Abstract
Purpose: To present a systematic review and meta-analysis comparing the transradial approach for aortoiliac and femoropopliteal interventions to the traditional transfemoral access. Methods: A search of the public domain databases MEDLINE, SCOPUS, Web of Science, and Cochrane Library Databases was performed to identify studies related to the use of the transradial approach for infra-aortic procedures. Meta-analysis was used to compare the transradial to the transfemoral route in terms of procedure success, complications, procedure parameters, and hospital length of stay. Results are presented as the odds ratio (OR) and 95% confidence interval (CI). Results: Nineteen studies containing 638 patients with transradial access for lower limb interventions were selected. Lesions were treated from the aortic bifurcation down to the popliteal artery. The mean technical success rate was 90.9%, conversion to a transfemoral approach was necessary in 9.9%, and complications were reported in 1.9%. The meta-analysis included 4 comparative studies involving 114 transradial and 208 transfemoral procedures. There was no significant advantage of either approach in terms of procedure success (OR 5.0, 95% CI 0.49 to 50.83, p=0.17), but the risk of developing a complication was significantly lower (OR 0.25, 95% CI 0.07 to 0.86, p=0.03) with the transradial approach. Conclusion: Transradial access for lower limb endovascular interventions can be performed with comparable technical success and a lower overall complication profile compared to transfemoral access.
Keywords: access route, angioplasty, aortoiliac interventions, common femoral artery, common iliac artery, external iliac artery, femoropopliteal segment, occlusion, peripheral artery disease, popliteal artery, radial artery, stenosis, stent, superficial femoral artery
Introduction
In 1989, the first studies were published in which the radial artery was used as an access vessel for percutaneous coronary angiography and intervention.1 Today, radial artery access has largely replaced the femoral artery as standard access for coronary interventions because it reduces by up to 60% the access-site complications, such as hemorrhage, hematoma, vascular closure device (VCD) failure, or major embolic events.2 Furthermore, transradial access has the same technical success rates as transfemoral access and shorter hospitalization3 in percutaneous coronary interventions. Large randomized trials comparing femoral and radial access even reported an increased overall survival in patients with acute coronary syndrome who were treated via the radial artery.2
Owing to its reduced risk of access-related complications in coronary interventions, the transradial route became more popular over time, and smaller devices were produced. This device development created the opportunity to treat noncoronary lesions in a variety of settings, including renal/visceral interventions, uterine artery embolization, peripheral interventions, and endoleak repair among others, with high technical success and a low incidence of major (eg, pseudoaneurysm and seizure, 0.13%) and minor (eg, hematoma, radial artery occlusion or spasm, arm pain, 2.4%) complications.4
It is common to perform peripheral aortoiliac interventions through the contralateral femoral artery,5,6 but current guidelines do not indicate a preferred access artery.7 Over the past few years, use of transradial and transulnar access in aortoiliac and infrainguinal procedures has grown despite its disadvantages,8,9 such as the long learning curve, challenges owing to a tortuous subclavian artery or aortic arch, and the passage of devices to the target vessel over longer distances through smaller arteries.
To evaluate if the transradial route in infra-aortic interventions has similar advantages to those documented for coronary procedures a systematic review and meta-analysis were conducted to assess the efficacy and safety of transradial access in the management of aortoiliac, femoral, and popliteal artery disease.
Methods
Search Strategy
This systematic review assembled clinical evidence using a prespecified protocol and an explicit, reproducible plan for literature search and synthesis as recommended by the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) guidelines.10
The MEDLINE (PubMed platform), SCOPUS Web of Science, and the Cochrane Library databases were searched by 2 authors (M.M.M., E.M.) up to April 30, 2017, using the following free text and medical subject headings [MeSH]: lower extremities, lower limb, iliac, profunda, popliteal, femoralis, tibiofibularis, tibialis, fibularis, dorsalis pedis artery, peripheral vascular interventions, side effects, feasibility, safety, complications, Endovascular Procedures [MeSH], Atherectomy [MeSH], transradial, radial access, radial approach, radial artery, Radial Artery [MeSH], transradial, transulnar, ulnar artery and Ulnar Artery [MeSH]. No linguistic or geographic filters were applied in the search. The database search was supplemented by a manual search of the reference lists of the included studies the “related articles” function provided in each database.
Selection Criteria
Study inclusion was performed at 2 levels by 2 authors (M.M.M., E.M.) using predefined inclusion and exclusion criteria. Any disagreements at any stage were resolved by consensus with a senior author (A.M.T.L.C.). For each eligible study, data elements were extracted from full-text versions by one author and a second author verified the extractions with the original sources.
Articles suitable for selection were randomized controlled trials, non-randomized controlled trials, other prospective cohort studies, and observational studies. Studies involving routes other than the transradial or the transulnar for infra-aortic revascularization, technical aspects of the transradial access, animal studies, and non–English language articles were excluded.
