent, 20/20) and decreased (logMAR, >0; Snellen equivalent, <20/20). The control group included 12 eyes from age-matched healthy participants with normal vision.
Main Outcomes and Measures
Visual acuity and mean vessel density in 4 retinal vascular plexuses: the superficial vascular plexus and the deep capillary complex, which comprises the intermediate capillary plexus and the deep capillary plexus.
Results
Of the 22 participants, 11 (50%) were men, mean (SD) age was 30 (6) years, and mean (SD) hemoglobin A1c level was 8.9% (1.6%). Of the 22 eyes with diabetic retinopathy, 13 (59%) had normal VA and 9 (41%) had decreased VA (mean [SD]: logMAR, 0.12 [0.04]; Snellen equivalent, 20/25). Mean [SE] vessel density was lower for eyes with diabetic retinopathy and normal VA compared with the control group in the superficial vascular plexus (44.1% [0.9%] vs 49.1% [0.9%]; difference, −5.0% [1.3%]; 95% CI, −7.5% to −2.4%; P < .001), in the deep capillary complex (44.3% [1.2%] vs 50.6% [1.3%]; difference, −6.3% [1.8%]; 95% CI, −9.9% to −2.7%; P = .001), in the intermediate capillary plexus (43.8% [1.2%] vs 49.3% [1.2%]; difference, −5.5% [1.7%]; 95% CI, −9.0% to −2.0%; P = .003), and in the deep capillary plexus (24.5% [1.0%] vs 30.5% [1.0%]; difference, −6.1% [1.4%]; 95% CI, −8.9% to −3.2%; P < .001). Mean vessel density was lower in eyes with diabetic retinopathy and decreased VA compared with eyes with diabetic retinopathy and normal VA; the mean (SE) loss was more pronounced in the deep capillary complex (34.6% [1.5%] vs 44.3% [1.2%]; difference, −9.6% [1.9%]; 95% CI, −13.6% to −5.7%; P < .001), especially in the deep capillary plexus (15.2% [1.2%] vs 24.5% [1.0%]; difference, −9.3% [1.5%]; 95% CI, −12.4% to −6.1%; P < .001), than in the superficial vascular plexus (39.6% [1.1%] vs 44.1% [0.9%]; difference, −4.5% [1.4%]; 95% CI, −7.3% to −1.7%; P = .002).
Conclusions and Relevance
These data suggest that in patients with type 1 diabetes without macular edema but with severe nonproliferative or proliferative diabetic retinopathy, decreased VA may be associated with the degree of capillary loss in the deep capillary complex.
This cohort study uses optical coherence tomography angiography to assess the association between visual acuity and mean vessel density in French patients with type 1 diabetes who have diabetic retinopathy and normal or decreased visual acuity.
Introduction
Capillary dropout is a hallmark of diabetic maculopathy, but its role in visual loss and in macular edema onset remains unclear. In 1984, Bresnick et al1 were the first to quantify the foveal avascular zone (FAZ) enlargement in patients with diabetes by using fluorescein angiography and associated it with the severity of diabetic retinopathy (DR). Several studies based on detection of capillary nonperfusion by fluorescein angiography have attempted to correlate diabetic macular ischemia and visual acuity (VA) with various results.2,3 Optical coherence tomography angiography (OCTA) now allows visualization of the retinal capillary plexuses4 and the calculation of vessel density and capillary nonperfusion areas in each plexus.5,6,7,8,9 The importance of the deep capillary plexus (DCP) impairment, which was undetectable by fluorescein angiography, has recently been shown by OCTA in patients with diabetes with no or with early-stage DR10 or with diabetic macular edema.11,12,13 However, the association between the capillary dropout and VA still remains largely unknown, especially in cases in which media opacity, severe retinal remodeling, or macular edema interfere with capillary nonperfusion. To investigate the association between VA and vessel density with a minimum of confounding factors, we studied a group of patients (aged <40 years) with type 1 diabetes complicated by sustained poor glycemic control and severe rapidly evolving DR, previously referred to as florid diabetic retinopathy.14 The included patients had no macular edema, intravitreal hemorrhage, tractional retinal detachment, or lens opacity and had been treated with panretinal photocoagulation (PRP). Casual observation of patients in our tertiary referral center suggested that such patients often have variable VA despite having no apparent macular complications. We build on this observation by systematically analyzing the FAZ and the vessel density of the retinal capillary plexuses using OCTA data and correlating these results with VA.
Methods
We reviewed the records of consecutive patients with type 1 diabetes complicated by rapidly progressive bilateral DR who were first seen at the Lariboisière Hospital, a tertiary referral center in Paris, France, between March 1, 2015, and January 31, 2016. This retrospective observational study was approved by the ethics committee of the French Society of Ophthalmology, Paris, France. Written informed consent was obtained from all patients before reviewing their medical records.
Inclusion criteria were patients younger than 40 years who had type 1 diabetes and a documented history of rapidly progressive, bilateral severe nonproliferative or proliferative DR that was diagnosed on fundus imaging results according to the Early Treatment Diabetic Retinopathy Study (ETDRS) DR grading scale and subsequently treated with PRP.15,16 Only patients imaged at least once with OCTA within the 12 months following completion of PRP were included. When both eyes were eligible, the eye with the better signal strength index was included.
