Abstract
The complement system is a dynamic subset of the innate immune system, playing roles in host defense, clearance of immune complexes and cell debris, and priming the adaptive immune response. Over the last 40 years our understanding of the complement system has evolved from identifying its presence and recognizing its role in the blood to now focusing on understanding the role of local complement synthesis in health and disease. In particular, the local synthesis of complement was found to have an involvement in mediating ischaemic injury, including following transplantation. Recent work on elucidating the triggers of local complement synthesis and activation in renal tissue have led to the finding that Collectin-11 (CL-11) engages with L-fucose at the site of ischaemic stress, namely at the surface of the proximal tubular epithelial cells. What remains unknown is the precise structure of the damage-associated ligand that participates in CL-11 binding and subsequent complement activation. In this article, we will discuss our hypothesis regarding the role of CL-11 as an integral tissue-based pattern recognition molecule which we postulate has a significant contributory role in complement-mediated ischaemic injury.
Keywords: collectin-11, lectin pathway, complement system, innate immunity, renal ischaemia, renal transplantation
Introduction
We have seen over many years that innate immune defense systems mounted at epithelial surfaces perform multiple and often non-immune roles. The toll-like receptor system is a classic example that has transformed our perception about the origins of innate immunity and its roles in insect development (1, 2), antimicrobial defense (3) and in the pathogenesis of some inflammatory conditions (4–6). The complement system Figure 1 has been known about for longer (7), but the diversity of function and, in particular, its role at the interface between innate and adaptive immunity can now be re-visited as a typical model for innate immune function as well as a therapeutic target in a growing number of medical disorders.
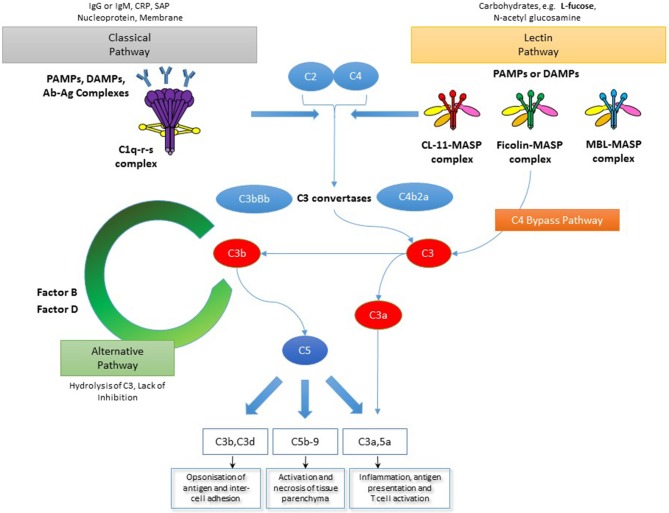
Figure 1.
The complement system. The complement system is activated through three main pathways: classical, lectin and alternative. The classical and lectin pathways are activated by pattern recognition molecules binding to pathogen cell surfaces as well as infected and/or damaged cells. In the case of the classical pathway this manifests as C1q binding, most commonly via immunoglobulin. However, C1q can also bind other immune surveillance molecules or directly to disrupted structures via pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). Meanwhile, the lectin pathway is initiated by binding of collectins, such as MBL and CL-11, as well as ficolins, to PAMPs or DAMPs expressing carbohydrate ligands. Also shown in the diagram is a C4-bypass mechanism in which MASP-2 in association with lectin molecules directly cleaves C3. The alternative pathway is activated by C3b binding to cell surfaces and acts as an amplification process for the central complement component, C3, upon which both the lectin and classical pathways converge upon. Recently, MASP-1/3 was also shown to trigger the alternative pathway as well. Through a number of complement convertases the effectors of the complement system are generated. These are the anaphylatoxins, C3a and C5a, the membrane attack complex (MAC, C5b-9) as well as C3b and its metabolite C3d which mediate antigen opsonisation and cell-cell adhesion (NB This is a generalized overview of the complement system as it specifically relates to the focus of this manuscript and is not meant to be a comprehensive depiction of all parts of the complement system).
Early beginnings—local complement synthesis
For us, the story begins with early reports detailing the significance of local complement synthesis. Colten et al. authored a number of publications that highlighted the capacity of macrophages for producing a wide range of complement components (8, 9). Well cited papers on skin cells, neural cells, gut cells, cardiac cells, and others came into vision, showing that the principle of local synthesis was almost universal in resident parenchymal cells and migratory leukocytes (10–17). Our interest, as a nephrology group starting up in the late 1980s, was caught by kidney expression of a number of complement genes in the context of inflammatory renal disease (18, 19).