Data Collection
Two authors (M.M.M., E.M.) independently extracted the following data from each included study: first author, year, and type of publication; size of study population; severity of peripheral artery disease as determined by Fontaine or Rutherford classification; morphology and characteristics; treated target vessel; degree of vessel stenosis as determined by angiographic assessment; radial artery access site; need for >1 percutaneous approach or conversion to transfemoral access; maximal sheath size; maximal wire length; and stent type. Data was also retrieved on the following outcomes: angiographic success at completion of intervention; closure technique; peri-interventional anticoagulation therapy; angiographic patency of revascularized target lesion at follow-up; major and minor complications; radial artery patency on completion of procedure and at the end of follow-up; hospital length of stay (LOS); and reasons for unsuccessful procedures. All data were extracted or extrapolated from text, tables, and figures. Primary outcomes were procedure success and complications; secondary outcomes were LOS, fluoroscopy and procedure times, and contrast volume.
Statistical Analysis
The meta-analysis included studies specifically comparing the transradial access with the transfemoral access for endovascular revascularization in the aortoiliac and femoropopliteal segments. Pooled meta-analysis was performed for all complications [bleeding, VCD failure, pseudoaneurysm, arteriovenous (AV) fistulas, hematoma >5 cm, death, stroke, radial artery rupture], procedure success, LOS, procedure and fluoroscopy times, and contrast volume.
Random-effects meta-analyses were performed using the Mantel-Haenszel method for dichotomous data to estimate pooled odds ratios (OR) or mean differences (MD). Statistical heterogeneity was assessed using I2 statistics. Methodological quality of all included studies was assessed using the checklist recommended by the 9-point Newcastle Ottawa scale (NOS).11 The meta-analysis was run in RevMan (version 5.3; The Cochrane Collaboration; http://community.cochrane.org/help/tools-and-software/revman-5).
Results
Study Selection
The search identified 279 articles (Figure 1), which were culled to 176 potentially eligible studies after removing duplicates. One article12 was added from a manual search of references. In all 149 studies were excluded after review of their titles and abstracts owing mainly to (1) revascularization of arteries other than those specified in the study protocol, (2) the use of other access methods for infra-aortic interventions, and (3) studies on the effectiveness of different stents in peripheral interventions. Among the 27 studies remaining, 2 studies were excluded because the full article was in Chinese13 or was not available,14 and 5 reviews12,15–18 were excluded because they were narrative or focused on technical aspects of transradial access and did not contain patient data. The final excluded article focused on transradial angiography and stenting of noncoronary arteries but did not present the results separately per treated vessel, making it impossible to differentiate between renovisceral and infra-aortic interventions.19
Figure 1.
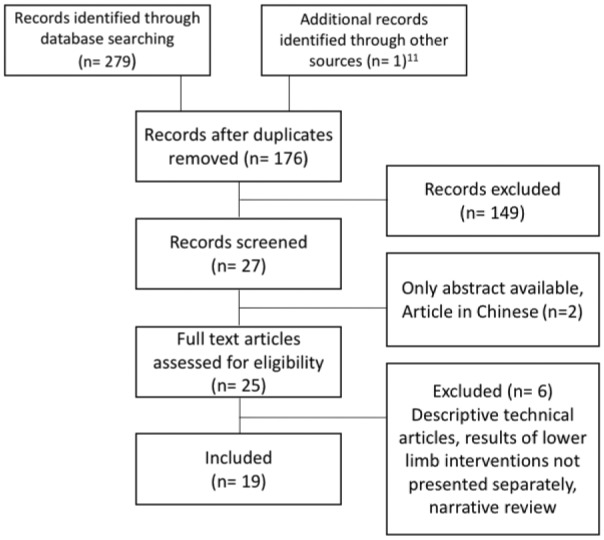
Flowchart of the systematic review.
The final systematic review included 19 studies8,9,20–36 (4 prospective cohort studies, 1 retrospective matched cohort study, 3 retrospective cohort studies, 2 case-control studies, 3 case series, and 6 case reports). All studies were hospital-based and consisted of either vascular surgery patients or individuals presenting to a vascular clinic for follow-up. Study sample size ranged from 1 to 156 patients. Four studies20–23 were eligible for the comparative meta-analysis; these 4 studies had a NOS score 7 to 9 out of a maximum of 9 (Table 1). For the 10 cohort/case-control studies the mean NOS was 6.7.
Table 1.
Patient Characteristics and Quality Assessment.