Exclusion criteria were age younger than 18 years, lens or other ocular media opacities preventing detailed imaging, high myopia (approximately >6 diopters), clinical evidence of any other maculopathy, or previous extensive focal/grid laser or vitreoretinal surgery. Patients with a current or previous history of significant diabetic macular edema were also excluded. Diabetic macular edema was defined as a central subfield thickening of at least 315 μm on spectral-domain optical coherence tomography corresponding to the normal value plus 2 SDs: 277 + (2 × 19) μm.17 Patients without diabetic macular edema treated with anti–vascular endothelial growth factor (VEGF) for progressive new vessels were not excluded. Eyes with an OCTA signal strength index less than 50/100 were excluded.
Visual acuity was measured on a Snellen chart and expressed as the logMAR. Based on VA levels, eyes were divided into 2 groups: eyes with a normal VA (logMAR, 0; Snellen equivalent, 20/20) and eyes with a decreased VA (logMAR, >0; Snellen equivalent, <20/20). Visual acuity and OCTA data were compared with those measured in healthy age-matched control participants (volunteer caregivers) (logMAR, 0; Snellen equivalent, 20/20).
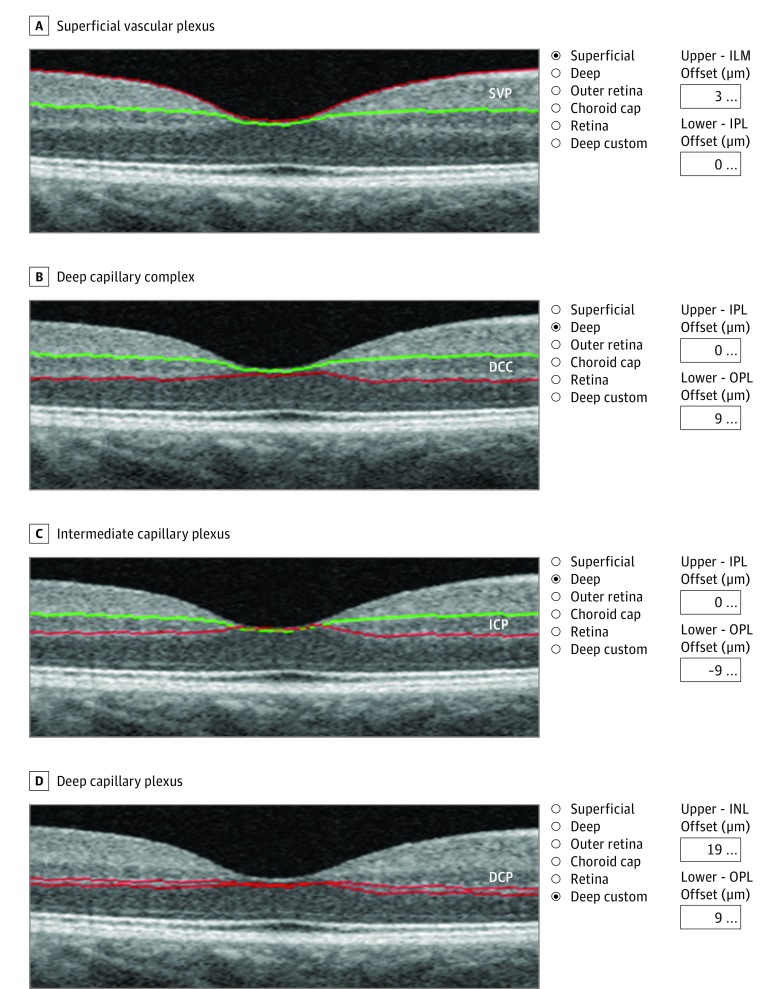
The fundus was imaged using an ultrawide field imaging system (Optos PLC) and OCTA (RTVue XR Avanti; Optovue). The OCTA findings from a single visit were analyzed. A 3 × 3-mm macular cube centered on the fovea, composed of 320 horizontal B-scans separated by 9 μm and containing 320 A-scans, was acquired. Images were analyzed using the AngioVue OCTA system available on the RTVue XR Avanti device. Automated vessel density was calculated using the AngioAnalytic software, beta version 2016 200 037, including the projection artifact removal. We used the terminology proposed by Campbell et al18 to name the different retinal vascular plexuses. The superficial vascular plexus (SVP), the deep capillary complex (DCC), and the DCP were automatically segmented using the default settings of the software, but the intermediate capillary plexus (ICP) was manually segmented. The location of the segmentation lines that determine each plexus are detailed in Figure 1. The accuracy of the automatic segmentation was verified visually by scrolling the 320 B-scans. Errors were found in 4 of the 22 eyes (18%), and manual correction was required.
Figure 1. Locations of the Segmentation Lines Used to Determine Each Plexus.
