SUMMARY
Pleomorphic xanthoastrocytoma (PXA) is a rare astrocytic neoplasm that commonly affects children and young adults, and presents with seizures. PXA is typically supratentorial with a predilection to the temporal lobe, and often involves the cortex and the meninges. PXAs have a favorable prognosis with a 10-year survival probability of >70%, and are WHO grade II neoplasms. Recent observations and studies demonstrate that PXAs are clinically, histologically and genetically distinct. Some PXAs recur and exhibit aggressive clinical behavior. In such cases, certain histological and clinical factors could account for the aggressive behavior. However, the histological features that predict adverse outcome are poorly defined. In the current WHO classification of CNS tumors, there is no option for a high-grade PXA, even if the tumor had numerous recurrences and poor outcome. In this review, we focus on aggressive clinical behavior and anaplasia in PXA, and discuss how our current experience suggests modifications in the current WHO classification. We also review recent discoveries on the molecular characteristics of PXA that could help us better understand their biological behavior.
Practice Points.
Pleomorphic xanthoastrocytoma (PXA) is a WHO grade II neoplasm, currently considered within the astrocytic category of tumors. The diagnostic criteria for PXA are well recognized and fairly reproducible.
Some PXAs have histologically malignant features and aggressive clinical behavior, however, the current classification scheme does not allow designation of a high-grade PXA (i.e., WHO grade III or IV), but recognizes ‘PXA with anaplastic features’ (aPXA).
While there is a high rate of concordance in the diagnosis of WHO grade II PXAs, the exact criteria for the diagnosis of aPXA are elusive.
Revisions in the WHO classification should designate aggressive neoplasms diagnosed as aPXA in a high-grade category, and should attempt to define the critical criteria for their diagnosis.
Pleomorphic xanthoastrocytoma (PXA) was first identified as a distinctive form of astrocytoma by Kepes et al. in 1979 [1]. In this study, the authors reported 12 patients (aged 7–25 years) with a unique form of supratentorial astrocytoma and a favorable outcome [1]. The tumors were recognized on the basis of their superficial cortical location and unique histological features that include marked cellular pleomorphism, rich reticulin network and prominent lipid-laden glial cells. Despite their highly pleomorphic and bizarre cytology that suggested malignant behavior, these tumors appeared to have a relatively favorable prognosis. The most popular hypothesis proposed by Kepes et al. postulated that PXA originates from subpial astrocytes, which was used to explain the superficial location of these neoplasms [2].
PXA entered the WHO classification as a grade II neoplasm in 1993 [3]. However, tumors with aggressive biology in the PXA category were known even before the publication of the 1993 classification, but such tumors were not assigned a higher grade [4]. Subsequent classifications in 2000 and in 2007 referred to these less common, aggressive tumors as ‘PXA with anaplastic features’ (aPXA), but avoided a WHO grade III designation [2,5]. The changes in the wording of the recent editions were mostly influenced by emerging data and the seminal study by Giannini et al. [6].
Since the revision in 2000, small but substantive experience has accumulated through original studies and reviews on PXA and tumors that were classified as aPXA. Currently, there is sufficient evidence that clinical, radiological and histological characteristics of the neoplasms classified as aPXA deserve special emphasis. These observations, along with the emerging information on the molecular features of tumors in this category, are slowly reshaping our perspective that such tumors do not have the favorable outcome of classical PXA.
What is PXA?
PXA is commonly considered a tumor of young people with an incidence rate of fewer than 0.07 cases per 100,000 population, as adjusted to the USA standard population in 2000 [7]. The limited number of cases reported in the literature reflects the rarity of PXA, which constitutes less than 1% of all astrocytic neoplasms [7]. There seems to be no reported gender or racial predilection [2]. Even though WHO 2007 quotes a study with a higher occurrence in females, this limited study only includes 13 patients and does not address gender predilection owing to its limited and biased sample size [8]. No specific etiologies have been implicated in the genesis or evolution of PXA. PXAs occur most frequently in the superficial cortex of the cerebral hemispheres, particularly in the temporal lobe, and often involve the overlying leptomeninges [6]. However, PXAs have been reported in other locations. Case reports document examples located in the cerebellum [9], spinal cord [10], retina [6], thalamus [11], pineal gland [12] and sellar region [1]. Some studies also report multicentric PXAs presenting with several noncontiguous lesions at diagnosis [13]. Many reports, including the original study, noted the tendency of the tumor to involve the meninges, even though dural invasion was considered unusual [1]. Owing to the superficial cerebral location, patients often present with a long history of seizures, many spanning months or years prior to radiographic diagnosis [6]. Clinical features at presentation include focal neurological deficits, visual disturbance, headaches and, rarely, intracerebral hemorrhage [14]. PXAs generally have slow but progressive symptoms that are dependent on their location.