| First Author | N | Men, % | BMI,a kg/m2 | Fontaine | Rutherford | Lesion Location | Occlusion, % | NOS |
|---|---|---|---|---|---|---|---|---|
| Trani 200922 | 12 | NR | NR | 8: 2b 4: 3 |
NR | SFA | NR | 7 |
| Staniloae 201021 | 27 | 63 | 27.9a | NR | 2.9a | CIA, EIA | 27 | 8 |
| Cortese 201220 | 21 | 53 | NR | NR | 13: 2/3 8: 4/5 |
IA | 25 | 9 |
| Roy 201623 | 54 | 61 | 25.3a | NR | 23: 2, 21: 3, 7: 4, 3: 5 | AB, CFA, IA, SFA, PopA | 41 | 7 |
| Lorenzoni 201133 | 25 | 56 | NR | NR | NR | EIA, CIA, CFA, SFA, PopA | 25 | 5 |
| Cortese 201436 | 147 | 76 | NR | NR | 121: 2–4, 28: 5/6 | CIA, EIA | NR | 6 |
| Lorenzoni 201425 | 110 | 80 | NR | NR | NR | SFA | 22 | 6 |
| Shinozaki 201424 | 30 | 70 | NR | 12: 1, 19: 2, 1: 3 |
NR | IA | NR | 4 |
| Coscas 20158 | 24 | 98 | 4 >30 | NR | NR | CFA, IA, SFA, PopA | NR | 6 |
| Ruzsa 20169 | 156 | 70 | 25.9a | 12:2a, 92: 2b, 28: 3, 19: 4 | NR | EIA, CFA, CIA | NR | 3 |
| Flachskampf 200532 | 1 | 100 | NR | 2b | NR | CIA | 33 | — |
| Staniloae 200631 | 1 | 0 | NR | NR | NR | CIA | NR | — |
| Watanabe 200727 | 3 | 66 | NR | 1: 1, 1: 2, 1: 3 | NR | EIA, CIA | NR | |
| Sanghvi 200828 | 14 | 66 | 26.9a | NR | NR | IA, SFA | NR | — |
| Trani 201035 | 2 | 100 | NR | 2b | NR | SFA | NR | — |
| Pitta 201129 | 1 | 100 | NR | 2b | NR | CIA | NR | — |
| Antov 201330 | 6 | 100 | 26.9a | NR | 3: 3, 3: 4 | CIA, SFA | 77 | |
| Harruna 201326 | 1 | 100 | NR | 2b | NR | CIA | NR | — |
| Shinozaki 201634 | 1 | 100 | NR | NR | NR | EIA | 100 | — |
Abbreviations: AB, aortic bifurcation; BMI, body mass index; CFA, common femoral artery; CIA, common iliac artery; EIA, external iliac artery; IA, iliac artery; NOS, Newcastle Ottawa scale (maximum score 9); NR; not reported; PopA, popliteal artery; SFA, superficial femoral artery.
Mean value.
Patient and Lesion Characteristics
A total of 638 patients underwent infra-aortic interventions via the transradial access for lesions involving the aortic bifurcation (n=4),23 external iliac artery (EIA; n=23),9,21,27,33,34,36 common iliac artery (CIA; n=295),9,20,21,23,24,26–33,36 common femoral artery (CFA; n=16),8,9,23,33 superficial femoral artery (SFA; n=57),8,22,23,25,28,30,33,35 or popliteal artery (n=8),8,23,33 though not all 19 studies reported the exact location of the treated vessel. Of 865 lesions treated, 146 (16.9%) were occlusions.
Severity of presenting symptoms was reported as either the Fontaine classification or Rutherford categories in 13 studies9,20–24,26,27,29,30,32,35,36 comprising 363 patients. Fontaine classifications ranged from I to IV and Rutherford categories ranged from 2 to 6 (Table 1). Presentation of the Rutherford category differed among the studies. Only Staniloae et al21 presented the Rutherford data as a mean value (Table 1).
Radial Access
Eleven articles reported a rationale as to why radial access was performed.8,9,21,23,24–26,28–30,32 Reasons were patient or interventional characteristics, such as obesity, absent iliac pulses, severe SFA disease, inability to remain supine, expectation of difficulties with femoral approach, the use of antegrade angiography, and downstream protection. Two studies treated all patients via the radial artery when it was patent and a sheath with a sufficient length was available.9,29 The other studies based their access choice on the experience of the interventionist.
Radial access side was reported in 18 studies. The left radial artery was utilized in 14 studies8,9,21–23,24,25,27–29,31,33–35 comprising 223 patients and the right radial artery was utilized in 9 studies8,20,22,23,26,30,32,33,35 comprising 224 patients. Some studies did not report access side for all patients. Among all 19 studies, 63 (9.9%) patients required conversion to the transfemoral approach or needed >1 percutaneous approach.8,9,20,21,23,25,28,36
Interventional Characteristics
Most studies reported the maximum sheath size (Table 2), which was 6-F in 12 studies, 7-F in 4,28–30 and 5-F in 1 case.26 The guidewire length was reported in the same studies. The most commonly used guidewire length was 300 cm (105 cm,27 120 cm,24 125 cm,9,32 260 cm,8,20 and 300 to 400 cm.25) Self-expanding stents were used in 11 studies8,9,22,24,25,28,30,31,33,35,36 and balloon-expandable stents in 10 studies.8,9,20,25–27,29–32,36 When reported, hemostasis was always achieved by manual compression,9,29,31 compression bandage,20,22,28,31,35 or by using a compression device.23,25,29,33 Post-interventional anticoagulation was reported in 10 studies8,9,20,22,25,26,31–33,36; in these dual antiplatelet therapy was prescribed for 1 to 3 months. More detailed data on interventional materials are available in Supplementary Table 1 (available in the online version of the article).
Table 2.