The superficial vascular plexus (SVP) (A) and the deep capillary complex (DCC) (B) are automatically segmented. C, The intermediate capillary plexus (ICP) slab is obtained from the DCC by moving the outer boundary (red line) 9 µm above the outer plexiform layer–outer nuclear layer (OPL-ONL) junction. D, The deep capillary plexus (DCP) inner boundary is set 19 µm below the inner nuclear layer–outer plexiform layer (OPL) junction and the outer boundary is set 9 µm below the OPL–outer nuclear layer junction. ILM indicates internal limiting membrane; INL, inner nuclear layer; and IPL, inner plexiform layer.
Macular vessel density corresponds to the percentage of surface occupied by vessels and capillaries in an area defined by a 3 × 3-mm square centered on the fovea (whole en face image). The vessel density was measured at 4 different levels: SVP, DCC, ICP, and DCP. The FAZ area was measured using the nonflow function on the OCTA software and manually corrected in case of segmentation errors. Projection artifact removal software allows measurement of the FAZ, which is delimited by an anastomotic perifoveal ring identical in the SVP and the DCC. The central 1-mm subfield thickness was also studied using the AngioVue software as well as the inner retinal thickness (internal limiting membrane–inner plexiform layer distance) and outer retinal thickness (inner plexiform layer–retinal pigment epithelium distance).
Statistical Analysis
Continuous variables were plotted using boxplots and compared 2 × 2 using the parametric unpaired, 2-tailed t test in case of normal distribution and otherwise using the nonparametric Wilcoxon rank sum test. For comparing means of more than 2 groups, a one-way analysis of variance was used. Statistical analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
The records of 42 patients with type 1 diabetes were reviewed. After exclusion of ineligible participants, 22 eyes of 22 patients were included in the final analysis. Of the 22 participants, 11 (50%) were men, and the mean (SD) age was 30 (6) years (range, 21-40 years). All patients had sustained poor glycemic control, and the mean (SD) hemoglobin A1c level was 8.9% (1.6%). Of the 22 eyes, 13 (59%) had a normal VA (logMAR, 0; Snellen equivalent, 20/20), while 9 (41%) had decreased VA (median: logMAR, 0.12; Snellen equivalent, 20/25 [range: logMAR, 0.1 to 0.2; Snellen equivalent, 20/25 to 20/32]). In the control group, 24 eyes of 12 healthy patients with a median age of 31 years (range, 23-40 years) and VA (logMAR, 0; Snellen equivalent, 20/20) were analyzed with the use of OCTA. Since both eyes from the same patient represent paired data, only the eye with the higher signal strength index was included (12 eyes). The 3 comparison groups (control group, patients with diabetes and normal VA, and patients with diabetes and decreased VA) were comparable demographically. eTable 1 in the Supplement summarizes the demographic and ocular findings. The Table shows the vessel density distribution in the different retinal plexuses (SVP, DCC, ICP, and DCP) as well as the FAZ area in patients with diabetes compared with controls.
Table. FAZ Area and VD Distributions in Patients With DR Compared With the Control Group.
| Area and VD Distribution | Mean (SD) | P Value | |||
|---|---|---|---|---|---|
| Control | DR With Normal VA | DR With Decreased VA | Control vs DR | DR With Normal VA vs DR With Decreased VA | |
| FAZ area, mm2 | 0.20 (0.07) | 0.30 (0.11) | 0.45 (0.12) | .008 | .04 |
| VD in SVP, % | 49.1 (2.5) | 44.1 (2.5) | 39.6 (4.3) | <.001 | .007 |
| VD in DCC, % | 50.6 (3.3) | 44.3 (3.2) | 34.7(6.8) | <.001 | .003 |
| VD in ICP, % | 49.3 (3.3) | 43.8 (3.5) | 34.8 (6.2) | <.001 | .001 |
| VD in DCP, % | 30.5 (3.9) | 24.5 (3.0) | 15.2 (3.8) | <.001 | <.001 |
Abbreviations: DCC, deep capillary complex; DCP, deep capillary plexus; DR, diabetic retinopathy; FAZ, foveal avascular zone; ICP, intermediate capillary plexus; SVP, superficial vascular plexus; VA, visual acuity; VD, vessel density.
