Abstract
Anaplastic thyroid cancer is an aggressive and highly lethal cancer for which conventional therapies have proved ineffective. Cancer stem-like cells (CSCs) represent a small fraction of cells in the cancer that are resistant to chemotherapy and radiation therapy and are responsible for tumor reoccurrence and metastasis. We characterized CSCs in thyroid carcinomas and generated clones of CSC lines. Our study showed that anaplastic thyroid cancers had significantly more CSCs than well-differentiated thyroid cancers. We also showed that Aldefluor-positive cells revealed significantly higher expression of stem cell markers, self-renewal properties, thyrosphere formation, and enhanced tumorigenicity. In vivo passaging of Aldefluor-positive cells resulted in the growth of larger, more aggressive tumors. We isolated and generated two clonal spheroid CSC lines derived from anaplastic thyroid cancer that were even more enriched with stem cell markers and more tumorigenic than the freshly isolated Aldefluor-positive cells. Resveratrol and valproic acid treatment of one of the CSC lines resulted in a significant decrease in stem cell markers, Aldefluor expression, proliferation, and invasiveness, with an increase in apoptosis and thyroid differentiation markers, suggesting that these cell lines may be useful for discovering new adjuvant therapies for aggressive thyroid cancers. For the first time, we have two thyroid CSC lines that will be useful tools for the study of thyroid CSC targeted therapies.
Thyroid cancers are the most common endocrine malignancies.1, 2 They comprise approximately 1% of human cancers. The incidence of thyroid cancer has been increasing worldwide, partially because of increased diagnosis of papillary thyroid microcarcinomas,3 but other reasons for this increase remain unknown. Although papillary thyroid carcinomas (PTCs) are the most common type of thyroid cancer, comprising approximately 80% to 85% of thyroid carcinomas, anaplastic thyroid carcinomas (ATCs), which constitute approximately 2% of thyroid cancers, remain one of the most lethal and treatment-resistant human cancers.1 Studies have shown that some ATCs arise from well-differentiated PTCs by dedifferentiation,4 although cancer stem-like cells (CSCs) may also produce ATCs.5, 6
The CSC hypothesis suggests that a small population of stem-like cells generate and sustain the variety of tumor cell populations within a tumor.7, 8, 9, 10, 11, 12, 13 CSCs are characterized by their ability for self-renewal, proliferation, resistance to chemotherapy and radiation therapy, multipotent capability, and expression of stem cell markers, such as Nanog, Sox2, and Oct4, and demonstrate tumor-initiating properties in vivo. Several studies suggest that CSCs may mediate metastatic disease and contribute to relapse after surgery. Plasticity in CSCs, which may replicate and transform to non-CSCs by asymmetric division, makes standard therapeutic approaches more challenging. Epithelial-mesenchymal transition (EMT) has also been associated with increased numbers of CSCs and probably contributes to the plasticity of CSCs when mesenchymal cells transition back to epithelial cells.14, 15, 16, 17, 18 Thus, understanding the biology of CSCs remains an ongoing challenge and will be necessary for the development of effective therapeutic agents for highly lethal cancers, such as ATCs.19, 20
The recent development of new ATC cell lines with molecular characterization has set a new standard for cell line characterization.21 Some of these new ATC cell lines have been shown to have features of CSCs, such as thyrosphere formation and expression of stem cell markers.22 New biomarkers to identify thyroid CSCs, such as aldehyde dehydrogenase (ALDH) and stage-specific embryonic antigen 1 (SSEA1) expression, have also been identified.23, 24 Other studies have shown that thyroid CSCs could be generated from primary thyroid tumors in immunodeficient mice.24
Herein, we characterized a series of thyroid cancer cell lines for CSC properties. Subsequently, CSC clones were derived from one of these cell lines and were characterized in vitro and in vivo. Our findings support the existence and unique properties of thyroid CSCs and suggest that these clones will be useful for understanding the biology of thyroid CSCs.
Materials and Methods
Cell Culture and TGF Treatment
The cell lines FRO and Kat18 were kindly provided by Dr. Herbert Chen (University of Wisconsin Madison, Madison, WI), and the NTHY-Ori-3 and 8505C were purchased from Sigma (St. Louis, MO) and were maintained in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C, 5% CO2. The papillary thyroid carcinoma cell line BCPAP was provided by Dr. Rebecca E. Schweppe (University of Colorado, Denver, CO), and the TPC-1 cell line was provided by Dr. Daniel T. Ruan (Brigham and Women's Hospital, Boston, MA). Both cell lines were maintained in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C, 5% CO2. The ATC cell lines THJ-16T and THJ-21T were kindly provided by Dr. John A. Copland III (Mayo Clinic, Jacksonville, FL) and were maintained in RPMI 1640 medium with 10% fetal bovine serum, 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin/streptomycin at 37°C, 5% CO2. To induce EMT, the cell lines were plated at a density of 1 × 105 per 75-mm flask, cultured with serum-free medium (modified from Fierabracci,11 with epidermal growth factor and fibroblast growth factor omitted) with (treated) or without (control) 2 ng/mL human recombinant transforming growth factor (TGF)-β1 (R&D Systems, Minneapolis, MN) for 21 days. Medium was replaced every 2 to 3 days. Control and treated cells were trypsinized, counted, and reinoculated at the original density every 7 days for 21 days; the remaining cells were collected for additional assays and analysis. To verify the authenticity of the THJ-16T cell line and the two clones, DNA short tandem repeat analysis was performed by DNA Diagnostics Center (Fairfield, OH). The cell line and the clones were tested using 19 markers and were found to be authentic.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from samples with TRIzol Reagent (Invitrogen, Grand Island, NY), according to the manufacturer's instructions, and RNA quality and concentrations were assessed on the NanoDrop 1000 (Thermo Scientific, Pittsburg, PA). To assess mRNA expression, total RNA of 1 μg was reverse transcribed using the All-in-One First-Strand cDNA Synthesis Kit (GeneCopoeia, Rockville, MD). The quantitative RT-PCR was performed on the CFX96 PCR Detection System (Bio-Rad Laboratories, Hercules, CA) using the Bullseye EvaGreen qPCR Mastermix (Midwest Scientific, St. Louis, MO), normalized to 18S, and relative fold-change was determined by the ΔΔ CT method. PCR primers used are listed in Table 1. The stem cell markers NANOG, OCT4, and SOX2 were verified using primers purchased from SABiosciences (Valencia, CA).
Table 1.
Real-Time PCR Primers
| Target | Forward primer | Reverse primer |
|---|---|---|
| ALDH1 | 5′-GCTGAGCCAGTCACCTGTGTTCCAG-3′ | 5′-GCTGAGCCAGTCACCTGTGTTGTTCCAG-3′ |
| ECAD | 5′-TCCCATCAGCTGCCCAGACC-3′ | 5′-TGACTCCTGTGTTCCTGTTA-3′ |
| Slug | 5′-ATGCATATTCGGACCCACAC-3′ | 5′-GCAGATGAGCCCTCAGATTT-3′ |
| CMET | 5′-GATTTTAGTCATCCCAATGTCC-3′ | 5′-ATCCAGCATACAGTTTCTTGC-3′ |
| EGFR | 5′-ATGTCCGGGAACACAAAGAC-3′ | 5′-TTCCGTCATATGGCTTGGAT-3′ |
| NIS | 5′-CCATCCTGGATGACAACTTGG-3′ | 5′-AAAAACAGACGATCCTCATTGGT-3′ |
| TTF1 | 5′-CCATGAGGAACAGCGCCTC-3′ | 5′-CTCACGTCCCCCAGCGA-3′ |
| TG | 5′-CTTCGAGTACCAGGTTGATGCC-3′ | 5′-GGTGGTTTCAGTGAAGGTGGAA-3′ |
| TPO | 5′-TGTGTCCAACGTGTTCTCCACAG-3′ | 5′-AAGACGTGGCTGTTCTCCCAC-3′ |
| TGF-βR1 | 5′-GGTCTTGCCCATCTTCACAT-3′ | 5′-TCTGTGGCTGAATCATGTCT-3′ |
| SMAD2 | 5′-CGAAATGCCACGGTAGAAAT-3′ | 5′-CCAGAAGAGCAGCAAATTCC-3′ |
| SMAD4 | 5′-CCATTTTCCAATCATCCTGCT-3′ | 5′-ACCTTTGCCTATGTGCAACC-3′ |
| SMAD7 | 5′-TCACCTTAGCCGACTCTGC-3′ | 5′-ACACCCACACACCATCCAC-3′ |
| 18S | 5′-GTAACCCGTTGAACCCCATT-3′ | 5′-CCATCCAATCGGTAGTAGCG-3′ |
ALDH1, aldehyde dehydrogenase 1; ECAD, Ecadherin; CMET, c-MET or MET; EGFR, epidermal growth factor receptor; NIS, sodium-iodide symporter; TTF1, transcription termination factor 1; TG, thyroglobulin; TPO, thyroperoxidase; TGF-βR1, transforming growth factor beta receptor 1.