On MRI and CT scans, PXA is most frequently a cystic lesion with a contrast-enhancing mural nodule or, partially cystic, enhancing tumor with minimal mass effect [15]. Peritumoral edema is not typical owing to the slow course of tumor development. Contrast enhancement on T1-weighted images is very common [16]. A small number of cases have been reported in association with other CNS lesions, including neurofibromatosis type 1 [17], focal cortical dysplasia [18], mesial temporal sclerosis [16] and velocardiofacial syndrome [19].
The key histopathological features of the classical PXA are well established [2]. PXAs are hypercellular tumors composed of atypical, pleomorphic cells with astrocytic or mesenchymal morphology and abundant cytoplasm (Figure 1). The cells are often arranged in fascicles or in a storiform pattern, giving a mesenchymal appearance to the tumor [1,6]. Xanthomatous cells with foamy cytoplasm are also diagnostically helpful, but are only encountered in approximately a quarter of cases. Large, bizarre multinucleated cells are common, and give the tumor its pleomorphic appearance. Intranuclear inclusions and eosinophilic granular bodies are almost constant findings [6]. Focal perivascular or intratumoral collections of mature lymphocytes with occasional plasma cells are also frequent [6]. One of the most characteristic features of PXAs is the deposition of reticulin around tumor cells, either diffusely or in a patchy fashion. Rosenthal fibers may also be seen, particularly at the edges of the tumor, particularly reflecting a reactive change in the adjacent brain. Rare tumor cells containing pigment granules were also reported [20]. Although cellular pleomorphism is a common feature of PXA, the absence of mitoses and necrosis enables their differentiation from malignant infiltrating gliomas [4].
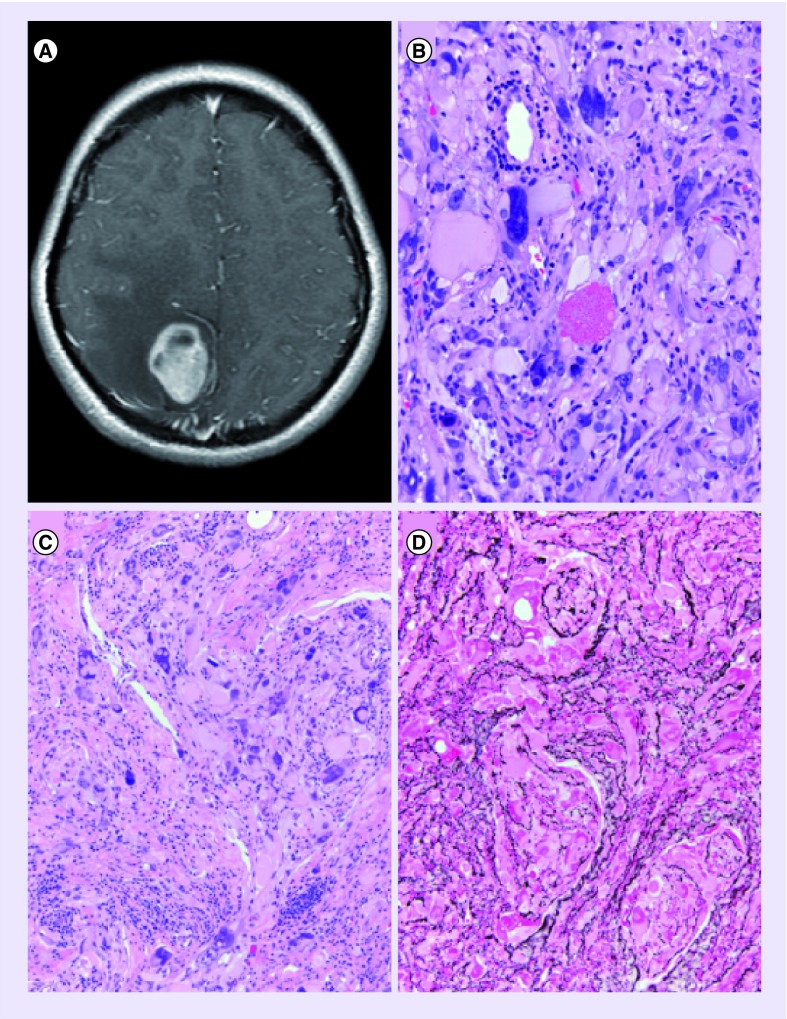
Figure 1. The typical radiological and histological features of pleomorphic xanthoastrocytoma.