The mean (SE) FAZ area was enlarged in patients with DR compared with controls (0.302 [0.035] vs 0.194 [0.036] mm2; difference, 0.109 [0.050] mm2; 95% CI, 0.006-0.211 mm2; P = .04), especially in patients with diabetes and decreased VA (0.447 [0.042] mm2; difference, 0.145 [0.054] mm2; 95% CI, 0.034-0.256 mm2; P = .01) (eTable 2 and eFigure 1 in the Supplement). Mean vessel density was lower for eyes with diabetic retinopathy and normal VA compared with the control group. The mean (SE) vessel density values were 44.1% (0.9%) vs 49.1% (0.9%) (difference [SE], −5.0% [1.3%]; 95% CI, −7.5% to −2.4%; P < .001) in the SVP, 44.3% (1.2%) vs 50.6% (1.3%) (difference [SE], −6.3% [1.8]; 95% CI, −9.9% to −2.7%; P < .001) in the DCC; 43.8% (1.2%) vs 49.3% (1.2%) (difference [SE], −5.5% [1.7%]; 95% CI, −9.0% to −2.0%; P = .003) in the ICP, and 24.5% (1.0%) vs 30.5% (1.0%) (difference [SE], −6.1% [1.4%]; 95% CI, −8.9% to −3.2%; P < .001) in the DCP (Figure 2 and eFigure 2 and eTable 3 in the Supplement). This decrease in vessel density was greater in the eyes of patients with diabetes and decreased VA than in the eyes of patients with diabetes and normal VA; the mean (SE) capillary loss was more pronounced in the DCC (34.6% [1.5%] vs 44.3% [1.2%]; difference, −9.6% [1.9%]; 95% CI, −13.6% to −5.7%; P < .001), especially in the DCP (15.2% [1.2%] vs 24.5% [1.0%]; difference, −9.3% [1.5%]; 95% CI, −12.4% to −6.1%; P < .001), than in the SVP (39.6% [1.1%] vs 44.1% [0.9%]; difference, −4.5% [1.4%]; 95% CI, −7.3% to −1.7%; P = .002) (eTable 3 and eFigure 2 in the Supplement). In addition, the mean (SE) reduction in vessel density among diabetic patients with normal and decreased VA was similar in the DCP (−9.3% [1.5%]), the ICP (−9.0% [1.95%]), and the DCC (−9.6% [1.95%]) (eTable 3 in the Supplement). However, baseline mean (SE) values in the DCP were much lower than other values in the ICP (15.2% [1.25%]; 95% CI, 12.8%-17.6% vs 34.8% [1.45%]; 95% CI, 31.9%-37.7%), suggesting that the DCP was more affected by the capillary dropout.
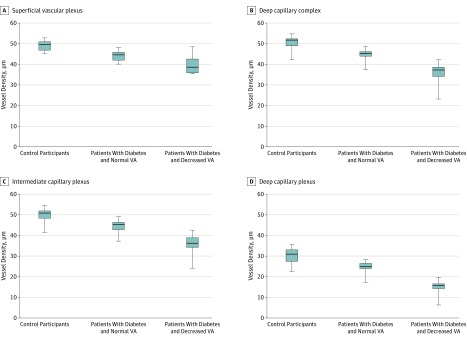
Figure 2. Mean Vessel Density (VD) in the Vascular Retinal Plexuses Among Patients With Diabetes Compared With Healthy Controls.
In the superficial vascular plexus (A), deep capillary complex (B), intermediate capillary plexus (C), and deep capillary plexus (D), the VD is decreased in all eyes with diabetic retinopathy compared with controls. The VD is significantly lower in eyes with diabetic retinopathy and decreased visual acuity (VA) than in eyes with diabetic retinopathy and normal VA.
Although there was a significant FAZ area enlargement in both the normal and decreased VA groups of patients with diabetes, the CIs largely overlapped. This measurement was not as important as vessel density in explaining VA (eFigure 1 and eTable 2 in the Supplement).
In all retinal plexuses, a decrease in vessel density was compatible with preserved vision. Indeed, 13 eyes (59%) had a normal VA, even though they had a significant decrease in vessel density (mean [SE] differences: SVP, −5.0% [1.3%]; ICP, −5.5% [1.7%]; DCP, −6.1% [1.4%]; and DCC, −6.3% [1.8%] compared with the control group) (eFigure 2 in the Supplement).
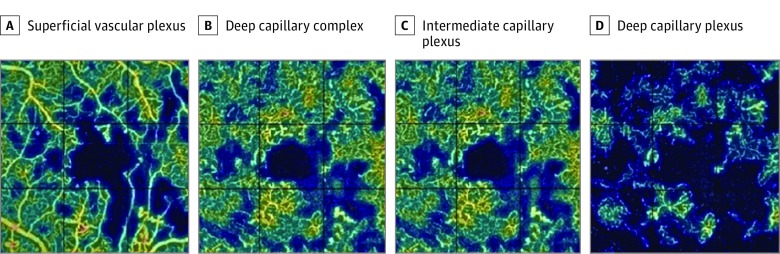
Examples of vessel density maps are shown in Figure 3; corresponding angiograms in the various plexuses of patients in the control group and patients with diabetes who had normal or decreased VA are shown in eFigure 3 in the Supplement.
Figure 3. Vessel Densities in Eyes of Patients With Diabetes and Decreased Visual Acuity.
A vessel rarefaction is seen in all plexuses (A-D), but the deep capillary plexus (D) is strongly damaged because capillary nonperfusion areas are detected far outside the foveal avascular zone.