Western Blot Analysis
Xenograft tissues and EMT-treated THJ-16T cells for time points 7, 14, and 21 days were washed twice with phosphate-buffered saline, and radioimmunoprecipitation assay lysis buffer was added. The protein lysates were prepared as previously described.25 Briefly, protein concentration was quantified using the BCA Protein Assay Kit (Pierce-Thermo Scientific, Pittsburg, PA) following the manufacturer's instructions. Equal amounts of denatured protein were resolved by electrophoresis on 4% to 15% Criterion TGX precast gels (Bio-Rad Laboratories, Hercules, CA), transferred onto nitrocellulose membranes (Bio-Rad Laboratories), blocked in 5% nonfat milk solution, then incubated with the following primary antibodies overnight at 4°C: Twist (1:500; Abcam, Cambridge, MA), Snail (1:1000; Novus Biologicals, Littleton, CO), Slug (1:1000), Oct4 (1:1000), and β-actin (1:2000), all from Cell Signaling Technology (Danvers, MA), and glyceraldehyde-3-phosphate dehydrogenase (1:2000; Santa Cruz Biotechnology, Dallas, TX). Membranes were washed the next day and incubated for 1 hour at room temperature with horseradish peroxidase–conjugated secondary antibodies (1:2000; Cell Signaling Technology). The immunoreactive protein bands were visualized by the detection systems of Immunstar (Bio-Rad Laboratories), SuperSignal West Pico, or SuperSignal West Femto (Pierce Biotechnology, Pittsburg, PA). β-Actin and glyceraldehyde-3-phosphate dehydrogenase were used as loading controls.
Xenografts
Sorted Aldefluor-positive (ALD+) and Aldefluor-negative (ALD−) cells and unsorted cells were injected s.c. into nude mice (Jackson Labs, Bar Harbor, ME) in 1:1 serum-free media/growth factor–reduced Matrigel (BD Biosciences, San Jose, CA). Tumors were measured with calipers once a week until the tumor reached 1 cm3 and harvested and processed for further analysis.
Flow Cytometry and Sorting of Cells
THJ-16T cells were harvested and ALDH activity analyzed by the ALDEFLUOR Kit (STEMCELLS Technology, Vancouver, BC, Canada), according to the manufacturer's instructions. SSEA1 expression was also analyzed (1:20 dilution; R&D, Minneapolis, MN). Flow cytometry was performed on the BD FACSCalibur and analyzed with Cyflogic software version 1.2.1. High-expressing Aldefluor-positive cells and low-expressing negative cells were sorted by the BD FACSAriaII SORP (Special Order Research Product, San Jose, CA) contained in the dedicated Biological Safety Cabinet. Data were collected using BD FACSDiva software version 8 (San Jose, CA). Cells were sorted into a 96-well ultra–low-attachment plate (Corning, Tewksbury, MA) with spheroid media (modified from Fierabracci,11 with 1× B27 added [Invitrogen]) at a concentration of 10 or 1 cell per well.
Sphere Formation
Sorted ALD+ and ALD− cells were incubated at 37°C, 5% CO2, and monitored twice weekly for sphere formation. Only spheres derived from a single cell are termed clones. The resulting clones were harvested from the 96-well plate and further propagated in an ultra–low-attachment T25 flask (Corning) with spheroid media and incubated at 37°C, 5% CO2.
Fluorescence Staining
Clonal spheroids were dissociated into single cells with 0.25% trypsin (Corning), plated in a 24-well ultra–low-attachment plate in spheroid media, and incubated for 3 days. Cells were stained with the Aldefluor kit and counterstained with Hoechst. Images were captured with an Eclipse TI fluorescence microscope (Nikon, Tokyo, Japan) and analyzed with NIS Elements Q15 software version 3.00 SP4 (Nikon).
Resveratrol and Valproic Acid Treatment, MTT, and Invasion Assays
Clonal spheres were dissociated with 0.25% trypsin, and single cells were treated with 50 μmol/L resveratrol (3,5,4′-trihydroxy-trans-stilbene) or dimethyl sulfoxide (vehicle) for 72 hours, or 1 mmol/L valproic acid for 3 or 7 days, in ultra–low-attachment plates in spheroid media. Cell proliferation and invasion were assayed using a Vybrant MTT cell proliferation assay kit (Molecular Probes, Eugene, OR) and a Cultrex BME cell invasion assay (R&D Systems), according to the manufacturer's protocol. The 96-well plate for the invasion assay was coated with 1×, 0.5×, and 0.1× BME basement membrane extract. Read-out for both assays was performed using a SpectraMax 190 absorbance microplate reader (Molecular Devices, Sunnyvale, CA).
Statistical Analysis
All statistics were calculated using the t-test, two-tailed and paired. Deviations were expressed as SEM.
Results
Identification of CSCs in Thyroid Cell Lines
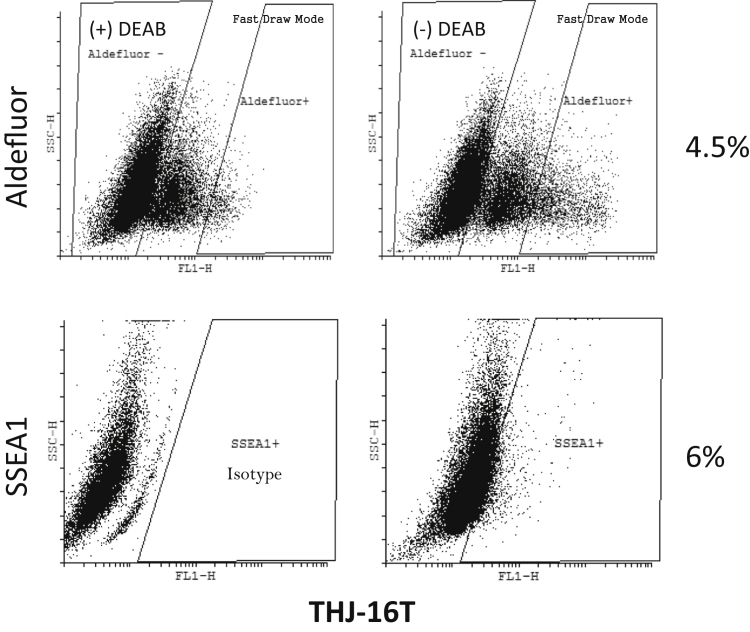
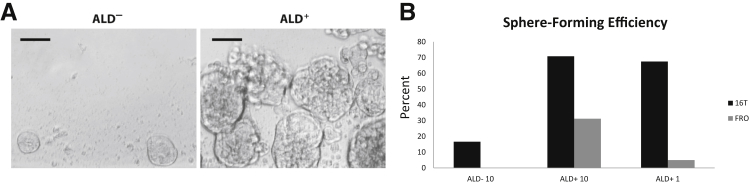
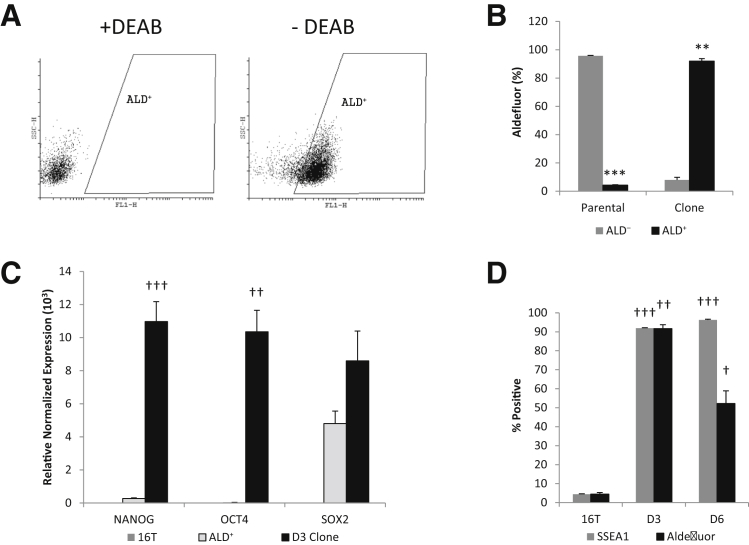
Several thyroid cancer cell lines were stained with the Aldefluor assay and analyzed by flow cytometry to determine the percentage of ALD+ population. The cell lines were also analyzed for SSEA1 expression. ATC cell lines displayed a much greater ALD+ population than the PTC lines but showed a varied expression of SSEA1 (Table 2). ALD+ and ALD− cells (Figure 1) were sorted into an ultra–low-attachment 96-well culture plate at a density of either 10 or 1 cell per well in a serum-free defined medium for assessment of spheroid formation. The ATC cell line THJ-16T (16T) displayed the highest ALD+ and SSEA1 expression, and was the most proficient at thyrosphere formation (Figure 2 and Table 2). On the basis of these findings, the 16T cell line and its ALD+ population were characterized further in this study.