It became apparent that among the variety of intrarenal cells studied, the renal tubule cells were a prolific source of complement components. The renal tubule supports vital functions in maintaining health and blocking invasive pathogens such as uropathogenic Escherichia coli. The capacity of renal tubule cells for complement synthesis was first characterized through the work of Daha and others (20–22) in Leiden and further confirmed through histological examination of both healthy and diseased tissue by several research groups (23–25). The link between inflammatory conditions primarily affecting the renal tubules and a high degree of intrarenal complement expression was striking. Notably, these diseases included ischaemic injury of the newly transplanted kidney and transplant rejection (26–28).
At the time, the techniques of tissue- or cell-specific gene deletion were not well enough established to allow interrogation of local complement gene function, in the way that would be pursued now. However, we found that we could harness skills in mouse kidney transplantation to swap kidneys between wild-type and gene-deleted mice, in both directions, to illuminate the relative importance of local complement synthesis (29–31). Our initial focus was on C3, the central and most abundant component of the complement cascade in blood. We had already shown, in human kidney transplantation, that C3 has a significant contribution to the systemic complement pool through intrarenal synthesis (32).
Complement and the transplanted kidney
The summation of the research including knockout mouse transplant studies and clinical observational studies has demonstrated a number of important principles. Namely, it showed that local synthesis of C3 had a disproportionate influence on ischaemia-reperfusion injury relative to the systemic pool (30), and an impact was also observed in the process of cell mediated rejection of MHC mismatched kidney transplants (31). It was evident from the analyses that the renal tubule cell was the primary target for complement deposition in these conditions, and the tubule cell was also the primary site of C3 expression (30).
The evidence additionally highlighted the role of donor antigen-presenting cells (APCs) as a source of complement (33). These cells, also known as donor passenger cells, reside in the interstitial space of the donor kidney, specifically around the renal tubules. Within the first 24 h after transplantation, these cells migrate into the recipient lymphoid system where they immunize the recipient against donor MHC antigen (34). Local production of complement was shown to modify the capacity of the APC to prime the antigen-specific T cells that mediate rejection (31, 35–37).
Considerable work has gone into identifying the complement effectors generated downstream of C3 that mobilize the inflammatory and adaptive immune functions against the transplant [reviewed in (38)]. These investigations have resulted in a deeper understanding both of the roles of the anaphylatoxins, C3a and C5a, (39) and the membrane attack complex (C5b-9) on immune cells and parenchymal tissue (40).
In addition to expressing core complement components and activating enzyme-precursors, tissue-resident and migratory cells also display receptors that detect a range of biologically active complement products formed downstream of C3 cleavage (41–43). This emphasizes the ability for cross talk between tissue-resident and migratory cells within the transplant setting. It is helpful to think of the different cells that produce and detect complement as nodes in a local network, whose functions bring together and amplify the innate immune response and regulate adaptive immunity.
Local tissue defense
Presumably, the local synthesis of complement components serves to enhance the defense against invasive organisms. For example, the synthesis of C3 by renal tract epithelium potentially increases the efficiency with which locally invasive organisms are opsonised and subsequently eliminated at the point of entry. There is strong evidence for such a role, as the renal tubular epithelium constitutively expresses complement, and the production is rapidly upregulated in the presence of infection (44, 45). Further testament to this mechanism is that many common urinary pathogens have developed resistance to complement, including clinically relevant strains of gram negative pathogens (46–48). Not only have these strains been found to resist complement mediated lysis but they can also utilize complement to invade complement receptor expressing tubular epithelial cells (44, 49, 50). The C3b receptor, CD46, is one such receptor used by uropathogenic E. coli to evade extracellular defenses (45, 51) and is an illustration of the means by which complement resistant strains can gain an advantage against the host. Thus, the local pool of complement can be both a protector against infection and a source of tissue injury.
Emergence of collectin-11
Whereas the last 20-years of research has taught us much about the effector functions of locally derived complement, our knowledge of the trigger mechanisms that localize tissue injury to a particular tissue compartment has lagged behind. This may be because the changes that induce complement activation are different for each organ and as such the studies in different organs have produced mixed and sometimes contradictory results [for more information refer to (52)]. Alternatively, it could be that the focus on circulating complement has not led us to the local mechanisms that drive complement-mediated disease. It is a common observation that measurement of circulating complement does not closely correlate with biopsy evidence of complement activation within an affected organ, and this may have delayed our understanding of local disease mechanisms. If only we understood more about the structures that triggered complement activation and how they are recognized in an organ such as the kidney, we would know more about how to detect and regulate harmful signals for health benefit.
We recently reviewed the evidence for the different pattern recognition molecules that could trigger complement activation in renal ischaemia-reperfusion injury and transplantation (52, 53). There, we considered whether the classical or lectin pathways could mediate the onset of ischaemia-reperfusion injury and found no conclusive evidence of a role for the classical pathway in the genesis of the renal injury within a murine model (54, 55). The lectin pathway also, at first, seemed not to have a key role in the induction of ischaemic renal injury, since the injury—at least in mice–was independent of C4, which is a component shared by both the classical and lectin pathways (56). However, we now believe that more recent findings on CL-11 and the coupled enzyme MBL associated serine protease-2 (MASP-2) reconcile these observations, both in the context of renal ischaemic epithelial cell damage and very possibly in retinal epithelial ischaemic damage (57–59).