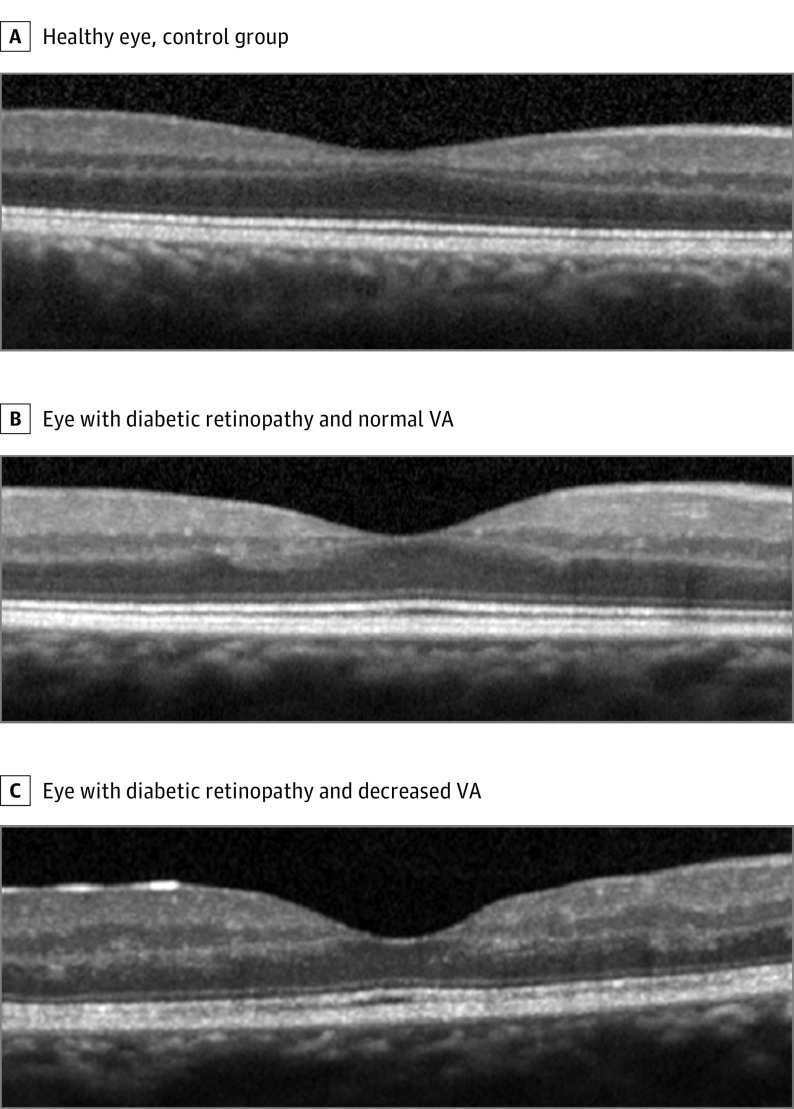
The qualitative analysis performed on the B-scans revealed that all eyes with diabetic retinopathy showed abnormalities in the inner retina compared with the control group. The signal strength index was identical in the control group and in patients with diabetes and normal VA (mean [SD], 77.8 [6.5] vs 77.3 [4.3]; P = .81), but it was lower in patients with decreased VA (77.8 [6.5] vs 67.8 [7.2]; P < .001). An irregularity of the ganglion cell layer–inner plexiform layer complex and inner nuclear layer with consecutive irregular edges of the inner plexiform layer and outer plexiform layer (Figure 4) was observed. The limits between the ganglion cell layer–inner plexiform layer complex and the inner nuclear layer could still be identified, and no disorganization of retinal inner layers was observed. There was no visible damage in the outer retina; in particular, no ellipsoid zone disruption was observed. No significant difference in the mean central subfield thickness, inner retinal thickness, and outer retinal thickness was found among the control group and the patients with diabetes who had normal VA or decreased VA (mean central subfield thickness for the control group, 254 μm [range, 224-268 μm]; normal VA, 269 μm [range, 214-312 μm]; and decreased VA, 263 μm [range, 231-289 μm]; P = .55; mean internal limiting membrane–inner plexiform layer thickness for the control group, 110 μm [range, 100-123 μm]; normal VA, 112 μm [range, 79-132 μm]; and decreased VA, 119 μm [range, 98-153 μm]; P = .57) (eTable 1 in the Supplement).
Figure 4. Optical Coherence Tomography in the Eyes of the Control Group and Patients With Diabetes.
Normal visual acuity (VA) represents 0 logMAR (Snellen equivalent, 20/20); decreased VA for the eye depicted represents 0.3 logMAR (Snellen equivalent, 20/40). Irregularities of the inner retinal layers are visible in all eyes with diabetic retinopathy; the stage is more advanced in the patient with the worst VA.
Discussion
Several studies using OCTA have shown that the macular capillary density is impaired regardless of the severity of DR and that capillary nonperfusion tends to increase with DR severity.19,20 The vessel density may even be decreased before detecting any clinical sign of DR, and the DCC seems to be more affected than the SVP.10 On the other hand, vessel density is severely decreased in the case of cystoid macular edema.10,12 However, the association between the macular vascularity and visual function is not completely understood.
Our study focused on a specific group of patients with type 1 diabetes who were younger than 40 years and who had severe rapidly progressive DR requiring PRP. This group of patients with normal vision or recent moderate vision loss, clear lens, and no previous macular edema form an interesting population for studying the correlation between macular capillary perfusion and VA. Our results show that VA was mainly conditioned by vessel density in the DCC.