Table 2.
Analysis of Thyroid Carcinoma Cell Lines by Flow Cytometry for Aldefluor Activity and SSEA1 Expression
| Cell line | ALD (%) | SSEA1 (%) | Spheres % (no./total) |
|---|---|---|---|
| 16T | 4.1 ± 1.7 | 5.3 ± 1.02∗ | 68 (81/120) |
| 21T | 2.4 ± 0.2 | 3.16 ± 0.23 | 25 (3/12) |
| FRO | 3.5 ± 1.5 | 0.59 ± 0.41 | 5 (6/120) |
| KAT18 | 2.4 ± 0.2 | 0.85 ± 0.56 | 4 (2/48) |
| TPC1 | 0.1 ± 0.03 | 0.07 ± 0.04 | 25 (3/12) |
| BCPAP | 0.5 ± 0.1 | 1.99 ± 0.09 | 58 (7/12) |
ALD and SSEA1 expressed as means ± SEM. Each line was also analyzed for thyrosphere formation efficiency, (x, y) where x is the number of spheres formed, and y is the number of wells tested.
ALD, Aldefluor; SSEA1, stage-specific embryonic antigen 1.
P < 0.05.
Figure 1.
Flow cytometric data of THJ-16T Aldefluor and stage-specific embryonic antigen 1 (SSEA1) content. Aldefluor panel of THJ-16T Aldefluor staining with or without DEAB (aldehyde dehydrogenase inhibitor) and the selection of Aldefluor (ALD)+ cells at 4.6%. SSEA1 panel of THJ-16T showing SSEA1-positive staining at 6% and the isotype control. FL1-H, aldefluor; SSC-H, side scatter.
Figure 2.
Aldefluor (ALD)+ sphere formation. A: Sphere formation capacity of ALD− and ALD+ sorted THJ-16T cells. B: Sphere-forming efficiency of two ATC lines (THJ-16T and FRO) comparing the percentage of spheres formed from ALD− cells sorted at 10 cells per well (ALD− 10), ALD+ sorted at 10 cells per well (ALD+ 10), and ALD+ cells sorted at one cell per well (ALD+ 1). Scale bar = 50 μm (A).
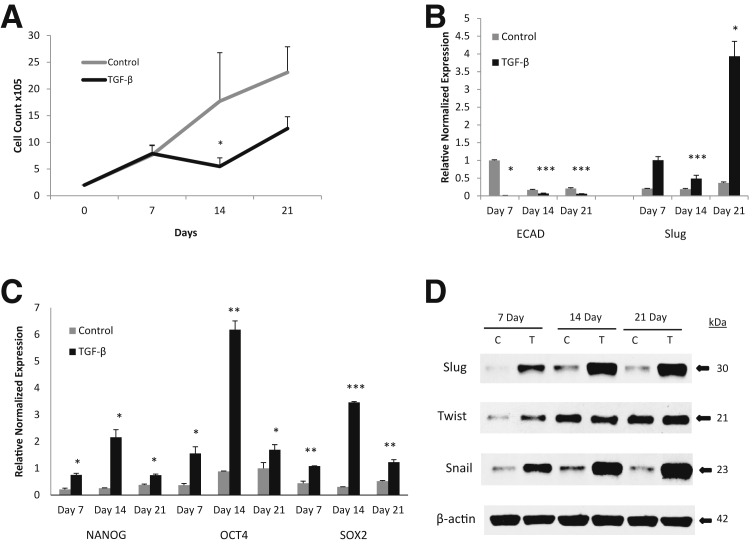
We have previously shown that induction of EMT with TGF-β1 treatment can increase CSC populations in PTC cell lines; however, induction of EMT in ATC has not been explored.18 Therefore, to further characterize the 16T cell line, its response to TGF-β1 stimulation and its capacity to undergo EMT were analyzed. Analysis of SMAD family members and TGF-βR1 expression by RT-PCR (Supplemental Figure S1) showed a significantly reduced expression of SMADs 2, 4, and 7 compared with that of the normal thyroid cell line Nthy-Ori3-1 (Nthy) and the PTC cell lines TPC-1 and BCPAP (BCP). TGF-βR1 was significantly reduced to that of Nthy but was comparable to the PTC and other ATC lines. Culturing 16T cells with TGF-β1 for 21 days showed that TGF-β1 exerts growth inhibition on 16T cells after 7 days of culture. This inhibition peaked at 14 days of culture, but growth began to recover by day 21 (Figure 3A). Analysis of EMT markers showed that E-cadherin was significantly reduced, where Slug and Snail were up-regulated in the TGF-β1–treated cells (Figure 3, B and D). In addition, because EMT can confer stemness, we tested and found that TGF-β1 significantly up-regulated the stem cell markers SOX2, OCT4, and NANOG (Figure 3C).
Figure 3.
Induction of epithelial-mesenchymal transition (EMT) in ATC. A: Growth curve of THJ-16T treated with or without (control) 2 ng/mL transforming growth factor (TGF-β)-1 for 21 days showing TGF-β exposure exhibits growth inhibition. B: RT-PCR results of ECAD down-regulation and Slug up-regulation by TGF-β treatment on THJ-16T cells normalized to 18S. C: RT-PCR results of stem cell markers NANOG, OCT4, and SOX2 up-regulation from TGF-β treatment of THJ-16T cells normalized to 18S. D: Western blot of EMT markers Slug, Twist, and Snail expression effect by TGF-β treatment of THJ-16T cells. β-Actin used as loading control. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus the control on the same day. C, control; T, TGF-β.
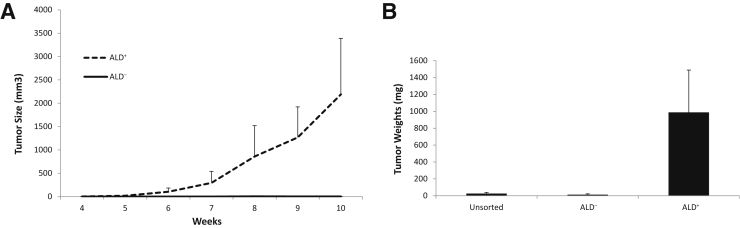
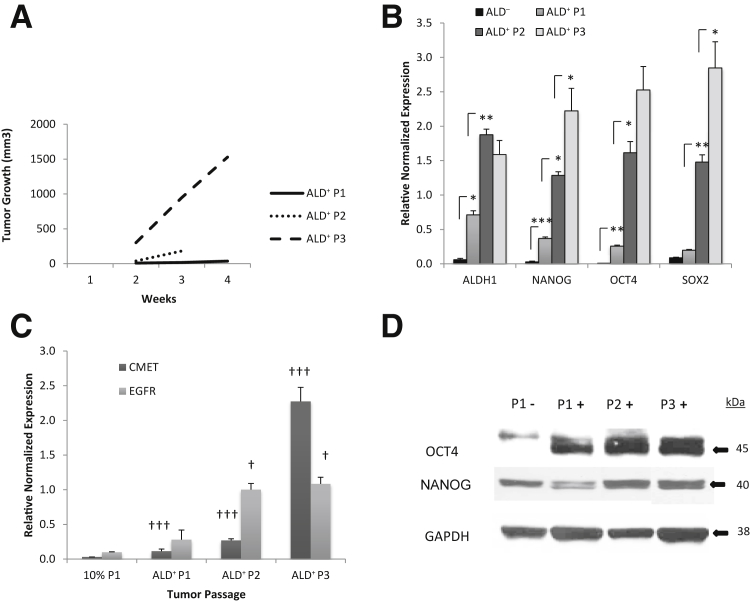
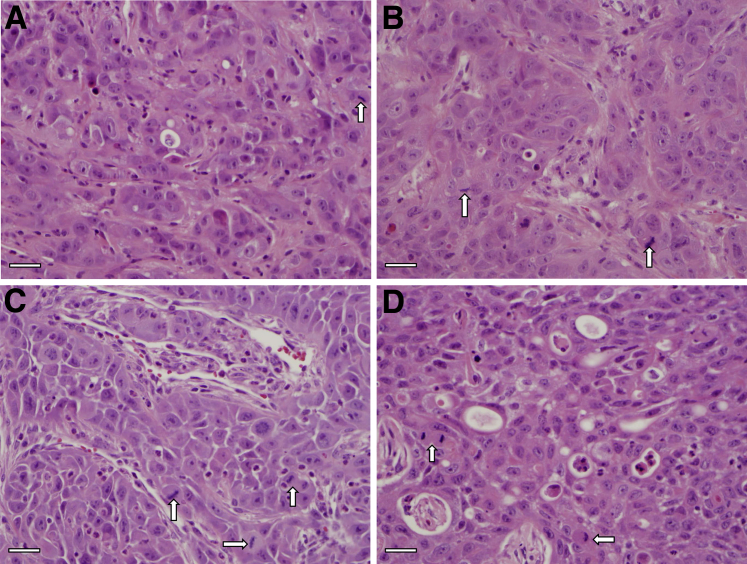
To investigate the in vivo tumorigenicity of the different populations within 16T cell line, freshly isolated 16T ALD+ and ALD− cells, as well as unsorted bulk cells, were s.c. injected into immunocompromised mice to assess tumor-forming capacity (Table 3). Tumor growth rates and the weight of the resulting tumors were measured and compared (Figure 4). The ALD+ cells formed much larger and extremely fast-growing tumors over that of the ALD− or unsorted cells. On further in vivo passaging, each subsequent passage of the ALD+ tumors (P1 to P3): i) became faster growing (Figure 5A), ii) showed a significant increase in stem cell markers SOX2, OCT4, and NANOG (Figure 5, B and D), iii) had a significant increase in CMET (also called MET or hepatocyte growth factor receptor) and epidermal growth factor receptor expression (Figure 5C), and iv) showed the histological features of the ALD+ and ALD− tumors were similar with cells having large nuclei and prominent nucleoli and prominent vascularity in the stroma. The ALD+ cells from P1, P2, and P3 showed significant increases in the mitotic activity compared with the ALD− cells (Supplemental Figure S2 and Figure 6).