CL-11 is a recently described member of the lectin family of pattern recognition molecules, with known antimicrobial functions and ability to trigger complement activation via the lectin pathway (60). Reported in 2006, CL-11 was at first named kidney collectin, or CL-K1, for its abundant expression in normal renal tissue (61, 62). The renaming of CL-11 is appropriate, since it is now known that the molecule is widely expressed (60). The most obvious expression site in the kidney is the renal tubule, for this structure encompasses the largest volume in the kidney, though CL-11 is also present in the glomerular mesangium and epithelium. Despite its strong presence in the kidney, the mean concentration of CL-11 in serum is just 284 ng/mL, by ELISA measurement. Furthermore, CL-11 is known to form heteromeric complexes with Collectin Liver 1 (CL-10) in the serum. Interestingly, this heterocomplex, CL-LK, has been shown to activate complement in vitro (63). However, we hypothesize that it is the local production of complement that accounts for tissue injury. Indeed, CL-11 has been shown to be produced by renal epithelial cells, whereas CL-10 has not been definitively shown to be expressed in the kidney (64) thereby making it less likely that the CL-LK heterocomplex is participating in renal injury.
CL-11 monomers have a similar structure to other C-type lectins such as MBL and consist of a globular head followed by a neck and a collagenous tail. The head contains a carbohydrate recognition domain (CRD), and the tail contains binding sites for MASPs which are required for complement activation. The CL-11 monomers form a triplet structure that self-combines to form oligomers with higher avidity of binding to ligand (65). Since hypoxia- or hypothermia-treated epithelial cells appear to bind CL-11 avidly (57–59), it is proposed that a change in presentation of the stress-induced cellular ligand for CL-11 underpins strong attachment of the oligomeric CL-11 complex. Whether this stress-induced pattern could involve a change in orientation or distribution of ligand, or increased expression or alteration of biochemical structure, is currently under investigation. However, clues to potential binding motifs or patterns can be gleaned from understanding the binding properties of other lectins. Mannose-Binding Lectin (MBL) is a well characterized C-type lectin similar to CL-11 in that it shares a similar structure with a CRD and collagenous tail, and binds oligosaccharide ligands in a calcium-dependent manner. In particular, MBL has a higher affinity for ligands that contain mannose or N-Acetyl-D-glucosamine residues (66). More recently, information has been gathered which further characterizes the MBL-oligosaccharide interaction, specifically in relation to MBL binding of lipopolysaccharide (67). Thus, our understanding of potential CL-11 ligands could be narrowed by considering and applying our knowledge of the glycan motifs that MBL recognizes.
Many molecules are normally glycosylated to some extent. In particular, fucosylated molecules are widely synthesized in normal tissues (68), and what is interesting to us is that L-fucose, the preferred monosaccharide recognized by CL-11, is also abundant in the proximal renal tubule (57). These are the very cells that express complement components in abundance. Therefore, the core components of the complement system including C3, C5 (23) and a lectin pathway trigger (CL-11) are expressed within the same hypoxia-sensitive segment of the renal tubule, where a potential binding ligand for CL-11 is also present. In vivo and ex vivo studies of epithelial cell injury suggest that hypoxia- or hypothermia-induced binding of CL-11 is followed by complement activation on the injured cell surface at sites that are specifically marked by CL-11. In a murine model of hypoxia-induced renal tubule cell stress, complement deposition was prevented by CL-11 deletion or by L-fucose blockade of the carbohydrate recognition domain of CL-11 (57). The protective effect of L-fucose blockade could also be demonstrated in wild type mice undergoing renal ischaemic insult (unpublished data). A similar injury mechanism also appears to occur in retinal pigmented epithelial cells, where hypoxia-induced membrane attack complex formation and CL-11 deposition correlate with sites of L-fucose expression (59). Thus, these findings may have potentially broad implications for diseases where complement mediated injury is thought to play a significant role.
Closing the Gap
David and colleagues originally described the ability of renal tubule cells to spontaneously activate complement in the presence of normal human serum, and they attributed this to activation of the alternative complement pathway (69, 70). However, subsequent understanding of the lectin pathway and, in particular, the role of CL-11 now suggests another explanation (57). It points to a role for pattern recognition by CL-11 in contact with a damage-associated ligand on hypoxia-activated cells, and in turn the subsequent activation of the complement cascade by lectin-pathway associated serine proteases, i.e. MASPs. Although the alternative pathway may still play a role in hypoxia-mediated renal injury, the emerging data suggests that CL-11 is indispensable for triggering complement activation. Secondary activation of the alternative pathway could then occur either after the formation of C3b (which is an acceptor for factor B of the alternative pathway), or by MASP-1/3-mediated cleavage of complement factor D, which in turn cleaves factor B (60, 71). In the current model, CL-11 is expressed at physiological levels in the kidney and only substantially increases in binding to the tubule following cell stress. Complement activation then occurs at the sites where CL-11-MASP complexes form.