(A) Contrast-enhanced, T1-weighted MRI demonstrating the typical superficial cystic mass with the enhancing mural nodule. (B) High-power magnification demonstrating the typical pleomorphic, multinucleated giant cells with an eosinophilic granular body. (C) Medium-power magnification of typical giant cells along with smaller tumor cells and clusters of lymphocytic infiltrates within the tumor. (D) Special stain for reticulin highlighting the dense intercellular reticulin network most often surrounding individual as well as small clusters of tumor cells.
(B) Image was taken at 400× maginifcation.
(C & D) Images were taken at 200× magnification.
Immunohistochemical studies demonstrate diffuse and strong positivity with the antibodies against GFAP, Olig-2, Sox2, and S100 protein supporting a glial, and more specifically an astrocytic, cell of origin [6,21]. Many PXAs also demonstrate positivity with the antibodies against neuronal markers including synaptophysin, neurofilament, class 3 β-tubulin and MAP2 with variable frequency [5]. The hematopoietic progenitor/vascular endothelial cell-associated antigen CD34 is expressed in tumor cells in up to 50% of cases [21]. The immunohistochemical and ultrastructural evidence of predominantly astrocytic nature aid in distinguishing this tumor from mesenchymal or meningothelial neoplasms [4]. In most tumors, proliferation indices such as Ki-67 (MIB1) and PCNA highlight less than a few percent of tumor cells [6]. The p53 labeling index is variable across reported cases, with most tumors being entirely negative [22].
While this underscores a rather unique neoplasm, a number of issues remain unresolved with respect to PXA and its aggressive examples. First, the issue of cell of origin still remains unresolved, and the suggestion regarding their origin as the ‘cerebral cortical astrocytic cells beneath the overlying pia mater’ is only substantiated by the presence of generic glial markers, such as GFAP [1]. While other markers of glial nature have also been positive in these tumors, many studies also demonstrate evidence of divergent differentiation [23]. The presence of ‘mixed’ or hybrid tumors with a neuronal or ganglionic component also suggests that the cell of origin may not be a purely astrocytic cell [23–27]. There is still much to debate regarding whether PXA and its aggressive subtypes can be considered within the purely astrocytic neoplasms or whether they are most suited to be classified within the family of glioneuronal tumors.
Molecular characteristics of PXA
Recent studies have made significant contributions to our current understanding of PXA as a unique neoplasm that is often genotypically distinct from diffuse (infiltrating) gliomas. Genetic alterations typical of infiltrating gliomas, such as TP53 mutations and EGFR amplifications, are distinctly rare or absent in most PXAs [21,22]. Earlier studies identified DNA loss on chromosome 9 as the most common regional chromosomal abnormality, occurring in approximately 50% of PXAs [28]. Other studies reported gains and losses in chromosomes 3, 5, 20 and 22 to a much lesser extent, and in only a small minority of cases [29–31]. Less common regional losses involve chromosomes 4, 6, 8p, 10p, 13, 17, 18 and 21 [29,30,32]. Chromosomal gains were also reported in loci at chromosomes 7 and 19 [28,29]. Other genetic aberrations included translocations at chromosome 1, 15, 20 and 22 [33,34]. Identification of deletions or epigenetic inactivation of CDKN2A or CDKN2B genes has been inconsistent [28,35].
In recent years, BRAF V600E mutations have been reported as the most common and consistent genetic abnormality in PXAs [36,37]. BRAF mutations can be found in a wide range of human cancers, and represent the most frequent genetic alteration in melanomas, papillary thyroid carcinoma and hairy cell leukemia, among others [38]. More than 95% of BRAF mutations are of the V600E type, which leads to the substitution of valine by glutamic acid in the kinase domain of the protein. The mutated BRAF protein is constitutively active and enhances the proliferative potential through activation of the MAPK pathway. This pathway also plays a critical role in oncogene-induced senescence. Among primary brain tumors, activating BRAF mutations are found frequently in PXAs and gangliogliomas, and less frequently in pilocytic astrocytomas [36]. The BRAF mutation is exceptionally rare among infiltrating gliomas with a slightly higher prevalence in pediatric glioblastomas [36], and therefore, considered helpful in the diagnosis of PXA and its differentiation from giant cell glioblastoma. Some authors also contend that BRAF V600E mutation rates may differ between adult and pediatric PXAs [39].
The presence of BRAF mutations emerged as a potential target in PXA, since there are currently available inhibitors that can be used clinically to treat patients [40]. It has been suggested that tumors with BRAF mutations may respond to combination chemotherapy using targeted inhibitors, however, even earlier studies revealed a paradoxal activation that may significantly impair the efficacy of single-agent chemotherapy [41]. While studies on the efficacy of BRAF inhibitors are being conducted for brain tumors, recent studies on melanocytic tumors integrating BRAF inhibitors into combination chemotherapy may overcome resistance to single-agent inhibition and may be another therapeutic strategy for patients with recurrent PXA [42]. While there is currently very limited data on such therapeutic strategies for PXAs, we expect this avenue to be explored more often in the near future.