Table 3.
Tumor Formation of THJ-16T Subtypes
| Cells/concentration | 1 × 104 | 4 × 104 | 1 × 105 | 5 × 105 | 1 × 106 |
|---|---|---|---|---|---|
| ALD+ | 6 of 7 | 2 of 2 | 3 of 5 | 3 of 3 | NA |
| ALD− | 1 of 2 | NA | 0 of 2 | 2 of 3 | 2 of 4 |
| Unsorted | NA | NA | 0 of 2 | 2 of 4 | 2 of 5 |
Cell concentrations ranged from 1 × 104 to 1 × 106.
ALD, Aldefluor; NA, not analyzed.
Figure 4.
Aldefluor (ALD)+ xenograft tumor growth. A: Tumor growth rate of ALD+ and ALD− THJ-16T cells injected s.c. into nude mice at 1 × 105 cells per injection site. B: Tumor weights of ALD+, ALD−, and unsorted parental THJ-16T cells injected s.c. into nude mice.
Figure 5.
Tumorigenicity increases with in vivo passaging. A: Tumor growth rate of Aldefluor (ALD)+ THJ-16T cells passaged in vivo. B: RT-PCR results for stem cell marker expression of ALD− and in vivo passaged ALD+ (ALD+ P1-ALD+ P3) tumors. Samples normalized to 18S. C: RT-PCR results of CMET and epidermal growth factor receptor (EGFR) expression of in vivo passaged (P1 to P3) ALD+ tumors compared with unsorted parental THJ-16T cells grown in RPMI 1640 media with 10% fetal bovine serum (10% P1). Samples normalized to 18S. D: Western blot of Oct4 and Nanog expression from ALD− tumors compared with in vivo passaged ALD+ tumors. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. †P < 0.05 and †††P < 0.001 versus the 10% tumor.
Figure 6.
Recapitulation of parental tumor in Aldefluor (ALD)− and in vivo passaged ALD+ THJ-16T cells. The histopathological features of the P1 to P3 were similar showing cells with large nuclei and prominent nucleoli and moderate amounts of eosinophilic cytoplasm. Hematoxylin and eosin staining of ALD- P1 (A), ALD+ P1 (B), ALD+ P2 (C), and ALD+ P3 (D) tumor. Prominent mitotic activity is present in P1, P2, and P3 (arrows). Scale bar = 100 μm (A–D).
Generation of Distinct CSC Clones
ALD+ cells were sorted into 96-well culture plates. Wells that contained only one cell per well were surveyed for clonal sphere formation. On sphere formation, the resulting sphere was transferred to a T25 ultra–low-attachment flask and incubated in spheroid media. After 10 days of spheroid culture, two clones were harvested to be analyzed further, referred to as D3 and D6 throughout this report. Both clones continued to replicate for more than a year in serum-free media (spheroid media) and in unattached conditions. However, the D3 clone was further characterized because of its rapid proliferative capacity.
After 12 months of continuous culture, passaged because of cell density or sphere size (to avoid necrosis at the sphere core), the D3 and D6 clones were analyzed for Aldefluor activity, SSEA1 expression, and stem cell markers. The D3 clone Aldefluor activity was >90% positive (Figure 7A). The stem cell markers OCT4 and NANOG were significantly increased in the clones over that of the parental 16T line (Figure 7C). The D6 clone Aldefluor activity was almost half that of D3, and yet, 10-fold higher than the parental line (Figure 7D). In addition, the SSEA1 expression correlated with the Aldefluor activity in the parental line and the D3 clone, but not for the D6 clone.
Figure 7.
Clonal sphere enriched for stem cell markers. A: Flow cytometric analysis of Aldefluor positivity with or without DEAB (aldehyde dehydrogenase inhibitor) of D3 clone. B: Graph indicating reversal of the percentage of Aldefluor (ALD) positive versus negative cells in the parental THJ-16T cell line compared with the D3 clone. C: RT-PCR results of stem cell markers comparing the parental THJ-16T cell line with freshly sorted ALD+ cells and the D3 clonal line. D: Percentage of Aldefluor and stage-specific embryonic antigen 1 (SSEA1) positivity of the D3 and D6 clonal lines compared with the parental THJ-16T cell line. ∗∗P < 0.01, and ∗∗∗P < 0.001 versus ALD−. †P < 0.05, ††P < 0.01, and †††P < 0.001 versus the 16T parental line control group.
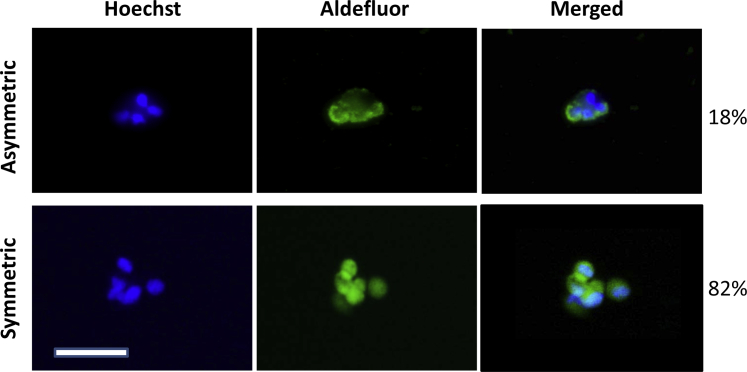
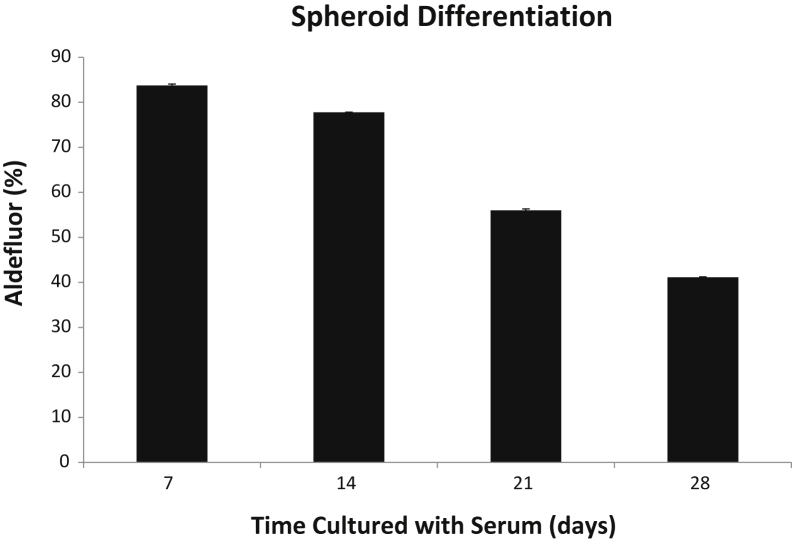
Furthermore, the Aldefluor positivity of the D3 clone inversely correlated to the parental line (Figure 7B). This inverse Aldefluor activity finding is intriguing, considering that previous reports have indicated that CSCs replicate in an asymmetrical manner.24 To investigate this finding, we analyzed the symmetrical versus asymmetrical division rates of the D3 clone. Symmetrical division was predominant over that of asymmetrical division at 82% versus 18%, respectively (Figure 8). The D3 clone was cultured in 10% fetal bovine serum media under attached conditions for 4 weeks. Under normal cell culture conditions, the Aldefluor activity significantly decreased (Figure 9), with P < 0.001 among the replicates. Therefore, the retention of stemness in D3 clones relied on the spheroid media and unattached culture conditions.