MASP-2 and its relationship with CL-11 are thought to play an essential role in this model. MASP-2 is one of the three MASPs that are physically linked with complement-activating lectins, including CL-11. MASP-2 differs from MASP-1 and MASP-3, in that only MASP-2 can directly cleave C3 in human and murine sera (64, 72). It is only recently that studies in gene-deleted mice have not only confirmed the importance of MASP-2 in mediating renal, cardiac and intestinal ischaemic injuries, but have shown that the injury in each case was C4-independent (30, 58, 73). The evidence supports a pathway of injury in which stress-induced ligand presentation leads to CL-11 binding and subsequent MASP-2-mediated triggering of complement activation using a route that involves the direct cleavage of C3 by MASP-2 (60, 71, 74). As indicated above, MASP-1/3-mediated activation of the alternative complement pathway could be a supplementary mechanism of injury triggered by CL-11.
In total, the evidence suggests that the renal epithelial cell behaves as a unit of host defense and inflammation, in which CL-11 appears to integrate the detection of cell stress signals with activation of the complement system. We envisage that CL-11 is primarily a tissue-based pattern recognition molecule that binds damage associated molecular patterns (DAMPs) Figure 2. In the presence of tissue stress from non-infectious causes, the effector actions of CL-11 appear to be misdirected by glycan ligands that are inappropriately exposed on the renal tubule cell surface. Similar cellular responses to stress such as hypoxia probably exist at other surfaces, such as the retinal epithelium, where hypoxia-induced complement activation in the presence of CL-11 is also described (59). What remains unclear is the biochemical structure of the damage-related glycan ligands (and carrier molecules) recognized by CL-11. Future studies will need to elucidate the nature of the stress signal on cells and how it is induced by hypoxia to fully validate the hypothesis, and show whether the same or similar mechanism operates at other epithelial surfaces. As the identity of these DAMPs begin to emerge, it will be important to determine whether and how those exposed on renal tissue differ from the CL-11 ligands on other tissues and microbial structures, or indeed on developing tissues targeted by CL-11. There is little doubt that investigating CL-11 will provide new tools to prove the wider functions of immune surveillance at different sites, with the potential to develop new clinical agents for detecting or blocking specific patterns.
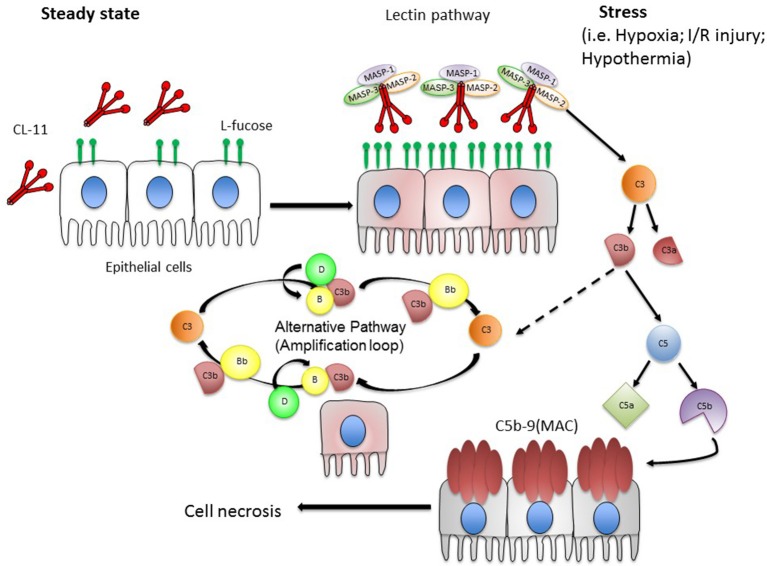
Figure 2.
Hypothesis of local complement activation triggered by CL-11 on stressed epithelial cells. We hypothesize that under steady state conditions, CL-11 is produced and released from the basolateral surface and likely the luminal surface of epithelial cells (e.g., renal tubular epithelial cells and retinal pigmented epithelial cells). Upon stress, damaged epithelial cells display an abnormal pattern of L-fucose resulting in CL-11 binding to cell surface. CL-11/MBL-associated serine protease (MASP 1, 2 & 3) complexes become activated promoting downstream complement activation. In particular, MASP-2 is a key player at the site of tissue injury cleaving C3 in a C4-independent manner. During this process, the anaphylatoxins C3a and C5a are generated and C5 cleavage initiates the terminal pathway that culminates in the formation of the membrane attack complex (MAC). C3b formed by the lectin pathway can covalently bind to target cells and initiate the alternative pathway. C3b bound to factor B (C3bB) is cleaved by factor D to form the alternative pathway C3 convertase.