Recurrence & aggressive behavior: aPXA
The possibility of local recurrence was implied in the original definition of PXAs since these tumors were not regarded as entirely benign, but only as having a relatively favorable biological behavior [1]. In larger studies, recurrence-free survival for typical PXAs was reported to be approximately 60% at 5 and 10 years [6,16]. Overall survival probability at 5 years was calculated at 85% for the same groups [6,16]. Nevertheless, individual cures with complete remission for up to 25 years have also been reported [43]. However, all studies identify a subset of tumors with more aggressive clinical behavior, such as local recurrence or distant spread. There have been increasingly more reports on the unfavorable behavior of tumors in the PXA category, which partly reflects a publication bias, but also underscores a subgroup of tumors in this category that do not conform to the classical definition. Furthermore, some PXAs present with cerebrospinal or leptomeningeal dissemination [13,44,45].
Certain clinical parameters have been reported to effect survival probability in PXAs. For instance, tumors in atypical or ‘difficult’ locations reportedly had less favorable outcomes [46]. The tumor size was also suggested as a possible prognostic factor, but studies to determine the prognostic significance of tumor size in PXAs have been limited. Some studies also suggested the extent of resection as an important variable effecting progression-free survival [6]. The preferred surgical treatment has been gross total resection for PXA, and subtotal resection was associated with a shorter progression-free survival [6,16]. However, overall survival probability could not be associated with the extent of resection [6]. Advanced patient age [47] and spinal dissemination at diagnosis [48] were also proposed as prognostic factors, but the data relating to these factors have not been adequately analyzed.
In 1983, Weldon-Linne et al. described a case of PXA in a 32-year-old man with rapid fatal outcome and extensive recurrent tumor showing complete histologic differentiation to malignant glioma [4]. In this report, the clinical and pathological features of the original tumor were typical of PXA. However, the recurrent tumor obtained at autopsy had features of a malignant astrocytoma with little, if any, resemblance to the histology of the original lesion. Later, Kepes et al. reported three other cases of classic WHO grade II PXA that recurred as high-grade malignant ‘astrocytic’ gliomas [49]. These reports underscored that tumors with histologically ‘anaplastic’ or malignant features also portended a less favorable prognosis in PXA [50].
Earlier reviews of tumors with malignant histological features revealed that the time interval for anaplastic or malignant transformation of a typical PXA could be markedly varied and may be observed as late as 18 years after initial surgery [43,51,52]. The histological features of tumors reported to have malignant transformation or the so-called aPXA have also been quite diverse (Figure 2). Many tumors in this category had significant mitotic activity, but the mitotic rate seen in aPXAs varied from fewer than three per ten high-power fields (HPFs) [21,49,53–57] to up to 18 per ten HPFs [43].
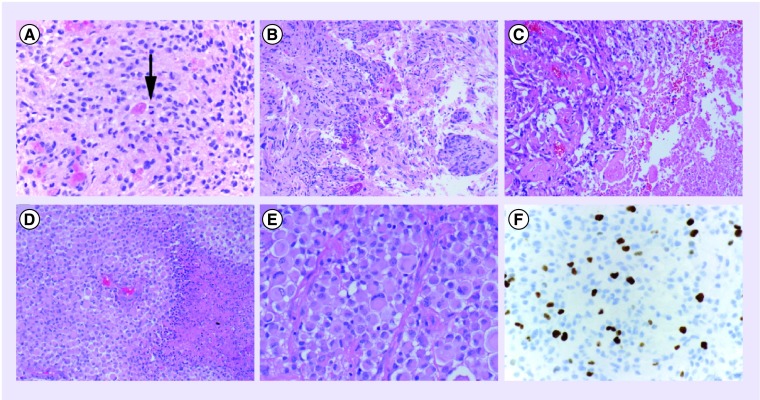
Figure 2. Histological features reported in tumors classified as ‘pleomorphic xanthoastrocytoma with anaplastic features’.
(A) Solid, glial-appearing neoplasm with eosinophilic granular bodies and mitotic figures (arrow). (B) Medium-power magnification demonstrating marked vascular endothelial proliferation. (C) Medium-power magnification with nonpalisading necrosis in a compact tumor and adjacent pleomorphic cells and hyalinized vessels. (D) Medium-power magnification of a solid area with numerous rhabdoid cells and palisading necrosis. (E) High-power magnification of a recurrent tumor previously diagnosed as classical pleomorphic xanthoastrocytoma showing predominantly rhabdoid cells. (F) Immunohistochemical staining for Ki-67 (MIB1) antibody with increased labeling index up to 10% in a tumor diagnosed as pleomorphic xanthoastrocytoma with anaplastic features.