Our results corroborate the weak correlation generally found between the FAZ surface as studied by OCTA and VA.11,20,21 Moreover, the way the projection artifact removal software allowed the measuring of the FAZ area on the entire retinal thickness is new; the SVP and the DCC are connected through a single anastomotic vascular ring around the fovea, and there is no reason to distinguish between the 2 plexuses, as was done in previous studies.18 The interpretation of the FAZ area has some limitations; delimiting the FAZ may be difficult when large capillary dropout areas extend to the vascular arcades, and the FAZ surface is highly variable among healthy individuals.2 Alternately, the parafoveal retinal tissue may function normally without a direct retinal blood supply.1 Sim et al22 found a link between macular ischemia and visual function in only 15% of patients with moderate to severe macular ischemia, especially in patients with papillomacular ischemia on fluorescein angiography. In earlier DR stages, the same authors found a great variability in VA levels that prevented the finding of any relevant correlation with the capillary dropout area.22
The association between vessel density and VA has also been studied by Samara et al,20 who found a modest correlation between VA and vessel density both in the SCP and the DCP (r, −0.5). However, the DCP they analyzed corresponded to what we now call the DCC, and it was affected by the partial projection of the superficial vessels. Furthermore, they were not able to analyze separately the vessel density in the inner capillary layers (ICP) and the outer capillary layers (DCP) of the DCC. Compared with other recent studies,20,23 we thus used a more restrictive segmentation of the DCP, similar to the segmentation proposed by Park et al,24 which gave density values in normal eyes close to those of Campbell et al.18 When we analyzed the DCC as a whole and the ICP and DCP separately, we found that VA depended mainly on vessel density in the DCP rather than in the SVP. In addition, the DCP appeared to be the most affected because no overlap was observed with vessel density values of the DCP in the control group. Conversely, ICP values among controls and patients with diabetes with decreased VA overlapped slightly. Some patients with decreased VA had decreased vessel density in the DCP despite close-to-normal vessel density values in the ICP, whereas the contrary was not observed, suggesting that the DCP vessel density was more important than the ICP vessel density in explaining decreased VA.
The reason why decreased vision appears to be primarily related to a loss of capillary perfusion in the DCC is unclear. Usui et al25 assumed that amacrine and horizontal cells form neurovascular units with capillaries in the ICP and DCP and are highly interdependent. They showed that losing 1 or both of them triggers profound effects on photoreceptor survival and function.
These data suggest that there might be a threshold of macular nonperfusion, especially in the DCP, beyond which normal vision cannot be maintained. Although the decrease in vessel density is usually observed both in the SVP and DCP, such a decrease in the DCP alone could be sufficient to induce visual loss. Alternately, a moderate loss of capillary perfusion in any retinal vascular plexus is compatible with normal vision, and it is likely that decrease in vessel density precedes a decrease in VA.
The fact that the deepest retinal capillaries are more affected by a progressive obstruction has been well described in histological studies.26 However, whether it is because of hemodynamic conditions or a more complex dysfunction of the neurogliovascular coupling remains unknown. Studies using OCTA have increasingly shown that the DCP is the termination of the retinal capillary units in which the blood comes from the superficial capillary layers and drains into deep venules via the DCP.18,27,28 A slowing in retinal blood flow could preferentially affect DCP perfusion.
The global decrease in vessel density could be secondary to a neuroglial loss resulting in impaired interaction between neurons, glial cells, and vascular cells (ie, an impaired neurovascular coupling).29 Indeed, early changes in retinal function have been reported in patients with diabetes before the detection of retinal vascular lesions, suggesting that some neurodegenerative events could precede vascular changes.30,31 Because neural activity significantly correlates with local blood flow,32,33 alterations of the neuroglial tissue in the inner retina could lead to a secondary decrease in capillary flow density with subsequent visual loss.34 Mechanisms other than macular hypoperfusion play a role in visual loss, and a decrease in VA is a late event in the history of visual function impairment. In patients with diabetes and no or mild DR, early changes, such as decreased contrast sensitivity, electroretinographic abnormalities, and impaired color vision, were observed despite a normal VA.35
A significant loss of DCP perfusion could have detrimental effects on the middle retina and even on the photoreceptors. The DCP could partially contribute to the oxygen supply to the photoreceptors, unlike what was assumed earlier.36 Although we did not find any structural change in the photoreceptors in retinal capillary dropout areas, we cannot exclude the possibility that functional damage impaired the VA.18,24,37 Histological studies reported a homogeneous eosinophilic substance accumulated between the photoreceptor outer segments and the retinal pigment epithelium corresponding to the areas with inner retinal capillary dropout.26 These deposits could alter the functioning of photoreceptors and participate in visual loss. Other studies have shown pathologic changes in the cone mosaic on adaptive optics, and the extent of photoreceptor loss could positively correlate with DR severity.38,39
Limitations
Our study has some limitations. It was a retrospective study focused on a specific group of young patients with type 1 diabetes without macular edema but with severe rapidly progressive DR in whom PRP was needed. Furthermore, it included a small sample of patients. We also acknowledge that while OCTA technology is constantly improving and allowed us to study the 3 vascular plexuses with high accuracy, some artifacts and segmentation errors were still observed and corrected, implying that one must remain cautious when interpreting vessel density. We also cannot exclude the possibility that some capillaries with slow but persistent flow were not detected. No significant difference was found in the inner retinal thickness (internal limiting membrane–inner plexiform layer) between patients with diabetes and controls. However, the ganglion cell layer–inner plexiform layer thickness was not analyzed because it was not available on the current software, and the analysis of the ganglion cell layer-inner plexiform layer thickness could be more sensitive to detect anomalies in the inner retinal layers. Finally, the decrease in vessel density in the macula could be a result of the combined effects of PRP and the use of anti-VEGF therapy. However, the number of anti-VEGF injections was not different between the 2 groups of patients with diabetes, and numerous studies have shown that intravitreal injections of anti-VEGF did not cause a decreased vessel density or FAZ enlargement.40,41 We believe there is a low likelihood that these limitations could have affected the meaning of the role of the vessel density decrease in the DCC and especially in the role DCP plays in loss of VA.