Author contributions
CN and MH participated in manuscript writing, editing, and figure development. GF contributed to figure development. CF and SS contributed to manuscript editing, and writing, respectively.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Some of the work described was supported by Medical Research Council grants MR/J006742/1, MR/L020254/1, G1001141, MR/J004553/1, MR/M007871/1, and MR/L012758/1, European Research Council (ERC-2012-ADG_20120314), UK Regenerative Medicine Platform, and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas's NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1.Imler JL, Zheng L. Biology of toll receptors: lessons from insects and mammals. J Leukoc Biol. (2004) 75:18–26. 10.1189/jlb.0403160 [DOI] [PubMed] [Google Scholar]
- 2.Song X, Jin P, Qin S, Chen L, Ma F. The evolution and origin of animal Toll-like receptor signaling pathway revealed by network-level molecular evolutionary analyses. PLoS ONE (2012) 7:e51657. 10.1371/journal.pone.0051657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocks CJ, Schembri MA, Sweet MJ, Kapetanovic R. For when bacterial infections persist: Toll-like receptor-inducible direct antimicrobial pathways in macrophages. J Leukoc Biol. (2018) 103:35–51. 10.1002/JLB.4RI0917-358R [DOI] [PubMed] [Google Scholar]
- 4.Lee YK, Kang M, Choi EY. TLR/MyD88-mediated innate immunity in intestinal graft-versus-host disease. Immune Netw. (2017) 17:144–51. 10.4110/in.2017.17.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao W, Xiong Y, Li Q, Yang H. Inhibition of toll-like receptor signaling as a promising therapy for inflammatory diseases: a journey from molecular to nano therapeutics. Front Physiol. (2017) 8:508. 10.3389/fphys.2017.00508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drexler SK, Foxwell BM. The role of toll-like receptors in chronic inflammation. Int J Biochem Cell Biol. (2010) 42:506–18. 10.1016/j.biocel.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 7.Nesargikar PN, Spiller B, Chavez R. The complement system: history, pathways, cascade and inhibitors. Eur J Microbiol Immunol. (2012) 2:103–11. 10.1556/EuJMI.2.2012.2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colten HR, Ooi YM, Edelson PJ. Synthesis and secretion of complement proteins by macrophages. Ann N Y Acad Sci. (1979) 332:482–90. 10.1111/j.1749-6632.1979.tb47142.x [DOI] [PubMed] [Google Scholar]
- 9.Einstein LP, Hansen PJ, Ballow M, Davis AE III, Davis JSt, Alper CA, et al. Biosynthesis of the third component of complement (C3) in vitro by monocytes from both normal and homozygous C3-deficient humans. J Clin Invest. (1977) 60:963–9. 10.1172/JCI108876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz Y, Revel M, Strunk RC. Interleukin 6 stimulates synthesis of complement proteins factor B and C3 in human skin fibroblasts. Eur J Immunol. (1989) 19:983–8. 10.1002/eji.1830190605 [DOI] [PubMed] [Google Scholar]
- 11.Katz Y, Cole FS, Strunk RC. Synergism between gamma interferon and lipopolysaccharide for synthesis of factor B, but not C2, in human fibroblasts. J Exp Med. (1988) 167:1–14. 10.1084/jem.167.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnum SR, Jones JL, Benveniste EN. Interleukin-1 and tumor necrosis factor-mediated regulation of C3 gene expression in human astroglioma cells. Glia (1993) 7:225–36. 10.1002/glia.440070306 [DOI] [PubMed] [Google Scholar]
- 13.Halstensen TS, Mollnes TE, Brandtzaeg P. Persistent complement activation in submucosal blood vessels of active inflammatory bowel disease: immunohistochemical evidence. Gastroenterology (1989) 97:10–9. 10.1016/0016-5085(89)91409-1 [DOI] [PubMed] [Google Scholar]
- 14.Ahrenstedt O, Knutson L, Nilsson B, Nilsson-Ekdahl K, Odlind B, Hallgren R. Enhanced local production of complement components in the small intestines of patients with Crohn's disease. N Engl J Med. (1990) 322:1345–9. 10.1056/NEJM199005103221903 [DOI] [PubMed] [Google Scholar]
- 15.Witte DP, Welch TR, Beischel LS. Detection and cellular localization of human C4 gene expression in the renal tubular epithelial cells and other extrahepatic epithelial sources. Am J Pathol. (1991) 139:717–24. [PMC free article] [PubMed] [Google Scholar]
- 16.Lappin D, Hamilton AD, Morrison L, Aref M, Whaley K. Synthesis of complement components (C3, C2, B and C1-inhibitor) and lysozyme by human monocytes and macrophages. J Clin Lab Immunol. (1986) 20:101–5. [PubMed] [Google Scholar]