Procedure Characteristics.
| First Author | Maximum Sheath Size, F | Maximum Guidewire Length, cm | Closure Technique | Periprocedural Anticoagulation | Reasons for Failure |
|---|---|---|---|---|---|
| Trani 200922 | 6 | 300 | Compression bandage | Dual AP 1 mo | NFP |
| Staniloae 201021 | 6 | 300 | NR | NR | N=3, twice not able to cross the lesion, once tortuosity of the subclavian artery |
| Cortese 201220 | 6 | 260 | Common bandage for 3 h | Dual AP 1 mo, lifelong aspirin | NFP |
| Roy 201623 | NR | NR | TR band | NR | NFP |
| Lorenzoni 201133 | 6 | 300 | TR band | Dual AP 1 mo | N=6, not able to cross the lesion |
| Cortese 201436 | 7 | 300 | NR | Dual AP 1 mo, lifelong aspirin | N=2, not able to cross the lesion |
| Lorenzoni 201425 | 8.5 | 400 | Compression and TR band | Dual AP for 3 mo | NR |
| Shinozaki 201424 | 6 | 120 | NR | NR | NFP |
| Coscas 20158 | 6 | 260 | Compression for 10 min | Dual AP for 2 mo, lifelong aspirin or clopidogrel | N=2, one due to spasm, one not able to cross |
| Ruzsa 20169 | 8.5 | 125 | NR | Dual AP | NR |
| Flachskampf 200532 | 6 | 125 | Compression | Dual AP 1 mo | NFP |
| Staniloae 200631 | 7 | 300 | Compression bandage | Dual AP | |
| Watanabe 200727 | 6 | 105 | NR | NR | NFP |
| Sanghvi 200828 | 6 | 300 | Compression bandage | NR | N=1, not able to reach distal SFA due to short sheath |
| Trani 201035 | 6 | 300 | Elastic bandage for 6 h | NR | NFP |
| Pitta 201129 | 7 | 300 | TR band | NR | NFP |
| Antov 201330 | 7 | 300 | Compression | NR | NFP |
| Harruna 201326 | 8 | 300 | NR | Dual AP | NFP |
| Shinozaki 201634 | 6 | 300 | NR | NR | NFP |
Abbreviations: AP, antiplatelet drug; NFP, no failed procedure; NR, not reported; SFA, superficial femoral artery; TR, transradial.
Procedure Success
This outcome, defined as angiographic stenosis <30% after intervention, was reported in 17 studies8,9,20–22,24–26,28–36 and ranged from 81% to 100% (Table 3) and the mean success rate was 90.9%. Five studies9,21,25,33,36 reported the most common reason for unsuccessful procedures, which was the inability to cross the lesion. Only 1 study28 reported an unsuccessful intervention because the sheath was too short to reach a lesion, which was located distal in the SFA. The mean fluoroscopy time (Table 3) was 25.8 minutes8,9,20–24,28,30,36 and the mean procedure time was 242 minutes.8,9,20,21,23,24,28,30,36 Total contrast dosage ranged from 20 to 430 mL. The hospital LOS ranged from discharge on the same day of the procedure to 4 days postprocedure.9,20,21,23,24,27,30,31,32,34,36 The mean time until discharge was 2.3 days.
Table 3.
Primary and Secondary Outcomes of the Included Studies.
| Primary Outcomes |
Secondary Outcomesb |
||||||
|---|---|---|---|---|---|---|---|
| First Author | N | Procedure Success, % | Complicationsa | Fluoroscopy Time, min | Procedure Time, min | Contrast, mL | LOS, d |
| Trani 200922 | 12 | 100 | 0 | 19±4 | 170±59 | 2.2±0.4 | |
| Staniloae 201021 | 27 | 88 | 0 | 30.4 | 97.9 | 238.7 | 0.6 |
| Cortese 201220 | 21 | 100 | 0 | 11±6 | 60±32 | 97±78 | 3.1±0.6 |
| Roy 201623 | 54 | 2 | 14.6±11.8 | 46.8±25.1 | 132.1±98.2 | 2.06±0.3 | |
| Lorenzoni 201133 | 25 | 84 | 1 | ||||
| Cortese 201436 | 147 | 99 | 5 | 16 | 64 | 153 | 2.8±1.4 |
| Lorenzoni 201425 | 110 | 91 | 18 | ||||
| Shinozaki 201424 | 30 | 100 | 0 | 20.5±6.5 | 60.4±17.4 | 104.5±27.4 | 2.1±1.1 |
| Coscas 20158 | 24 | 91 | 7 | 9 | 45 | 40 | |
| Ruzsa 20169 | 156 | 99 | 13 | 7.5±1.1 | 25.8±3.5 | 98.3±10.9 | 2.0±0.4 |
| Flachskampf 200532 | 1 | 100 | 0 | 1 | |||
| Staniloae 200631 | 1 | 100 | 0 | ||||
| Watanabe 200727 | 3 | 0 | 1 | ||||
| Sanghvi 200828 | 14 | 93 | 0 | 31 | 106 | 220 | |
| Trani 201035 | 2 | 100 | 0 | ||||
| Pitta 201129 | 1 | 100 | 0 | ||||
| Antov 201330 | 6 | 100 | 0 | 35 | 82.66 | 338.3 | 1.33 |
| Harruna 201326 | 1 | 100 | 0 | ||||
| Shinozaki 201634 | 1 | 100 | 0 | 1 | |||
Abbreviation: LOS, length of stay.
Includes bleeding, vascular closure device failure, pseudoaneurysm, arteriovenous fistulas, hematoma >5 cm, death, stroke, radial artery rupture.
Data are presented as the means ± standard deviation if available.