Conclusions
Although only a few eyes at a single tertiary referral center were evaluated retrospectively, these data suggest that, for patients with type 1 diabetes who have severe DR without macular edema, the decrease in VA is mainly associated with a decrease in vessel density in the DCC, particularly the DCP, more so than a decrease in vessel density in the SVP or FAZ area enlargement.
eTable 1. Baseline Characteristics of Patients
eTable 2. ANOVA Model for Foveal Avascular Zone (FAZ) Area in the Control and Diabetes Groups
eTable 3. ANOVA Model for Vessel Density (VD) in SVP, ICP, DCP, and DCC in the Control and Diabetes Groups
eFigure 1. Adjusted Difference Means for Foveal Avascular Zone (FAZ) Area
eFigure 2. Adjusted Difference Means for Foveal Avascular Zone (FAZ) Area and for Vessel Density (VD) in SVP, DCC, ICP, and DCP
eFigure 3. Angiograms of Plexuses in the Eyes of the Control and Diabetes Groups With Normal or Decreased Visual Acuity (VA)
References
- 1.Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102(9):1286-1293. [DOI] [PubMed] [Google Scholar]
- 2.Arend O, Wolf S, Harris A, Reim M. The relationship of macular microcirculation to visual acuity in diabetic patients. Arch Ophthalmol. 1995;113(5):610-614. [DOI] [PubMed] [Google Scholar]
- 3.Sim DA, Keane PA, Zarranz-Ventura J, et al. Predictive factors for the progression of diabetic macular ischemia. Am J Ophthalmol. 2013;156(4):684-692. [DOI] [PubMed] [Google Scholar]
- 4.Hwang TS, Zhang M, Bhavsar K, et al. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134(12):1411-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1):35-44.e1. [DOI] [PubMed] [Google Scholar]
- 6.Matsunaga DR, Yi JJ, De Koo LO, Ameri H, Puliafito CA, Kashani AH. Optical coherence tomography angiography of diabetic retinopathy in human subjects. Ophthalmic Surg Lasers Imaging Retina. 2015;46(8):796-805. [DOI] [PubMed] [Google Scholar]
- 7.Takase N, Nozaki M, Kato A, Ozeki H, Yoshida M, Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377-2383. [DOI] [PubMed] [Google Scholar]
- 8.Kim AY, Rodger DC, Shahidzadeh A, et al. Quantifying retinal microvascular changes in uveitis using spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;171:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang TS, Gao SS, Liu L, et al. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016;134(4):367-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carnevali A, Sacconi R, Corbelli E, et al. Optical coherence tomography angiography analysis of retinal vascular plexuses and choriocapillaris in patients with type 1 diabetes without diabetic retinopathy. Acta Diabetol. 2017;54(7):695-702. [DOI] [PubMed] [Google Scholar]
- 11.Balaratnasingam C, Inoue M, Ahn S, et al. Visual acuity is correlated with the area of the foveal avascular zone in diabetic retinopathy and retinal vein occlusion. Ophthalmology. 2016;123(11):2352-2367. [DOI] [PubMed] [Google Scholar]
- 12.Mané V, Dupas B, Gaudric A, et al. Correlation between cystoid spaces in chronic diabetic macular edema and capillary nonperfusion detected by optical coherence tomography angiography. Retina. 2016;36(suppl 1):S102-S110. [DOI] [PubMed] [Google Scholar]
- 13.Gill A, Cole ED, Novais EA, et al. Visualization of changes in the foveal avascular zone in both observed and treated diabetic macular edema using optical coherence tomography angiography. Int J Retina Vitreous. 2017;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohner EM, Hamilton AM, Joplin GF, Fraser TR. Florid diabetic retinopathy and its response to treatment by photocoagulation or pituitary ablation. Diabetes. 1976;25(2):104-110. [DOI] [PubMed] [Google Scholar]
- 15.Lattanzio R, Brancato R, Bandello FM, Azzolini C, Malegori A, Maestranzi G. Florid diabetic retinopathy (FDR): a long-term follow-up study. Graefes Arch Clin Exp Ophthalmol. 2001;239(3):182-187. [DOI] [PubMed] [Google Scholar]
- 16.Beaumont P, Hollows FC. Classification of diabetic retinopathy, with therapeutic implications. Lancet. 1972;1(7747):419-425. [DOI] [PubMed] [Google Scholar]
- 17.Wolf-Schnurrbusch UE, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50(7):3432-3437. [DOI] [PubMed] [Google Scholar]
- 18.Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017;7:42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agemy SA, Scripsema NK, Shah CM, et al. Retinal vascular perfusion density mapping using optical coherence tomography angiography in normals and diabetic retinopathy patients. Retina. 2015;35(11):2353-2363. [DOI] [PubMed] [Google Scholar]
- 20.Samara WA, Shahlaee A, Adam MK, et al. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124(2):235-244. [DOI] [PubMed] [Google Scholar]