- 17.Whaley K. Biosynthesis of the complement components and the regulatory proteins of the alternative complement pathway by human peripheral blood monocytes. J Exp Med. (1980) 151:501–16. 10.1084/jem.151.3.501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passwell J, Schreiner GF, Nonaka M, Beuscher HU, Colten HR. Local extrahepatic expression of complement genes C3, factor B, C2, and C4 is increased in murine lupus nephritis. J Clin Invest. (1988) 82:1676–84. 10.1172/JCI113780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Couser WG. Mechanisms of glomerular injury in immune-complex disease. Kidney Int. (1985) 28:569–83. 10.1038/ki.1985.167 [DOI] [PubMed] [Google Scholar]
- 20.Brooimans RA, Stegmann AP, van Dorp WT, van der Ark AA, van der Woude FJ, van Es LA, et al. Interleukin 2 mediates stimulation of complement C3 biosynthesis in human proximal tubular epithelial cells. J Clin Invest. (1991) 88:379–84. 10.1172/JCI115314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seelen MA, Brooimans RA, van der Woude FJ, van Es LA, Daha MR. IFN-gamma mediates stimulation of complement C4 biosynthesis in human proximal tubular epithelial cells. Kidney Int. (1993) 44:50–7. 10.1038/ki.1993.212 [DOI] [PubMed] [Google Scholar]
- 22.Timmerman JJ, van der Woude FJ, van Gijlswijk-Janssen DJ, Verweij CL, van Es LA, Daha MR. Differential expression of complement components in human fetal and adult kidneys. Kidney Int. (1996) 49:730–40. 10.1038/ki.1996.102 [DOI] [PubMed] [Google Scholar]
- 23.Khan TN, Sinniah R. Role of complement in renal tubular damage. Histopathology (1995) 26:351–6. 10.1111/j.1365-2559.1995.tb00197.x [DOI] [PubMed] [Google Scholar]
- 24.Welch TR, Beischel LS, Witte DP. Differential expression of complement C3 and C4 in the human kidney. J Clin Invest. (1993) 92:1451–8. 10.1172/JCI116722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welch TR, Beischel LS, Frenzke M, Witte D. Regulated expression of complement factor B in the human kidney. Kidney Int. (1996) 50:521–5. 10.1038/ki.1996.344 [DOI] [PubMed] [Google Scholar]
- 26.Andrews PA, Finn JE, Lloyd CM, Zhou W, Mathieson PW, Sacks SH. Expression and tissue localization of donor-specific complement C3 synthesized in human renal allografts. Eur J Immunol. (1995) 25:1087–93. 10.1002/eji.1830250434 [DOI] [PubMed] [Google Scholar]
- 27.Andrews PA, Pani A, Zhou W, Sacks SH. Local transcription of complement C3 in human allograft rejection. Evidence for a pathogenic role and correlation to histology and outcome. Transplantation (1994) 58:637–40. 10.1097/00007890-199409150-00023 [DOI] [PubMed] [Google Scholar]
- 28.Sacks SH, Zhou W, Andrews PA, Hartley B. Endogenous complement C3 synthesis in immune complex nephritis. Lancet (1993) 342:1273–4. 10.1016/0140-6736(93)92362-W [DOI] [PubMed] [Google Scholar]
- 29.Sheerin NS, Springall T, Carroll MC, Hartley B, Sacks SH. Protection against anti-glomerular basement membrane (GBM)-mediated nephritis in C3- and C4-deficient mice. Clin Exp Immunol. (1997) 110:403–9. 10.1046/j.1365-2249.1997.4261438.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrar CA, Zhou W, Lin T, Sacks SH. Local extravascular pool of C3 is a determinant of postischemic acute renal failure. FASEB J. (2006) 20:217–26. 10.1096/fj.05-4747com [DOI] [PubMed] [Google Scholar]
- 31.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. (2002) 8:582–7. 10.1038/nm0602-582 [DOI] [PubMed] [Google Scholar]
- 32.Tang S, Zhou W, Sheerin NS, Vaughan RW, Sacks SH. Contribution of renal secreted complement C3 to the circulating pool in humans. J Immunol. (1999) 162:4336–41. [PubMed] [Google Scholar]
- 33.Bartel G, Brown K, Phillips R, Peng Q, Zhou W, Sacks SH, et al. Donor specific transplant tolerance is dependent on complement receptors. Transpl Int. (2013) 26:99–108. 10.1111/tri.12006 [DOI] [PubMed] [Google Scholar]
- 34.Marino J, Babiker-Mohamed MH, Crosby-Bertorini P, Paster JT, LeGuern C, Germana S, et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci Immunol. (2016) 1:aaf8759. 10.1126/sciimmunol.aaf8759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, et al. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity (2008) 28:425–35. 10.1016/j.immuni.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood (2008) 112:1759–66. 10.1182/blood-2008-04-151068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou W, Patel H, Li K, Peng Q, Villiers MB, Sacks SH. Macrophages from C3-deficient mice have impaired potency to stimulate alloreactive T cells. Blood (2006) 107:2461–9. 10.1182/blood-2005-08-3144 [DOI] [PubMed] [Google Scholar]