Complications
Most studies stated the manner in which they assessed complications8,20–23,25,29,33,36; however, not all were according to the same reporting standards (Supplementary Table 2, available in the online version of the article). Of the 638 patients who underwent the transradial access, complications occurred in 12 (1.9%). These complications (Table 3) comprised minor bleeding in 1 patient,36 right hemispheric stroke in 1 patient,22 and radial artery rupture in 2 patients.9 Furthermore, 1 study8 reported a case of distal embolization and a psoas hematoma. Six more major adverse events were reported during follow-up of 2 months: 1 myocardial ischemia, 2 deaths, 1 emergent angioplasty, and 2 amputations. All events occurred in patients with critical limb ischemia. Postintervention radial artery patency was assessed in 8 studies8,9,20,23,25,28,29,33 comprising 405 patients. Of these, the radial artery was patent in 379 (93.6%) patients after the intervention.
Meta-analysis
Four studies20–23 (3 retrospective cohort studies and 1 prospective matched cohort trial) involving 114 transradial and 208 transfemoral patients were included in the meta-analysis. The pooled study population was homogeneous for age, underlying disease, and clinical symptoms. Lesions were located in the iliac artery, aortic bifurcation, SFA, CFA, or popliteal artery (Table 1).
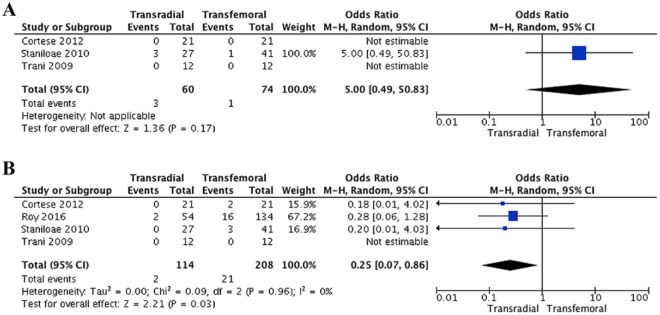
The primary outcome, procedure success, was equal between access methods (OR 5.0, 95% CI 0.49 to 50.83, p=0.17; Figure 2A) for the 3 studies reporting this outcome (n=134).20–22 It was not possible to calculate the statistical heterogeneity because of insufficient data. Transradial access showed a significant reduced complication rate (OR 0.25, 95% CI 0.07 to 0.86, p=0.03; Figure 2B) compared with the transfemoral approach; the heterogeneity was low (I2=0%). Complications are described in Table 4.
Figure 2.
Forest plots of the (A) procedure success and (B) complications outcomes. CI, confidence interval, M-H, Mantel-Haenszel.
Table 4.
Access-Site Complications in the 4 Studies Comparing Transradial to Transfemoral Access.
| Trani 200922 | Staniloae 201021 | Cortese 201220 | Roy 201623 | |
|---|---|---|---|---|
| Radial artery | (n=12) | (n=27) | (n=21) | (n=65) |
| Bleeding | 0 | 0 | 0 | 0 |
| Hematoma | 0 | 0 | 0 | 0 |
| Pseudoaneurysm | 0 | 0 | 0 | 0 |
| Radial artery occlusion | NR | NR | 0 | 2 |
| Femoral artery | (n=12) | (n=41) | (n=21) | (n=123) |
| TIMI minor bleeding | 0 | 0 | 1 | 0 |
| TIMI major bleeding | 0 | 0 | 1 | 0 |
| Hematoma | 0 | 0 | 0 | 1 |
| Pseudoaneurysm | 0 | 0 | 0 | 6 |
| AV fistula | 0 | 0 | 0 | 1 |
| VCD failure | 0 | 0 | 0 | 8 |
| Persistent bleeding requiring more compression | 0 | 3 | 0 | 0 |
Abbreviations: AV, arteriovenous; NR, not reported; TIMI, thrombolysis in myocardial infarction bleeding criteria; VCD, vascular closure device.
None of the secondary outcomes showed a significant advantage for one technique over the other: LOS (MD 0.24 days, 95% CI −0.52 to 0.04, p=0.09; I2=0%), fluoroscopy time (MD 2.47 minutes, 95% CI −7.10 to 2.16, p=0.08; I2=60%), procedure time (MD 4.20 minutes, 95% CI −11.74 to 3.35, p=0.28; I2=0%), and contrast volume (MD 7.24 mL, 95% CL −59.45 to 44.97, p=0.79; I2=76%).
Discussion
In percutaneous coronary interventions and coronary angiography, the radial artery has become the most commonly used access vessel. Evidence from the included studies in this review and meta-analysis suggests that the transradial access is feasible and possibly even safer for infra-aortic interventions because of the high procedure success and the significantly reduced risk of developing complications. The transradial approach is especially useful in the particular subset of patients in whom groin access is impeded by morbid obesity, occluded CFAs, prosthetic grafts, or severe iliac tortuosity.28,37
Limited evidence from the current meta-analysis showed no significant difference in procedure success between the access strategies. Only 1 study reported a significantly higher success rate for interventions performed via the femoral artery. However, their transradial group had significantly more occlusive lesions treated than in the transfemoral group, which may have influenced their results.