(A, E & F) Images were taken at 400× magnification.
(B, C & D) Images were taken at 200× magnification.
In addition to increased mitotic rate, the well-described dense reticulin network of typical PXA was also found to be lost in aPXAs [49,58]. Few studies considered vascular endothelial proliferation as a criterion for anaplasia [21], while focal necrosis was accepted as an important criterion in many studies [43,51,59,60]. Conversely, some researchers, including the author of the original report, suggested that a pleomorphic astrocytic tumor with necrosis should be excluded from the PXA category [61]. In their article, Giannini et al. suggested the designation of aPXA, which shared key characteristics of PXA but exhibited greater unpredictability in aggression. Their multivariate analysis on a series of 71 cases found that high mitotic indices (greater than or equal to five mitoses per ten HPF) carried a poorer prognosis with respect to both recurrence-free and overall survival. A ‘significant adverse association of necrosis with survival’ was also reported, although this did not reach statistical significance on multivariate analysis. Thus, they have dissented from the view that tumors with necrosis should be excluded from this category [6]. Other authors supported the association of necrosis as well as pseudopalisading necrosis with a more aggressive behavior and suggested the use of this criterion as a sign of ‘anaplasia‘, but not a criterion to exclude tumors from the PXA category [54,59,62]. The proposed definition for aPXA currently includes a mitotic index greater of than or equal to five per ten HPFs with or without necrosis in a tumor principally considered in the PXA category. However, in a study comparing conventional PXA with aPXA, Hirose et al. suggested that it may not always be possible to apply the criteria of Giannini et al. for the diagnosis of aPXA [21]. These authors contended that classical characteristics of PXA should be recognizable even in the histologically anaplastic areas.
Some of the reported cases of aPXA have unusual histological features that confirm their aggressive nature but raise even more questions regarding their diagnosis. Rare tumors that develop rhabdoid components have been documented in this category [63]. We have also observed such cases in the recurrent examples of tumors previously reported as classical PXA without any evidence of anaplastic features (Figure 2D & E).
Tumors reported as de novo aPXA are far more diverse and challenging, and the reports on such cases leave more unanswered questions on how such tumors should be recognized [59,64–66]. Once anaplastic, the survival probability was considerably shorter compared with typical PXAs [50,53]. It has been much easier to diagnose aPXA if a malignant tumor arose in the same location as a classic PXA, and is histologically and genetically similar to the primary tumor. It is far more subjective if the tumor has radiological and histological features of a malignant infiltrating glioma, no prior history of a WHO grade II PXA and only rare eosinophilic granular bodies. The threshold for diagnosing such a tumor as aPXA can vary tremendously among experts.
The malignant component in aPXAs has been reported as either WHO grade III or IV diagnoses, depending on the histological features observed in the recurrent tumors. Currently, it is not clear whether there are any substantive differences among de novo aPXAs or malignant progression of a WHO grade II PXA into histologically grade III or IV neoplasm.
Conclusion & future perspective
PXA is a WHO grade II neoplasm, such as a low grade neoplasm, which carries a generally favorable prognosis. Histological features, such as increased mitotic activity, necrosis with or without pseudopalisading and rhabdoid morphology, identify a subset of tumors in this category with a more aggressive behavior. These tumors are often classified as aPXA per the current WHO classification, but the use of this terminology is in direct contradiction to the use of the term ‘anaplasia’ throughout the rest of the scheme. Virtually all other uses of the term anaplasia implies a high-grade neoplasm, most often WHO grade III that suggests a higher rate of local recurrence and an unfavorable