Figure 8.
Symmetric versus asymmetric division. Live, unfixed D3 spheres stained with Aldefluor and counterstained with Hoechst; 1 × 102 single cells were incubated for 72 hours to allow for cell division and then analyzed for symmetric versus asymmetric division. The D3 clone shows predominantly symmetric division over asymmetric division (82% and 18%, respectively). Scale bar = 100 μm.
Figure 9.
Spheroid differentiation with serum. Graph showing a significant decrease in Aldefluor activity over time in the D3 clonal line after culturing in normal media containing 10% fetal bovine serum.
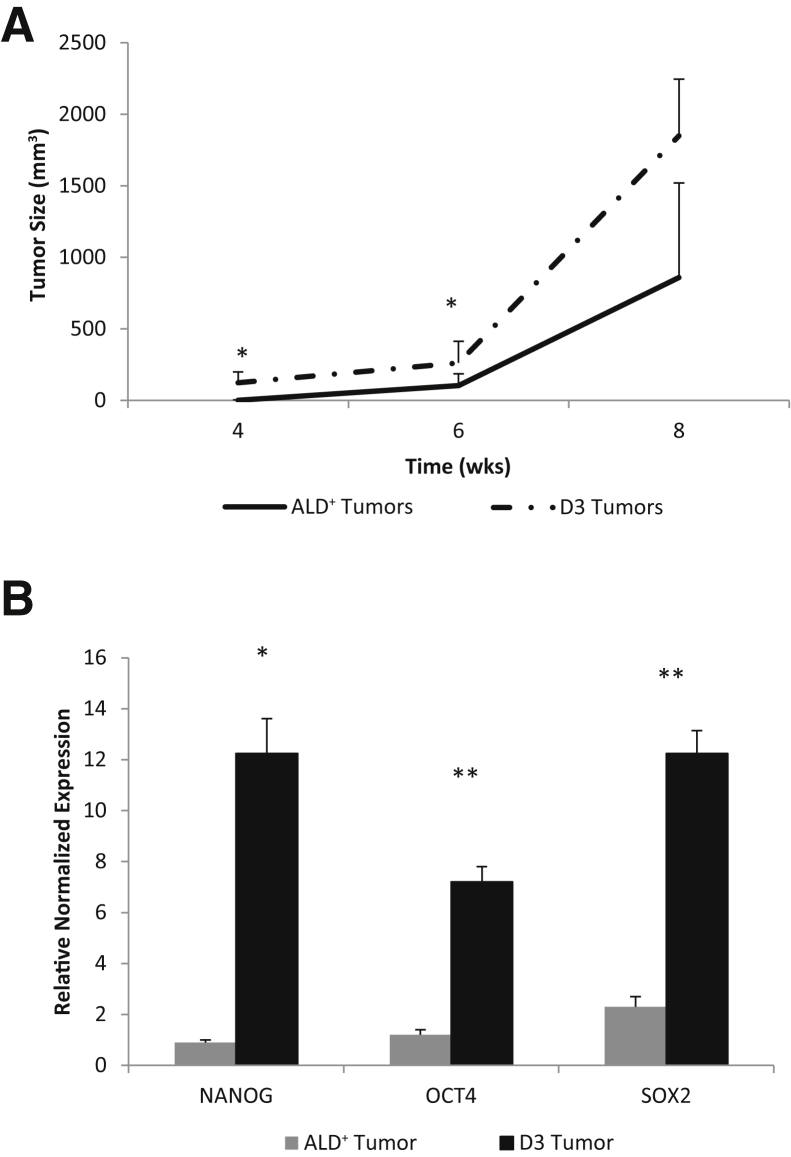
The clone's tumorigenicity was next compared with that of freshly isolated parental ALD+ cells. ALD+ 16T cells and D3 clonal cells were s.c. injected into immunocompromised mice and tumor growth was monitored (Supplemental Figure S3). Tumorigenic ability of the D3 clone significantly increased and rapidly produced large tumors compared with the 16T parental cells (Figure 10A). In addition, the tumors generated by the D3 clone had significantly up-regulated stem cell markers NANOG, SOX2, and OCT4 (Figure 10B).
Figure 10.
Clonal spheres are highly tumorigenic. The D3 clonal spheres were dissociated into single cells and freshly sorted Aldefluor (ALD)+ THJ-16T cells were injected s.c. into nude mice at 1 × 105 cells per injection site (3 injection sites, one in each flank and one in the back). A: The D3 clone forms larger tumors faster than the ALD+ tumors. B: RT-PCR results comparing stem cell marker expression of the D3 tumors and the ALD+ tumors. ∗P < 0.05, ∗∗P < 0.01 versus respective ALD+ tumor.
Targeting Thyroid CSCs
Cancer stem cells are known to be radiation and chemotherapy resistant. Anaplastic thyroid cancer is resistant to radioactive iodine (125I) because of the lack of NIS (sodium/iodide symporter) expression. Both valproic acid (VPA) and resveratrol have been shown to induce NIS and other differentiation marker expression and affect proliferation and apoptosis; therefore, we investigated the effects of VPA and resveratrol on the D3 clone.26, 27, 28
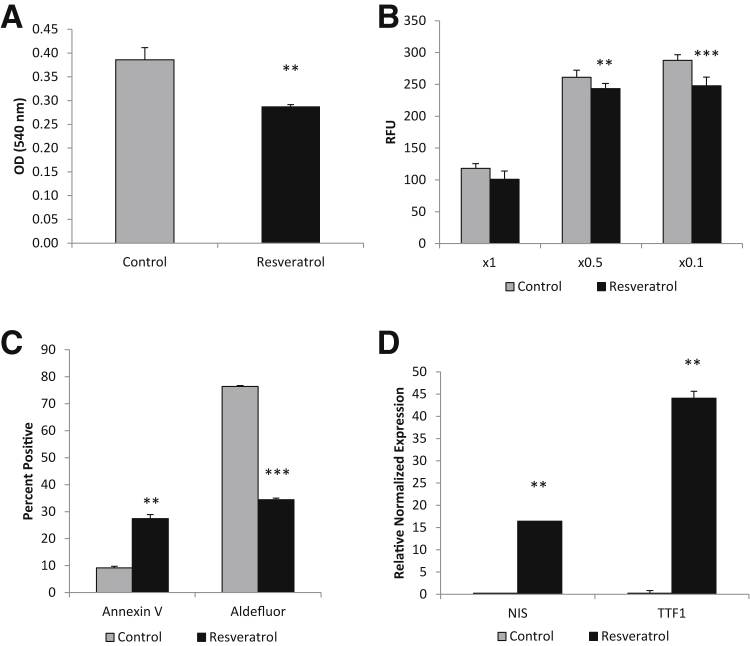
D3 spheroid cells were collected and dissociated into single cells and cultured for 72 hours with 50 μmol/L resveratrol in spheroid media or control spheroid media containing dimethyl sulfoxide. Resveratrol significantly reduced the proliferation of D3 cells, as measured by an MTT assay (Figure 11A), and significantly reduced the invasive potential through a BME matrix assay (Figure 11B). The effect on the proliferation rate may be, in part, because of the significant increase in annexin V staining, as seen by flow cytometry analysis (Figure 11C). In addition, resveratrol treatment also greatly decreased the high Aldefluor activity in treated D3 cells (Figure 11C). To determine whether the decrease in Aldefluor activity was because of differentiation of the cancer stem cells, thyroid differentiation markers NIS and TTF-1 were tested. Both NIS and TTF-1 were significantly increased in the resveratrol-treated cells by 15- and 40-fold, respectively (Figure 11D).