There are several reasons in favor of the transradial access, most notably decreased major access-site complications (eg, bleeding, hematoma, or pseudoaneurysm).38 This random-effects meta-analysis substantiated a significant reduction in complications in patients who underwent transradial access (1.9%). The patients suffered fewer complications such as minor bleeding, myocardial infarction, embolic events, pseudoaneurysm, VCD failure, and hematoma. On the contrary, in transfemoral interventions, the reported incidences of access-site complications vary between 5% and 23% for hematoma, 0.5% and 9% for pseudoaneurysm, 0.2% to 2.1% for AV fistula, 0.8% to 20% for arterial occlusion, and <0.1% to 20% for infection.39 These findings are consistent with a meta-analysis by Jolly et al,40 which reported the risk of major bleeding or access-site bleeding to be 73% less with the transradial access in coronary interventions in comparison to the transfemoral route. The randomized controlled RIVAL trial38 found a significantly increased risk of complications in the transfemoral access compared with the transradial in coronary angiography and interventions. Specifically, the risk of major bleedings, death, myocardial infarction, stroke, and major vascular complications was elevated in non-bypass procedures. Moreover, increased risks of large hematoma, pseudoaneurysm, and AV fistula was reported for the transfemoral access.
The most common complication of the transradial access in our review was radial artery occlusion. Unfortunately, postintervention radial artery occlusion was assessed in only 8 studies8,9,20,23,25,28,29,33 comprising 405 patients, with a 6.4% incidence. In their meta-analysis, Rashid et al41 focused on radial artery occlusion in transradial coronary interventions, concluding that radial artery occlusion was a common complication of transradial access. They reported a prevalence of 7.7% during the first 24 hours and 5.5% after 1 month. Furthermore, they reported that an increased intraprocedural dose of heparin (5000 units) was the most effective measure for the prevention of radial artery occlusion. A compression time of 15 minutes also seemed to preserve the patency of the radial artery.41 In the 638 patients in our systematic review just 2 radial artery ruptures were disclosed. Furthermore, no hematoma, wound infection, or other access-related complications were reported.
Another advantage of transradial access is that a patient with a transfemoral access (depending on institution) is normally kept immobilized for 6 hours postprocedure, whereas in the transradial access this immobility is not necessary.42 Our meta-analysis found a nonsignificant reduction of 0.24 days in LOS in comparison to the femoral access. The 1 article21 that could not be used in the meta-analysis of this outcome measure found a significant reduction in the LOS (14.4 vs 20.9 hours, p=0.003) in the transradial access group. The mean time to discharge of all studies reviewed was 2.26 days. Taking all this into account, available data imply that the decreased LOS documented after transradial access in coronary interventions may be assumed for infra-aortic interventions as well.
In our review, the frequency of choosing the left or the right hand for the intervention was almost equal. The benefit of left radial artery access is the shorter distance to the descending aorta and the fact that the aortic arch does not have to be crossed. Some studies suggest that the stroke risk is lower in left-sided access because the catheters and wires do not have to cross the brachiocephalic trunk.6 Moreover, the left subclavian artery often provides a direct route for catheter or sheath access to the descending aorta without the need for additional guiding catheters due to its natural curve into the aorta. However, other studies suggest that it is more comfortable for the interventionist to approach the patient via the right arm.43 Another study by Lorenzoni et al33 suggested positioning the patient upside down on the angiographic table with the left arm abducted wide. This positioning has the advantage that the flat panel is distant to the operator and can possibly be associated with less radiation exposure for the operator. One study compared left and right radial access and found no difference in procedure success.23
Three studies assessed the influence of the learning curve with opposing results. While Staniloae et al21 stated that climbing the learning curve resulted in a shortening of the procedure time but not in improved procedure success rates, Coscas et al9 and Ruzsa et al8 found no statistical significant improvement of any outcome evaluating the effect of the learning curve.
Converting to femoral artery access due to failure to access the radial artery was seen in 9.5% of all patients who underwent a radial access intervention. The RIVAL38 study reported an 8% rate of conversions in low-volume centers and 4.4% in high-volume centers. Challenging anatomy can be a reason to move to the femoral artery. Another reason could be the lack of sufficient and adequate instruments to reach peripheral lesions. Treatment failure due to inability to cross more distally located occlusive lesions was reported in several studies.9,21,32,35 An explanation for this might perhaps be the meager pushablity from the transradial access.
Another issue that has to be mentioned for transradial access is the possibility that devices can be too short to reach the lesion in very tall patients.28,33 Because radial access is a new and uncommon technique to treat lesions in the lower limb, current catheters, angioplasty balloons, and stent delivery systems may still limit the applicability of the access for all patients and lesions.9
Limitations
The biggest limitation of this study is the lack of any randomized controlled trials; rather, most of the studies included in this meta-analysis were cohort studies, which have a higher risk of publication bias than with randomized controlled trials. Another limitation of this study is the relatively small number of patients included. Furthermore, the studies included in the meta-analysis showed some heterogeneity. Among these studies patient characteristics concerning the body mass index, diabetes, and dyslipidemia differed. Symptomatology of peripheral artery disease according to the Fontaine or Rutherford classification was the same in all studies.
Most of the treated lesions were located in the iliac artery, but the studies performed by Trani et al22,35 focused on lesions of the superficial femoral artery. The study conducted by Roy et al23 included more occlusive lesions than the other studies did. The definitions of access complications were not the same among the studies, but the criterion for procedure success was. All of the above influences the external validity of our findings.