prognosis. This designation often allows patients to be treated with the high-grade glioma protocols. The growing literature on tumors in the PXA category increasingly suggests that aPXA carries a significantly less favorable prognosis. While histological features alone may not identify all such tumors, they will identify at least some that may need to be considered in the high-grade category. The future revisions of the classification scheme should attempt to clarify these criteria more carefully, and allow designation of tumors with such features as truly anaplastic, such as WHO grade III, neoplasms. This also may benefit the prospective clinical trials that study the impact of adjuvant treatment in these more aggressive tumors.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Kepes JJ, Rubinstein LJ, Eng LF. Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer. 1979;44:1839–1852. doi: 10.1002/1097-0142(197911)44:5<1839::aid-cncr2820440543>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. IARC Press; Lyon, France: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleihues P, Burger PC, Scheithauer BW. The new WHO classification of brain tumours. Brain Pathol. 1993;3:255–268. doi: 10.1111/j.1750-3639.1993.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 4.Weldon-Linne CM, Victor TA, Groothuis DR, Vick NA. Pleomorphic xanthoastrocytoma. Ultrastructural and immunohistochemical study of a case with a rapidly fatal outcome following surgery. Cancer. 1983;52:2055–2063. doi: 10.1002/1097-0142(19831201)52:11<2055::aid-cncr2820521115>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 5.Kleihues P, Cavenee WK. Pathology and Genetics of Tumours of the Nervous System. World Health Organization Classification of Tumours. IARC Press; Lyon, France: 2000. [Google Scholar]
- 6.Giannini C, Scheithauer BW, Burger PC, et al. Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer. 1999;85:2033–2045. [PubMed] [Google Scholar]
- 7.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl. 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouladi M, Jenkins J, Burger P, et al. Pleomorphic xanthoastrocytoma: favorable outcome after complete surgical resection. Neuro Oncol. 2001;3:184–192. doi: 10.1093/neuonc/3.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasdahl DA, Scheithauer BW, Andrews BT, Jeffrey RA., Jr Cerebellar pleomorphic xanthoastrocytoma: case report. Neurosurgery. 1994;35:947–950; discussion 50–51. doi: 10.1227/00006123-199411000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Herpers MJ, Freling G, Beuls EA. Pleomorphic xanthoastrocytoma in the spinal cord. Case report. J. Neurosurg. 1994;80:564–569. doi: 10.3171/jns.1994.80.3.0564. [DOI] [PubMed] [Google Scholar]
- 11.Kros JM, Vecht CJ, Stefanko SZ. The pleomorphic xanthoastrocytoma and its differential diagnosis: a study of five cases. Hum. Pathol. 1991;22:1128–1135. doi: 10.1016/0046-8177(91)90265-q. [DOI] [PubMed] [Google Scholar]
- 12.Srinivas BH, Uppin MS, Panigrahi MK, Vijaya Saradhi M, Jyotsna Rani Y, Challa S. Pleomorphic xanthoastrocytoma of the pineal region. J. Clin. Neurosci. 2010;17:1439–1441. doi: 10.1016/j.jocn.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Okazaki T, Kageji T, Matsuzaki K, et al. Primary anaplastic pleomorphic xanthoastrocytoma with widespread neuroaxis dissemination at diagnosis – a pediatric case report and review of the literature. J. Neurooncol. 2009;94:431–437. doi: 10.1007/s11060-009-9876-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee DK, Cho KT, Im SH, Hong SK. Pleomorphic xanthoastrocytoma with an intracystic hemorrhage: a case report and literature review. J. Korean Neurosurg. Soc. 2007;42:410–412. doi: 10.3340/jkns.2007.42.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crespo-Rodriguez AM, Smirniotopoulos JG, Rushing EJ. MR and CT imaging of 24 pleomorphic xanthoastrocytomas (PXA) and a review of the literature. Neuroradiology. 2007;49:307–315. doi: 10.1007/s00234-006-0191-z. [DOI] [PubMed] [Google Scholar]
- 16.Rao AA, Laack NN, Giannini C, Wetmore C. Pleomorphic xanthoastrocytoma in children and adolescents. Pediatr. Blood Cancer. 2010;55:290–294. doi: 10.1002/pbc.22490. [DOI] [PubMed] [Google Scholar]