Figure 11.
Resveratrol induces differentiation of cancer stem-like cells (CSCs). A: MTT assay showing 72 hours resveratrol treatment at 50 μmol/L significantly reduces proliferation of CSCs. B: Resveratrol significantly decreases the invasive potential of CSCs at both ×0.5 and ×0.1 basement membrane extract concentrations. C: Flow cytometric data indicating resveratrol significantly increases apoptosis and decreases Aldefluor activity of CSCs. D: RT-PCR results indicating resveratrol induces thyroid differentiation markers of CSCs. ∗∗P < 0.01, and ∗∗∗P < 0.001 versus controls. RFU, relative fluorescence unit.
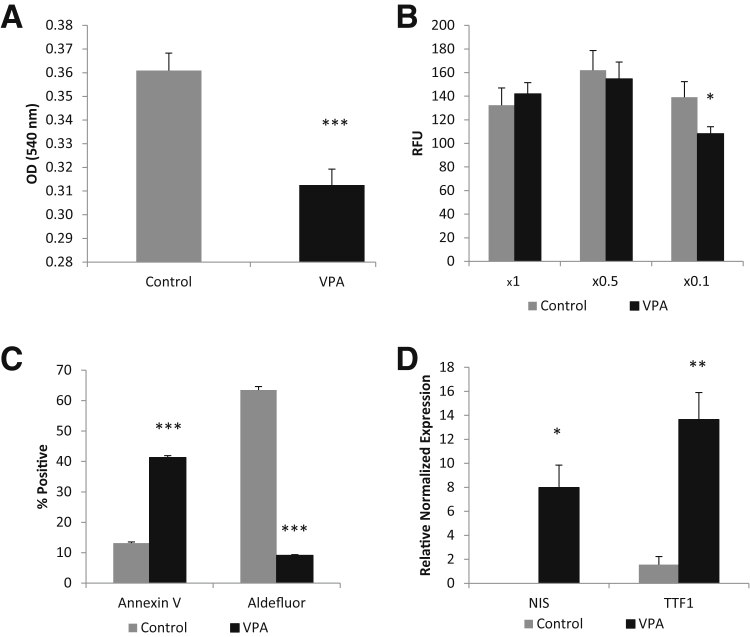
D3 cells were dissociated and incubated with 1 mmol/L VPA in spheroid media or control spheroid media for 7 days. Similar to the resveratrol experiments, VPA significantly reduced the proliferation (Figure 12A) and invasive potential (Figure 12B) of the D3 cells. VPA also greatly increased apoptosis, as assessed by annexin V staining, and significantly reduced the Aldefluor activity (Figure 12C). In addition, VPA also significantly increased thyroid differentiation markers (Figure 12D).
Figure 12.
Valproic acid (VPA) induces differentiation of cancer stem-like cells (CSCs). A: Seven day VPA in vitro treatment of CSCs greatly reduces proliferative properties, as shown by MTT assay. B: VPA decreases invasion slightly at a basement membrane extract concentration of ×0.1. C: Flow cytometric analysis showing that VPA treatment dramatically increases apoptosis and decreases Aldefluor positivity. D: RT-PCR data showing VPA induces thyroid differentiation markers in CSCs. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus controls. RFU, relative fluorescence unit.
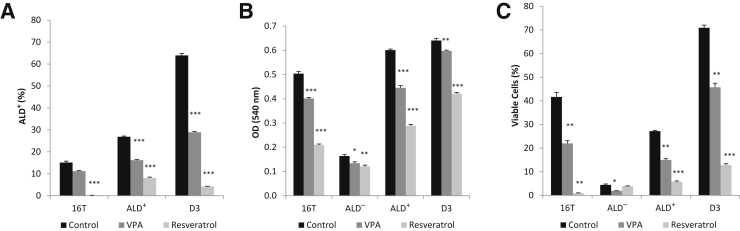
To determine whether the effects of resveratrol and VPA treatment were unique to the D3 clone or if its effect can be seen in the parental strain and its ALD− and ALD+ fractions, freshly sorted ALD− and ALD+ fractions and dissociated 16T and D3 cells were incubated for 3 days with 1 mmol/L VPA, 50 μmol/L resveratrol, or control (dimethyl sulfoxide) in spheroid media. Three days of treatment was chosen because of the vulnerability of the 16T (approximately 95% ALD−) and the ALD− fraction to the serum-free media. Aldefluor activity was significantly reduced in all cell fractions across treatment groups (Figure 13A), with the exception of the VPA-treated 16T cells in which the Aldefluor activity was slightly reduced but not found to be significant (P < 0.056). The ALD− fraction was tested; however, the Aldefluor activity remained negative for all treatment groups in the ALD− fraction (data not shown). Proliferation rates (Figure 13B) and cell viability (Figure 13C) were significantly affected for all cell fractions across all treatment groups; however, the ALD− fraction was less affected than the 16T, ALD+, and D3 cell groups. In addition, the ALD− fraction was severely affected by the serum-free nature of the spheroid media, because the vast majority (>95%) of the cells died within 3 days in the serum-free culture conditions.
Figure 13.
Resveratrol and valproic acid (VPA) treatment affects the parental 16T, its Aldefluor (ALD)− and ALD+ fractions, and the D3 clone. The unsorted 16T, freshly isolated ALD− and ALD+ fractions, and the D3 clone were incubated with 1 mmol/L VPA, 50 μmol/L resveratrol, or control (dimethyl sulfoxide) in spheroid media for 3 days. A: Both VPA and resveratrol significantly reduce Aldefluor activity on all fractions of cells tested, with the exception of VPA on the parental 16T, which reduces the Aldefluor activity yet it is not significant. The ALD− fraction was tested and remains negative for all treatments (data not shown). B: MTT assay showing significantly reduced proliferation in all cell fractions tested with the ALD− fraction the least affected. The ALD− fraction proliferation rate is severely affected by the serum-free nature of the spheroid media. C: Percentage of viable cells for all cell fractions and treatment groups showing the reduction of viable cells. This also shows the severity of the serum-free media effect on the ALD− fraction. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus the control group. OD, optical density.
Discussion
Since the development of the CSC theory, researchers have examined various cancers for markers to identify and isolate CSCs for further characterization. CD133 and SSEA1 were reported to be reliable markers of glioblastoma CSCs,29 whereas CD44+/CD24− were used to characterize breast cancer CSCs.30 Many reports indicate that increased activity of the enzyme ALDH is a useful marker of CSC in many malignant tissues.31 However, CSC markers appear to be tissue specific because several reports indicate that thyroid tissues are devoid of CD133 and are highly positive for CD44 and ALDH expression.22, 24 Todaro et al24 found that primary thyroid cancer cells that exhibited high ALDH expression/function could be isolated and examined in vitro and in vivo, because they formed spheres with self-renewal properties and recapitulated the parental tumors in xenografts. Given this information, several ATC and PTC cell lines were analyzed for ALD+ populations and, once isolated, investigated for their ability to form spheres and molecular characteristics. However, previous clonogenic CSC lines derived from thyroid cancer have not been previously reported. Herein, we report, for the first time, the generation and maintenance of CSC clones from an ATC cell line. These clones have been maintained in culture for >1 year and show the characteristic features of thyroid CSCs, including thyrosphere formation, expression of high levels of ALDH and of the stem cell markers SOX2, OCT4, and NANOG, predominantly symmetric division, rapid growth in vivo, and the ability to differentiate and lose stem cell characteristics when exposed to serum or treated with resveratrol and VPA.
Self-renewal is considered a key feature of CSCs.32 EMT processes have been found to be correlated with development of stemness in breast carcinoma14, 33 and in thyroid carcinomas.18 To address the ability to undergo EMT, 16T cells were treated with TGF-β1 to induce EMT in a serum-free, TGF-β1 treatment model that our laboratory developed previously.18 Interestingly, TGF-β1 treatment exhibited growth inhibition, indicating that the reduced levels of smad proteins were functional in the TGF-β pathway machinery. Furthermore, TGF-β1 treatment significantly up-regulated EMT and stem cell markers in a time-dependent model. To the best of our knowledge, this is the first time an ATC cell line has been induced to undergo EMT. The implication of this observation is that ATCs, like PTCs, may also undergo EMT before metastasizing to a secondary site.