It is clearly necessary to perform randomized controlled trials in the near future to compare the transradial and transfemoral routes in the treatment of peripheral artery disease similar to those found in the coronary literature.
Conclusion
The transradial access for peripheral endovascular revascularization is a feasible and safe alternative to the conventional transfemoral route with potentially lower complication rates. Randomized controlled trials assessing procedural success, safety as well as cost benefits, are required to further validate the use of the transradial access in lower limb interventions.
Supplemental Material
Supplemental material, 18-0085_Supplementary_Tables for Transradial Approach for Aortoiliac and Femoropopliteal Interventions: A Systematic Review and Meta-analysis by Max M. Meertens, Eugene Ng, Stanley E. K. Loh, Miny Samuel, Barend M. E. Mees and Andrew M. T. L. Choong in Journal of Endovascular Therapy
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: The online tables are available at http://journals.sagepub.com/doi/suppl/10.1177/10.1177/1526602818792854
ORCID iD: Max M. Meertens  https://orcid.org/0000-0002-7746-8723
https://orcid.org/0000-0002-7746-8723
References
- 1. Campeau L. Percutaneous radial artery approach for coronary angiography. Catheter Cardiovasc Diagn. 1989;16:3–7. [DOI] [PubMed] [Google Scholar]
- 2. Rao S, Stone G. Arterial access and arteriotomy site closure devices. Nat Rev Cardiol. 2016;13:641–650. [DOI] [PubMed] [Google Scholar]
- 3. Gan L, Lib Q, Liuc R. Effectiveness and feasibility of transradial approaches for primary percutaneous coronary intervention in patients with acute myocardial infarction. J Nanjing Med Univ. 2009;23:270–274. [Google Scholar]
- 4. Posham R, Biederman DM, Patel RA. Transradial approach for noncoronary interventions: a single-center review of safety and feasibility in the first 1,500 cases. J Vasc Interv Radiol. 2016;27:159–166. [DOI] [PubMed] [Google Scholar]
- 5. Sixt S, Alawied A, Rastan A. Acute and long-term outcome of endovascular therapy for aortoiliac occlusive lesions stratified according to the TASC classification: a single-center experience. J Endovasc Ther. 2008;15:408–416. [DOI] [PubMed] [Google Scholar]
- 6. Grenon S, Reilly L, Ramaiah V. Technical endovascular highlights for crossing the difficult aortic bifurcation. J Vasc Surg. 2011;54:893–896. [DOI] [PubMed] [Google Scholar]
- 7. Aboyans V, Ricco JB, Bartelink ML. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39:763–816.28886620 [Google Scholar]
- 8. Ruzsa Z, Tóth K, Nemes B. Transradial and transulnar access for iliac artery interventions using sheathless guiding systems: A feasibility study. Catheter Cardiovasc Interv. 2016;88:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coscas R, Blic RD, Capdevila C. Percutaneous radial access for peripheral transluminal angioplasty. J Vasc Surg. 2015;61:463–468. [DOI] [PubMed] [Google Scholar]
- 10. Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62;1006–1012. [DOI] [PubMed] [Google Scholar]
- 11. Wells GW, Shea B, O’Connel D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed April 19, 2018.
- 12. Touma J, Coscas R, Ijaverliat I. Radial access for endovascular ilio-femoral procedures. J Cardiovasc Surg (Torino). 2016;57:302–310. [PubMed] [Google Scholar]
- 13. Jiang Z, Qian J, Zhengran L. Interventional therapy of arteriosclerotic obliterations of iliaco-femoral artery via radial artery [in Chinese]. Zhonghua Fang She Xue Za Zhi. 2008;42:974–977. [Google Scholar]
- 14. Coppola J, Kurian D, Staniloae C. Transradial peripheral vascular interventions. Indian Heart J. 2010;62:197–201. [PubMed] [Google Scholar]
- 15. Tchétché D, Sauguet A, Jordan C. Transradial peripheral angioplasty. Ann Cardiol Angeiol (Paris). 2009;58:355–359. [DOI] [PubMed] [Google Scholar]
- 16. Patel T, Shah S, Pancholy S. Utility of transradial approach for peripheral vascular interventions. J Invasive Cardiol. 2015;27:277–282. [PubMed] [Google Scholar]
- 17. Korabathina R, Yadav S, Coppola J. Transradial approach to lower extremity interventions. Vasc Health Risk Manag. 2010;9:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lorenzoni R, Roffi M. Transradial access for peripheral and cerebrovascular interventions. J Invasive Cardiol. 2013;25:539–536. [PubMed] [Google Scholar]
- 19. Yamashita T, Imai S, Tamada T. Transradial approach for noncoronary angiography and interventions. Catheter Cardiovasc Interv. 2007;70:303–308. [DOI] [PubMed] [Google Scholar]
- 20. Cortese B, Peretti E, Troisi N. Transradial percutaneous iliac intervention, a feasible alternative to the transfemoral route. Cardiovasc Revasc Med. 2012;13:331–334. [DOI] [PubMed] [Google Scholar]