- 38.Sacks SH, Zhou W. The role of complement in the early immune response to transplantation. Nat Rev Immunol. (2012) 12:431–42. 10.1038/nri3225 [DOI] [PubMed] [Google Scholar]
- 39.Peng Q, Li K, Smyth LA, Xing G, Wang N, Meader L, et al. C3a and C5a promote renal ischemia-reperfusion injury. J Am Soc Nephrol. (2012) 23:1474–85. 10.1681/ASN.2011111072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou W, Farrar CA, Abe K, Pratt JR, Marsh JE, Wang Y, et al. Predominant role for C5b-9 in renal ischemia/reperfusion injury. J Clin Invest. (2000) 105:1363–71. 10.1172/JCI8621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YH, Zhang YG. Kidney and innate immunity. Immunol Lett. (2017) 183:73–8. 10.1016/j.imlet.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 42.Kemper C, Kohl J. Novel roles for complement receptors in T cell regulation and beyond. Mol Immunol. (2013) 56:181–90. 10.1016/j.molimm.2013.05.223 [DOI] [PubMed] [Google Scholar]
- 43.Holers VM. Complement and its receptors: new insights into human disease. Annu Rev Immunol. (2014) 32:433–59. 10.1146/annurev-immunol-032713-120154 [DOI] [PubMed] [Google Scholar]
- 44.Springall T, Sheerin NS, Abe K, Holers VM, Wan H, Sacks SH. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat Med. (2001) 7:801–6. 10.1038/89923 [DOI] [PubMed] [Google Scholar]
- 45.Li K, Zhou W, Hong Y, Sacks SH, Sheerin NS. Synergy between type 1 fimbriae expression and C3 opsonisation increases internalisation of E. coli by human tubular epithelial cells. BMC Microbiol. (2009) 9:64. 10.1186/1471-2180-9-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li K, Sacks SH, Sheerin NS. The classical complement pathway plays a critical role in the opsonisation of uropathogenic Escherichia coli. Mol Immunol. (2008) 45:954–62. 10.1016/j.molimm.2007.07.037 [DOI] [PubMed] [Google Scholar]
- 47.Kaca W, Arabski M, Fudala R, Holmstrom E, Sjoholm A, Weintraub A, et al. Human complement activation by smooth and rough Proteus mirabilis lipopolysaccharides. Arch Immunol Ther Exp. (2009) 57:383–91. 10.1007/s00005-009-0043-8 [DOI] [PubMed] [Google Scholar]
- 48.Doorduijn DJ, Rooijakkers SH, van Schaik W, Bardoel BW. Complement resistance mechanisms of Klebsiella pneumoniae. Immunobiology (2016) 221:1102–9. 10.1016/j.imbio.2016.06.014 [DOI] [PubMed] [Google Scholar]
- 49.Miller TE, Phillips S, Simpson IJ. Complement-mediated immune mechanisms in renal infection. II. Effect of decomplementation. Clin Exp Immunol. (1978) 33:115–21. [PMC free article] [PubMed] [Google Scholar]
- 50.Abreu AG, Barbosa AS. How Escherichia coli circumvent complement-mediated killing. Front Immunol. (2017) 8:452. 10.3389/fimmu.2017.00452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li K, Feito MJ, Sacks SH, Sheerin NS. CD46 (membrane cofactor protein) acts as a human epithelial cell receptor for internalization of opsonized uropathogenic Escherichia coli. J Immunol. (2006) 177:2543–51. 10.4049/jimmunol.177.4.2543 [DOI] [PubMed] [Google Scholar]
- 52.Nauser CL, Farrar CA, Sacks SH. Complement recognition pathways in renal transplantation. J Am Soc Nephrol. (2017) 28:2571–8. 10.1681/ASN.2017010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howard M, Farrar CA, Sacks SH. Structural and functional diversity of collectins and ficolins and their relationship to disease. Semin Immunopathol. (2018) 40:75–85. 10.1007/s00281-017-0642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riedemann NC, Ward PA. Complement in ischemia reperfusion injury. Am J Pathol. (2003) 162:363–7. 10.1016/S0002-9440(10)63830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorsuch WB, Chrysanthou E, Schwaeble WJ, Stahl GL. The complement system in ischemia-reperfusion injuries. Immunobiology (2012) 217:1026–33. 10.1016/j.imbio.2012.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farrar CA, Zhou W, Sacks SH. Role of the lectin complement pathway in kidney transplantation. Immunobiology (2016) 221:1068–72. 10.1016/j.imbio.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 57.Farrar CA, Tran D, Li K, Wu W, Peng Q, Schwaeble W, et al. Collectin-11 detects stress-induced L-fucose pattern to trigger renal epithelial injury. J Clin Invest. (2016) 126:1911–25. 