Another characteristic of CSCs is increased tumorigenicity and the recapitulation of the parental tumor. ALD+ and ALD− 16T cells, as well as unsorted bulk cells, were injected s.c. into immunocompromised mice at different cell concentrations and tumor formation assessed. The ALD+ cells were markedly more tumorigenic than the ALD− or unsorted cells. They grew much larger tumors at an accelerated rate over the ALD− or bulk cells. Serial passaging of the ALD+ tumors continued to exhibit accelerated growth rates, increasing with each passage. In addition, with each serial passage, stem cell marker expression increased significantly, as well as the markers of more aggressive thyroid carcinoma CMET and epidermal growth factor receptor. Later passages of the ALD+ tumors also became more invasive, with some tumors infiltrating the surrounding muscle. All passages were able to recapitulate the morphological characteristics of the parental tumor, including the Aldefluor-negative tumor. However, the mitotic index was increased with each serial passage.
Some ALD+ cells that were sorted at a density of one cell per well readily replicated and formed clonal spheres. These clonal spheres have significantly high Aldefluor activity and up-regulated stem cell markers. One clone, D3, exhibits a rapid rate of proliferation, >90% Aldefluor activity and >80% symmetrical division, whereas a second clone, D6, also showed >65% Aldefluor activity; however, the proliferation rate was much slower. Furthermore, the clonal tumors maintained a significantly high stem cell marker expression. These findings indicate that we have generated clonal cancer stem cell lines that can be a useful tool in the investigations of therapies for the targeting of CSCs in aggressive or invasive thyroid carcinomas.
Previously, Yu et al27 found that resveratrol (3,5,4′-trihydroxy-trans-stilbene), a phytoalexin, induced differentiation markers, including NIS, and reduced proliferation in anaplastic thyroid carcinoma. On incubation of the D3 CSC line with resveratrol for 72 hours, a significant reduction in proliferation and an increase in apoptosis were observed. Treatment with resveratrol significantly up-regulated the thyroid differentiation markers NIS and TTF-1, and significantly reduced the Aldefluor activity. In addition, resveratrol treatment significantly reduced the invasive properties of the D3 line. Resveratrol may be useful in the treatment of undifferentiated cancers with a high CSC content. On differentiation of CSCs, conventional therapies may be more effective in eliminating cancers, their reoccurrences, and their metastatic capabilities.
Valproic acid (2-propyl pentanoic acid, VPA) has been reported to interfere with multiple regulatory mechanisms that are critical to carcinogenesis and cancer progression, such as the extracellular signal-regulated kinase, AKT, and phosphatidylinositol 3-kinase pathways, glycogen synthase kinase 3 α and β, and as a histone deacetylase inhibitor.26, 28 They observed an increase in differentiation, apoptosis, and gene expression, as well as a decrease in cell proliferation in cancer cells in vitro. The efficacy of VPA as a treatment for patients of some cancers is controversial, where some clinical trials have found benefits, others have not. We investigated the effects of 7 days in vitro VPA treatment on the D3 clonal line. It was found that VPA induced a highly significant reduction in proliferation and an increase in apoptosis. VPA treatment also dramatically reduced the Aldefluor activity while increasing the differentiation markers NIS and TTF-1. Furthermore, VPA also decreased the D3 clones' invasive potential slightly, although significantly. Similarly to resveratrol, VPA shows promise as an adjuvant in the targeting of CSCs in multiple therapeutic strategies.
In summary, high expression/function of the ALDH enzyme within thyroid cancer cells is a reliable marker for thyroid CSCs, and ATCs contain a greater number of CSCs than differentiated thyroid cancers. The higher percentage of CSCs in ATC might explain the more aggressive behavior and may contribute to the resistance to therapies. More important, we generated clonal spheroid CSC lines that exhibited more enhanced CSC properties and superior tumorigenicity than the freshly isolated ALD+ cells. Resveratrol and VPA treatments of one of the clonal lines down-regulated stem cell markers, including ALDH expression, decreased proliferation and invasiveness, induced apoptosis, and up-regulated thyroid differentiation markers, including NIS, which is important for radioiodine uptake used in the treatment of aggressive thyroid cancers.26 Although the effects of resveratrol and VPA treatments are not unique to the CSC fraction of ATCs, their actions on CSC and non-CSC fractions may render ATCs more susceptible to conventional therapies. In conclusion, CSC clonal lines have been established for the first time, which should be powerful and useful tools for the study of effective CSC-targeted therapies.
Acknowledgments
We thank Drs. John A. Copland III (Mayo Clinic, Jacksonville, FL), Rebecca E. Schweppe (University of Colorado, Denver, CO), and Daniel T. Ruan (Brigham and Women's Hospital, Boston, MA) for the THJ-16T and THJ-21T, BCPAP, and TPC-1 cell lines, respectively, and the staffs of the Translational Research in Pathology, Flow Cytometry, 3P, and Experimental Pathology laboratories (University of Wisconsin) for their services.
Footnotes
Supported by Department of Pathology startup funding (R.V.L.), NIH-RO1 CA122225, American Cancer Society MEN2 Thyroid Cancer Professorship (H.C.), and Carbone Cancer Center support grant P30 CA014520 [Translational Research in Pathology, Flow Cytometry, 3P, and Experimental Pathology laboratories (University of Wisconsin)].
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2016.02.003.
Supplemental Data
Quantitative RT-PCR analysis of transforming growth factor (TGF)-βR1, SMAD2, SMAD4, and SMAD7 expression of the TGF-β pathway components of normal (NTHY-ori-3), papillary thyroid carcinoma (PTC; TPC-1 and BCPAP), and anaplastic thyroid carcinoma (ATC; THJ-16T, THJ-21T, and 8505C) thyroid cell lines. The anaplastic thyroid cell lines have decreased TGF-β pathway components compared with the PTC and normal cell lines. ∗P < 0.05, ∗∗P < 0.01 NTHY versus PTC lines; †P < 0.05, ††P < 0.01, and †††P < 0.001 NTHY versus ATC lines; ‡P < 0.05 NTHY versus BCPAP.
Histological appearance of tumors after in vivo passages of THJ-16T Aldefluor (ALD)+ tumor cells. Formalin-fixed, paraffin-embedded tissue sections were cut (4 μm thick) and stained with hematoxylin and eosin. Mitotic sounds were performed at ×40 magnification using a 1 × 1-cm square grid in the ocular of the microscope. At least 500 cells per case were enumerated. In vivo passaging of ALD+ tumor cells significantly increases mitotic index with each passage. ∗∗P < 0.01.
Histological comparison of THJ-16T tumor and clonal sphere tumor. Formalin-fixed, paraffin-embedded tissue sections were cut (4 μm thick) and stained with hematoxylin and eosin. The THJ-16T tumor in A and the clonal sphere tumor in B show sheets of tumor cells with large nuclei and moderate amounts of eosinophilic cytoplasm. Mitotic figures are present in both sets of xenografts, but more prominent in the D3 clonogenic cells, and the tumor cells from the D3 clone are more mesenchymal or spindle shaped. Arrows indicate mitotic cells. Scale bar = 100 μm.