- 21. Staniloae C, Korabathina R, Yu J. Safety and efficacy of transradial aortoiliac interventions. Catheter Cardiovasc Interv. 2010;75:659–662. [DOI] [PubMed] [Google Scholar]
- 22. Trani C, Burzotta F, Tommasino A. Transradial approach to treat superficial femoral artery in-stent restenosis. Catheter Cardiovasc Interv. 2009;74:494–498. [DOI] [PubMed] [Google Scholar]
- 23. Roy A, Garot P, Louvard Y. Comparison of transradial vs transfemoral access for aortoiliac and femoropopliteal interventions: a single-center experience. J Endovasc Ther. 2016;23:880–888. [DOI] [PubMed] [Google Scholar]
- 24. Shinozaki N, Ogata N, Ikari Y. Initial results of transradial iliac artery stenting. Vasc Endovascular Surg. 2013;48:51–54. [DOI] [PubMed] [Google Scholar]
- 25. Lorenzoni R, Lisi C, Corciu A. Tailored use of transradial access for above-the-knee angioplasty. J Endovasc Ther. 2014;21:635–640. [DOI] [PubMed] [Google Scholar]
- 26. Harruna S, Jacobs E, White C. Trans-radial bilateral iliac artery stenting. Vasc Med. 2013;18:200–203. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe H, Sato K, Kobori T. Three cases of external iliac artery stenting using an upper limb approach. Jpn J Intery Cardiol. 2007;22:166–171. [Google Scholar]
- 28. Sanghvi K, Kurian D, Coppola J. Transradial intervention of iliac and superficial femoral artery disease is feasible. J Interv Cardiol. 2008;23:385–387. [DOI] [PubMed] [Google Scholar]
- 29. Pitta S, Barsness G, Lerman A. Transradial iliac artery intervention with dual downstream embolic protection. J Vasc Surg. 2011;53:808–810. [DOI] [PubMed] [Google Scholar]
- 30. Antov S, Kedev S. Transradial approach as first choice for stenting of chronic total occlusion of iliac and femoral superficial artery. Pril (Makedon Akad Nauk Umet Odd Med Nauki). 2013;34:13–24. [PubMed] [Google Scholar]
- 31. Staniloae C, Kurian D, Coppola J. Transradial bilateral iliac stenting. J Invasive Cardiol. 2006;18:256–257. [PubMed] [Google Scholar]
- 32. Flachskampf F, Wolf T, Daniel W. Transradial stenting of the iliac artery: a case report. Catheter Cardiovasc Interv. 2005;65:193–195. [DOI] [PubMed] [Google Scholar]
- 33. Lorenzoni R, Mazzoni A, Lazzari M. Radial artery access for above the knee angioplasty: a feasibility study. EuroIntervention. 2011;7:924–929. [DOI] [PubMed] [Google Scholar]
- 34. Shinozaki N, Minowa T, Ikari Y. Transradial intervention for chronic total occlusion at the external iliac artery using a bidirectional approach through a single guiding catheter. Cardiovasc Interv Ther. 2016;32:174–177. [DOI] [PubMed] [Google Scholar]
- 35. Trani C, Tommasino A, Burzotta F. Pushing the limits forward: transradial superficial femoral artery stenting. Catheter Cardiovasc Interv. 2010;76:1065–1071. [DOI] [PubMed] [Google Scholar]
- 36. Cortese B, Trani C, Lorenzoni R. Safety and feasibility of iliac endovascular interventions with a radial approach. Results from a multicenter study coordinated by the Italian Radial Force. Int J Cardiol. 2014;175:280–284. [DOI] [PubMed] [Google Scholar]
- 37. Dattilo P, Tsai T, Rogers K. Acute and medium term outcomes of endovascular therapy of obstructive disease of diverse etiology of the common femoral artery. Catheter Cardiovasc Interv. 2013;81:1013–1022. [DOI] [PubMed] [Google Scholar]
- 38. Jolly S, Yusuf S, Cairns J. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;9775:1409–1420. [DOI] [PubMed] [Google Scholar]
- 39. Banerjee S. Practical Approach to Peripheral Arterial Chronic Total Occlusions. Singapore: Springer Singapore; 2017:95–122. [Google Scholar]
- 40. Jolly S, Amlani S, Hamon M. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132–140. [DOI] [PubMed] [Google Scholar]
- 41. Rashid M, Kwok C, Pancholy S. Radial artery occlusion after transradial interventions: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(1):e002686. doi: 10.1161/JAHA.115.002686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kiemeneij F, Laarman GJ, Odekerken D. A randomized comparison of percutaneous transluminal coronary angioplasty by the radial, brachial and femoral approaches: the Access Study. J Am Coll Cardiol. 1997;29:1269–1275. [DOI] [PubMed] [Google Scholar]
- 43. Luo J, Wang H, Huang W. Transradial artery intervention: an alternative approach for renal artery stent implantation? Chin Med J. 2012;125:3340–3343. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 18-0085_Supplementary_Tables for Transradial Approach for Aortoiliac and Femoropopliteal Interventions: A Systematic Review and Meta-analysis by Max M. Meertens, Eugene Ng, Stanley E. K. Loh, Miny Samuel, Barend M. E. Mees and Andrew M. T. L. Choong in Journal of Endovascular Therapy