- 17.Saikali S, Le Strat A, Heckly A, Stock N, Scarabin JM, Hamlat A. Multicentric pleomorphic xanthoastrocytoma in a patient with neurofibromatosis type 1. Case report and review of the literature. J. Neurosurg. 2005;102:376–381. doi: 10.3171/jns.2005.102.2.0376. [DOI] [PubMed] [Google Scholar]
- 18.Kim B, Chung CK, Myung JK, Park SH. Pleomorphic xanthoastrocytoma associated with long-standing Taylor-type IIB-focal cortical dysplasia in an adult. Pathol. Res. Pract. 2009;205:113–117. doi: 10.1016/j.prp.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 19.Murray JC, Donahue DJ, Malik SI, et al. Temporal lobe pleomorphic xanthoastrocytoma and acquired BRAF mutation in an adolescent with the constitutional 22q11.2 deletion syndrome. J. Neurooncol. 2011;102:509–514. doi: 10.1007/s11060-010-0350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong J, Chu SG, Mao Y, Wang Y. Pigmented pleomorphic xanthoastrocytoma: a rare variant and literature review. Neuropathology. 2011;31:88–92. doi: 10.1111/j.1440-1789.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirose T, Ishizawa K, Sugiyama K, Kageji T, Ueki K, Kannuki S. Pleomorphic xanthoastrocytoma: a comparative pathological study between conventional and anaplastic types. Histopathology. 2008;52:183–193. doi: 10.1111/j.1365-2559.2007.02926.x. [DOI] [PubMed] [Google Scholar]
- 22.Giannini C, Hebrink D, Scheithauer BW, Dei Tos AP, James CD. Analysis of p53 mutation and expression in pleomorphic xanthoastrocytoma. Neurogenetics. 2001;3:159–162. doi: 10.1007/s100480100116. [DOI] [PubMed] [Google Scholar]
- 23.Powell SZ, Yachnis AT, Rorke LB, Rojiani AM, Eskin TA. Divergent differentiation in pleomorphic xanthoastrocytoma. Evidence for a neuronal element and possible relationship to ganglion cell tumors. Am. J. Surg. Pathol. 1996;20:80–85. doi: 10.1097/00000478-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Lindboe CF, Cappelen J, Kepes JJ. Pleomorphic xanthoastrocytoma as a component of a cerebellar ganglioglioma: case report. Neurosurgery. 1992;31:353–355. doi: 10.1227/00006123-199208000-00023. [DOI] [PubMed] [Google Scholar]
- 25.Kordek R, Biernat W, Alwasiak J, Liberski PP. Pleomorphic xanthoastrocytoma and desmoplastic infantile ganglioglioma – have these neoplasms a common origin? Folia Neuropathol. 1994;32:237–239. [PubMed] [Google Scholar]
- 26.Lach B, Duggal N, DaSilva VF, Benoit BG. Association of pleomorphic xanthoastrocytoma with cortical dysplasia and neuronal tumors. A report of three cases. Cancer. 1996;78:2551–2563. [PubMed] [Google Scholar]
- 27.Perry A, Giannini C, Scheithauer BW, et al. Composite pleomorphic xanthoastrocytoma and ganglioglioma: report of four cases and review of the literature. Am. J. Surg. Pathol. 1997;21:763–771. doi: 10.1097/00000478-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Weber RG, Hoischen A, Ehrler M, et al. Frequent loss of chromosome 9, homozygous CDKN2A/p14(ARF)/CDKN2B deletion and low TSC1 mRNA expression in pleomorphic xanthoastrocytomas. Oncogene. 2007;26:1088–1097. doi: 10.1038/sj.onc.1209851. [DOI] [PubMed] [Google Scholar]
- 29.Yin XL, Hui AB, Liong EC, Ding M, Chang AR, Ng HK. Genetic imbalances in pleomorphic xanthoastrocytoma detected by comparative genomic hybridization and literature review. Cancer Genet. Cytogenet. 2002;132:14–19. doi: 10.1016/s0165-4608(01)00512-x. [DOI] [PubMed] [Google Scholar]
- 30.Sawyer JR, Roloson GJ, Chadduck WM, Boop FA. Cytogenetic findings in a pleomorphic xanthoastrocytoma. Cancer Genet. Cytogenet. 1991;55:225–230. doi: 10.1016/0165-4608(91)90081-5. [DOI] [PubMed] [Google Scholar]
- 31.Hosokawa Y, Tsuchihashi Y, Okabe H, et al. Pleomorphic xanthoastrocytoma. Ultrastructural, immunohistochemical, and DNA cytofluorometric study of a case. Cancer. 1991;68:853–859. doi: 10.1002/1097-0142(19910815)68:4<853::aid-cncr2820680430>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Grau E, Balaguer J, Canete A, et al. Subtelomeric analysis of pediatric astrocytoma: subchromosomal instability is a distinctive feature of pleomorphic xanthoastrocytoma. J. Neurooncol. 2009;93:175–182. doi: 10.1007/s11060-008-9763-6. [DOI] [PubMed] [Google Scholar]
- 33.Li NY, Zhou J, Zhou HB, Ma HH. [Clinicopathologic study of pleomorphic xanthoastrocytoma of brain] Zhonghua Bing Li Xue Za Zhi. 2006;35:453–457. [PubMed] [Google Scholar]