References
- 1.Sipos J.A., Mazzaferri E.L. Thyroid cancer epidemiology and prognostic variables. Clin Oncol (R Coll Radiol) 2010;22:395–404. doi: 10.1016/j.clon.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Davies L., Welch H.G. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Ahn H.S., Kim H.J., Welch H.G. Korea's thyroid cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014;371:1765–1767. doi: 10.1056/NEJMp1409841. [DOI] [PubMed] [Google Scholar]
- 4.Albores-Saavedra J., Hernandez M., Sanchez-Sosa S., Simpson K., Angeles A., Henson D.E. Histologic variants of papillary and follicular carcinomas associated with anaplastic spindle and giant cell carcinomas of the thyroid: an analysis of rhabdoid and thyrogloblulin inclusions. Am J Surg Pathol. 2007;31:729–736. doi: 10.1097/01.pas.0000213417.00386.74. [DOI] [PubMed] [Google Scholar]
- 5.Schulenburg A., Bramswig K., Herrmann H., Karlic H., Mirkina I., Hubmann R., Laffer S., Marian B., Shehata M., Krepler C., Pehamberger H., Grunt T., Jager U., Zielinski C.C., Valent P. Neoplastic stem cells: current concepts and clinical perspectives. Crit Rev Oncol Hematol. 2010;76:79–98. doi: 10.1016/j.critrevonc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Takano T. Fetal cell carcinogenesis of the thyroid: a modified theory based on recent evidence. Endocr J. 2014;61:311–320. doi: 10.1507/endocrj.ej13-0517. [DOI] [PubMed] [Google Scholar]
- 7.Zhang P., Zuo H., Ozaki T., Nakagomi N., Kakudo K. Cancer stem cell hypothesis in thyroid cancer. Pathol Int. 2006;56:485–489. doi: 10.1111/j.1440-1827.2006.01995.x. [DOI] [PubMed] [Google Scholar]
- 8.Klonisch T., Hoang-Vu C., Hombach-Klonisch S. Thyroid stem cells and cancer. Thyroid. 2009;19:1303–1315. doi: 10.1089/thy.2009.1604. [DOI] [PubMed] [Google Scholar]
- 9.Thomas D., Friedman S., Lin R.Y. Thyroid stem cells: lessons from normal development and thyroid cancer. Endocr Relat Cancer. 2008;15:51–58. doi: 10.1677/ERC-07-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitsutake N., Iwao A., Nagai K., Namba H., Ohtsuru A., Saenko V., Yamashita S. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797–1803. doi: 10.1210/en.2006-1553. [DOI] [PubMed] [Google Scholar]
- 11.Fierabracci A. Identifying thyroid stem/progenitor cells: advances and limitations. J Endocrinol. 2012;213:1–13. doi: 10.1530/JOE-11-0183. [DOI] [PubMed] [Google Scholar]
- 12.Davies T.F., Latif R., Minsky N.C., Ma R. Clinical review: the emerging cell biology of thyroid stem cells. J Clin Endocrinol Metab. 2011;96:2692–2702. doi: 10.1210/jc.2011-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin R.Y. Thyroid cancer stem cells. Nat Rev Endocrinol. 2011;7:609–616. doi: 10.1038/nrendo.2011.127. [DOI] [PubMed] [Google Scholar]
- 14.Mani S.A., Guo W., Liao M.J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin N., Campbell L.L., Polyak K., Brisken C., Yang J., Weinberg R.A. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. PMC2728032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J., Weinberg R.A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari N., Gheldof A., Tatari M., Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22:194–207. doi: 10.1016/j.semcancer.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Buehler D., Hardin H., Shan W., Montemayor-Garcia C., Rush P.S., Asioli S., Chen H., Lloyd R.V. Expression of epithelial-mesenchymal transition regulators SNAI2 and TWIST1 in thyroid carcinomas. Mod Pathol. 2013;26:54–61. doi: 10.1038/modpathol.2012.137. PMCID: PMC 3559085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardin H., Guo Z., Shan W., Montemayor-Garcia C., Asioli S., Yu X.-M., Harrison A.D., Chen H., Lloyd R.V. The roles of the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p in papillary thyroid carcinoma progression. Am J Pathol. 2014;184:2342–2354. doi: 10.1016/j.ajpath.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Z., Hardin H., Lloyd R.V. Cancer stem-like cells and thyroid cancer. Endocr Relat Cancer. 2014;21:T285–T300. doi: 10.1530/ERC-14-0002. PMCID: PMC 24788702. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia P., Tsumagari K., Abd Elmageed Z.Y., Friedlander P., Buell J.F., Kandil E. Stem cell biolgy in thyroid cancer: insights for novel therapies. World J Stem Cells. 2014;6:614–619. doi: 10.4252/wjsc.v6.i5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marlow L.A., D'Innocenzi J., Zhang Y., Rohl S.D., Cooper S.J., Sebo T., Grant C., McIver B., Kasperbauer J.L., Wadsworth J.T., Casler J.D., Kennedy P.W., Highsmith W.E., Clark O., Milosevic D., Netzel B., Cradic K., Arora S., Beaudry C., Grebe S.K., Silverberg M.L., Azorsa D.O., Smallridge R.C., Copland J.A. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J Clin Endocrinol Metab. 2010;95:5338–5347. doi: 10.1210/jc.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W., Reeb A.N., Sewell W.A., Elhomsy G., Lin R.Y. Phenotypic characterization of metastatic anaplastic thyroid cancer stem cells. PLoS One. 2013;8:e65095. doi: 10.1371/journal.pone.0065095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma R., Minsky N., Morsed S.A., Davies T.F. Stemness in human thyroid cancers and derived cell lines: the role of assymetrically dividing cancer stem cells resistant to chemotherapy. J Clin Endocrinol Metab. 2014;99:E400–E409. doi: 10.1210/jc.2013-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Todaro M., Lovino F., Eterno V., Cammareri P., Gambara G., Espina V., Gulotta G., Dieli F., Giordano S., De Maria R., Stassi G. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. doi: 10.1158/0008-5472.CAN-10-1994. [DOI] [PubMed] [Google Scholar]
- 25.Sippel R.S., Carpenter J.E., Kunnimalaiyaan M., Lagerholm S., Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastointest Liver Physiol. 2003;285:G245–G254. doi: 10.1152/ajpgi.00420.2002. [DOI] [PubMed] [Google Scholar]
- 26.Furuya F., Shimura H., Suzuki H., Taki K., Ohta K., Haraguchi K., Onaya T., Endo T., Kobayashi T. Histone deacetylase inhibitors restore radioiodide uptake and retention in poorly differentiated and anaplastic thyroid cancer cells by expression of the sodium/iodide symporter thyroperoxidase and thyroglobulin. Endocrinology. 2004;145:2865–2875. doi: 10.1210/en.2003-1258. [DOI] [PubMed] [Google Scholar]
- 27.Yu X.M., Jaskula-Sztul R., Ahmed K., Harrison A.D., Kunnimalayaan M., Chen H. Resveratrol induces differentiation marker expression in anaplastic thyroid carcinoma via activation of notch 1 signaling and suppresses cell growth. Mol Cancer Ther. 2013;12:1276–1287. doi: 10.1158/1535-7163.MCT-12-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostrouchova M., Kostrouch Z., Kostrouchova M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol (Praha) 2007;53:37–49. [PubMed] [Google Scholar]
- 29.Son M.J., Woolard K., Nam D.H., Lee J., Fine H.A. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Beca F.F., Caetano P., Gerhard R., Alvarenga A., Gomes M., Paredes J., Schmitt F. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol. 2013;66:187–191. doi: 10.1136/jclinpath-2012-201169. [DOI] [PubMed] [Google Scholar]
- 31.Liu S.Y., Zheng P.S. High aldehyde dehydrogenase activity identifies cancer stem cells in human cervical cancer. Oncotarget. 2013;4:2462–2475. doi: 10.18632/oncotarget.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Brien C.A., Kreso A., Jamieson C.H.M. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 33.Raimondi C., Gradilone A., Naso G., Vincenzi B., Petracca A., Nicolazzo C., Palazzo A., Saltarelli R., Spremberg F., Cortesi E., Gazzaniga P. Epithelial-mesenchymal transition and stemness features in circulating tumor cells from breast cancer patients. Breast Cancer Res Treat. 2011;130:449–455. doi: 10.1007/s10549-011-1373-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quantitative RT-PCR analysis of transforming growth factor (TGF)-βR1, SMAD2, SMAD4, and SMAD7 expression of the TGF-β pathway components of normal (NTHY-ori-3), papillary thyroid carcinoma (PTC; TPC-1 and BCPAP), and anaplastic thyroid carcinoma (ATC; THJ-16T, THJ-21T, and 8505C) thyroid cell lines. The anaplastic thyroid cell lines have decreased TGF-β pathway components compared with the PTC and normal cell lines. ∗P < 0.05, ∗∗P < 0.01 NTHY versus PTC lines; †P < 0.05, ††P < 0.01, and †††P < 0.001 NTHY versus ATC lines; ‡P < 0.05 NTHY versus BCPAP.
Histological appearance of tumors after in vivo passages of THJ-16T Aldefluor (ALD)+ tumor cells. Formalin-fixed, paraffin-embedded tissue sections were cut (4 μm thick) and stained with hematoxylin and eosin. Mitotic sounds were performed at ×40 magnification using a 1 × 1-cm square grid in the ocular of the microscope. At least 500 cells per case were enumerated. In vivo passaging of ALD+ tumor cells significantly increases mitotic index with each passage. ∗∗P < 0.01.
Histological comparison of THJ-16T tumor and clonal sphere tumor. Formalin-fixed, paraffin-embedded tissue sections were cut (4 μm thick) and stained with hematoxylin and eosin. The THJ-16T tumor in A and the clonal sphere tumor in B show sheets of tumor cells with large nuclei and moderate amounts of eosinophilic cytoplasm. Mitotic figures are present in both sets of xenografts, but more prominent in the D3 clonogenic cells, and the tumor cells from the D3 clone are more mesenchymal or spindle shaped. Arrows indicate mitotic cells. Scale bar = 100 μm.