10.1172/JCI83000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asgari E, Farrar CA, Lynch N, Ali YM, Roscher S, Stover C, et al. Mannan-binding lectin-associated serine protease 2 is critical for the development of renal ischemia reperfusion injury and mediates tissue injury in the absence of complement C4. FASEB J. (2014) 28:3996–4003. 10.1096/fj.13-246306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fanelli G, Gonzalez-Cordero A, Gardner PJ, Peng Q, Fernando M, Kloc M, et al. Human stem cell-derived retinal epithelial cells activate complement via collectin 11 in response to stress. Sci Rep. (2017) 7:14625. 10.1038/s41598-017-15212-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen S, Selman L, Palaniyar N, Ziegler K, Brandt J, Kliem A, et al. Collectin 11 (CL-11, CL-K1) is a MASP-1/3-associated plasma collectin with microbial-binding activity. J Immunol. (2010) 185:6096–104. 10.4049/jimmunol.1002185 [DOI] [PubMed] [Google Scholar]
- 61.Keshi H, Sakamoto T, Kawai T, Ohtani K, Katoh T, Jang SJ, et al. Identification and characterization of a novel human collectin CL-K1. Microbiol Immunol. (2006) 50:1001–13. 10.1111/j.1348-0421.2006.tb03868.x [DOI] [PubMed] [Google Scholar]
- 62.Hogenkamp A, van Eijk M, van Dijk A, van Asten AJ, Veldhuizen EJ, Haagsman HP. Characterization and expression sites of newly identified chicken collectins. Mol Immunol. (2006) 43:1604–16. 10.1016/j.molimm.2005.09.015 [DOI] [PubMed] [Google Scholar]
- 63.Henriksen ML, Brandt J, Andrieu JP, Nielsen C, Jensen PH, Holmskov U, et al. Heteromeric complexes of native collectin kidney 1 and collectin liver 1 are found in the circulation with MASPs and activate the complement system. J Immunol. (2013) 191:6117–27. 10.4049/jimmunol.1302121 [DOI] [PubMed] [Google Scholar]
- 64.Garred P, Genster N, Pilely K, Bayarri-Olmos R, Rosbjerg A, Ma YJ, et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev. (2016) 274:74–97. 10.1111/imr.12468 [DOI] [PubMed] [Google Scholar]
- 65.Selman L, Hansen S. Structure and function of collectin liver 1 (CL-L1) and collectin 11 (CL-11, CL-K1). Immunobiology (2012) 217:851–63. 10.1016/j.imbio.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 66.Drickamer K. Engineering galactose-binding activity into a C-type mannose-binding protein. Nature (1992) 360:183–6. 10.1038/360183a0 [DOI] [PubMed] [Google Scholar]
- 67.Man-Kupisinska A, Swierzko AS, Maciejewska A, Hoc M, Rozalski A, Siwinska M, et al. Interaction of mannose-binding lectin with lipopolysaccharide outer core region and its biological consequences. Front Immunol. (2018) 9:1498. 10.3389/fimmu.2018.01498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology (2006) 16:158R−84R. 10.1093/glycob/cwl040 [DOI] [PubMed] [Google Scholar]
- 69.Biancone L, David S, Della Pietra V, Montrucchio G, Cambi V, Camussi G. Alternative pathway activation of complement by cultured human proximal tubular epithelial cells. Kidney Int. (1994) 45:451–60. 10.1038/ki.1994.59 [DOI] [PubMed] [Google Scholar]
- 70.David S, Biancone L, Caserta C, Bussolati B, Cambi V, Camussi G. Alternative pathway complement activation induces proinflammatory activity in human proximal tubular epithelial cells. Nephrol Dial Transplant. (1997) 12:51–6. 10.1093/ndt/12.1.51 [DOI] [PubMed] [Google Scholar]
- 71.Ma YJ, Skjoedt MO, Garred P. Collectin-11/MASP complex formation triggers activation of the lectin complement pathway–the fifth lectin pathway initiation complex. J Innate Immun. (2013) 5:242–50. 10.1159/000345356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wallis R. Interactions between mannose-binding lectin and MASPs during complement activation by the lectin pathway. Immunobiology (2007) 212:289–99. 10.1016/j.imbio.2006.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwaeble WJ, Lynch NJ, Clark JE, Marber M, Samani NJ, Ali YM, et al. Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc Natl Acad Sci USA. (2011) 108:7523–8. 10.1073/pnas.1101748108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yaseen S, Demopulos G, Dudler T, Yabuki M, Wood CL, Cummings WJ, et al. Lectin pathway effector enzyme mannan-binding lectin-associated serine protease-2 can activate native complement C3 in absence of C4 and/or C2. FASEB J. (2017) 31:2210–9. 10.1096/fj.201601306R [DOI] [PubMed] [Google Scholar]