- 21.Casselholmde Salles M, Kvanta A, Amrén U, Epstein D. Optical coherence tomography angiography in central retinal vein occlusion: correlation between the foveal avascular zone and visual acuity. Invest Ophthalmol Vis Sci. 2016;57(9):OCT242-OCT246. [DOI] [PubMed] [Google Scholar]
- 22.Sim DA, Keane PA, Zarranz-Ventura J, et al. The effects of macular ischemia on visual acuity in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(3):2353-2360. [DOI] [PubMed] [Google Scholar]
- 23.Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(12):5548-5555. [DOI] [PubMed] [Google Scholar]
- 24.Park JJ, Soetikno BT, Fawzi AA. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina. 2016;36(11):2039-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usui Y, Westenskow PD, Kurihara T, et al. Neurovascular crosstalk between interneurons and capillaries is required for vision. J Clin Invest. 2015;125(6):2335-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bek T. Transretinal histopathological changes in capillary-free areas of diabetic retinopathy. Acta Ophthalmol (Copenh). 1994;72(4):409-415. [DOI] [PubMed] [Google Scholar]
- 27.Bonnin S, Mané V, Couturier A, et al. New insight into the macular deep vascular plexus imaged by optical coherence tomography angiography. Retina. 2015;35(11):2347-2352. [DOI] [PubMed] [Google Scholar]
- 28.Garrity ST, Paques M, Gaudric A, Freund KB, Sarraf D. Considerations in the understanding of venous outflow in the retinal capillary plexus. Retina. 2017;37(10):1809-1812. [DOI] [PubMed] [Google Scholar]
- 29.Abcouwer SF, Gardner TW. Diabetic retinopathy: loss of neuroretinal adaptation to the diabetic metabolic environment. Ann N Y Acad Sci. 2014;1311:174-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(suppl 2):S253-S262. [DOI] [PubMed] [Google Scholar]
- 31.Gaucher D, Chiappore JA, Pâques M, et al. Microglial changes occur without neural cell death in diabetic retinopathy. Vision Res. 2007;47(5):612-623. [DOI] [PubMed] [Google Scholar]
- 32.Kern TS. Interrelationships between the retinal neuroglia and vasculature in diabetes. Diabetes Metab J. 2014;38(3):163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luu CD, Szental JA, Lee SY, Lavanya R, Wong TY. Correlation between retinal oscillatory potentials and retinal vascular caliber in type 2 diabetes. Invest Ophthalmol Vis Sci. 2010;51(1):482-486. [DOI] [PubMed] [Google Scholar]
- 34.Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52(2):1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44(1):53-60. [DOI] [PubMed] [Google Scholar]
- 36.Yi J, Liu W, Chen S, et al. Visible light optical coherence tomography measures retinal oxygen metabolic response to systemic oxygenation. Light Sci Appl. 2015;4(9):e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scarinci F, Nesper PL, Fawzi AA. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016;168:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lombardo M, Parravano M, Serrao S, Ziccardi L, Giannini D, Lombardo G. Investigation of adaptive optics imaging biomarkers for detecting pathological changes of the cone mosaic in patients with type 1 diabetes mellitus. PLoS One. 2016;11(3):e0151380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soliman MK, Sadiq MA, Agarwal A, et al. High-resolution imaging of parafoveal cones in different stages of diabetic retinopathy using adaptive optics fundus camera. PLoS One. 2016;11(4):e0152788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dugel PU, Hillenkamp J, Sivaprasad S, et al. Baseline visual acuity strongly predicts visual acuity gain in patients with diabetic macular edema following anti-vascular endothelial growth factor treatment across trials. Clin Ophthalmol. 2016;10:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bong A, Doughty MJ, Button NF, Mansfield DC. On the relationship between visual acuity and central retinal (macular) thickness after interventions for macular oedema in diabetics: a review. Clin Exp Optom. 2016;99(6):491-497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline Characteristics of Patients
eTable 2. ANOVA Model for Foveal Avascular Zone (FAZ) Area in the Control and Diabetes Groups
eTable 3. ANOVA Model for Vessel Density (VD) in SVP, ICP, DCP, and DCC in the Control and Diabetes Groups
eFigure 1. Adjusted Difference Means for Foveal Avascular Zone (FAZ) Area
eFigure 2. Adjusted Difference Means for Foveal Avascular Zone (FAZ) Area and for Vessel Density (VD) in SVP, DCC, ICP, and DCP
eFigure 3. Angiograms of Plexuses in the Eyes of the Control and Diabetes Groups With Normal or Decreased Visual Acuity (VA)