- 34.Sawyer JR, Thomas EL, Roloson GJ, Chadduck WM, Boop FA. Telomeric associations evolving to ring chromosomes in a recurrent pleomorphic xanthoastrocytoma. Cancer Genet. Cytogenet. 1992;60:152–157. doi: 10.1016/0165-4608(92)90008-v. [DOI] [PubMed] [Google Scholar]
- 35.Kaulich K, Blaschke B, Numann A, et al. Genetic alterations commonly found in diffusely infiltrating cerebral gliomas are rare or absent in pleomorphic xanthoastrocytomas. J. Neuropathol. Exp. Neurol. 2002;61:1092–1099. doi: 10.1093/jnen/61.12.1092. [DOI] [PubMed] [Google Scholar]
- 36.Packer LM, Rana S, Hayward R, et al. Nilotinib and MEK inhibitors induce synthetic lethality through paradoxical activation of RAF in drug-resistant chronic myeloid leukemia. Cancer Cell. 2011;20:715–727. doi: 10.1016/j.ccr.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias-Santagata D, Lam Q, Vernovsky K, et al. BRAF V600E mutations are common in pleomorphic xanthoastrocytoma: diagnostic and therapeutic implications. PLoS One. 2011;6:e17948. doi: 10.1371/journal.pone.0017948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spirli C, Morell CM, Locatelli L, et al. Cyclic AMP/PKA-dependent paradoxical activation of Raf/MEK/ERK signaling in polycystin-2 defective mice treated with sorafenib. Hepatology. 2012;56:2363–2374. doi: 10.1002/hep.25872. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain MC. Salvage therapy with BRAF inhibitors for recurrent pleomorphic xanthoastrocytoma: a retrospective case series. J. Neurooncol. 2013;114:237–240. doi: 10.1007/s11060-013-1176-5. [DOI] [PubMed] [Google Scholar]
- 41.Hall-Jackson CA, Eyers PA, Cohen P, et al. Paradoxical activation of Raf by a novel Raf inhibitor. Chem. Biol. 1999;6:559–568. doi: 10.1016/s1074-5521(99)80088-x. [DOI] [PubMed] [Google Scholar]
- 42.Smalley KS, Flaherty KT. Integrating BRAF/MEK inhibitors into combination therapy for melanoma. Br. J. Cancer. 2009;100:431–435. doi: 10.1038/sj.bjc.6604891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X, Bandopadhayay P, Ng J, Ashley D, Chow CW. The evolution of the histology in pleomorphic xanthoastrocytomas in children: a study of 15 cases. Pathology. 2011;43:9–16. doi: 10.1097/PAT.0b013e328340bb98. [DOI] [PubMed] [Google Scholar]
- 44.Iwaki T, Fukui M, Kondo A, Matsushima T, Takeshita I. Epithelial properties of pleomorphic xanthoastrocytomas determined in ultrastructural and immunohistochemical studies. Acta Neuropathol. 1987;74:142–150. doi: 10.1007/BF00692844. [DOI] [PubMed] [Google Scholar]
- 45.Lubansu A, Rorive S, David P, et al. Cerebral anaplastic pleomorphic xanthoastrocytoma with meningeal dissemination at first presentation. Childs Nerv. Syst. 2004;20:119–122. doi: 10.1007/s00381-003-0854-6. [DOI] [PubMed] [Google Scholar]
- 46.Lim S, Kim JH, Kim SA, Park ES, Ra YS, Kim CJ. Prognostic factors and therapeutic outcomes in 22 patients with pleomorphic xanthoastrocytoma. J. Korean Neurosurg. Soc. 2013;53:281–287. doi: 10.3340/jkns.2013.53.5.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ng WH, Lim T, Yeo TT. Pleomorphic xanthoastrocytoma in elderly patients may portend a poor prognosis. J. Clin. Neurosci. 2008;15:476–478. doi: 10.1016/j.jocn.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 48.Passone E, Pizzolitto S, D‘Agostini S, et al. Non-anaplastic pleomorphic xanthoastrocytoma with neuroradiological evidences of leptomeningeal dissemination. Childs Nerv. Syst. 2006;22:614–618. doi: 10.1007/s00381-005-0008-0. [DOI] [PubMed] [Google Scholar]
- 49.Kepes JJ, Rubinstein LJ, Ansbacher L, Schreiber DJ. Histopathological features of recurrent pleomorphic xanthoastrocytomas: further corroboration of the glial nature of this neoplasm. A study of 3 cases. Acta Neuropathol. 1989;78:585–593. doi: 10.1007/BF00691285. [DOI] [PubMed] [Google Scholar]
- 50.Vu TM, Liubinas SV, Gonzales M, Drummond KJ. Malignant potential of pleomorphic xanthoastrocytoma. J. Clin. Neurosci. 2012;19:12–20. doi: 10.1016/j.jocn.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Prayson RA, Morris HH., 3rd Anaplastic pleomorphic xanthoastrocytoma. Arch. Pathol. Lab. Med. 1998;122:1082–1086. [PubMed] [Google Scholar]
- 52.Nakajima T, Kumabe T, Shamoto H, Watanabe M, Suzuki H, Tominaga T. Malignant transformation of pleomorphic xanthoastrocytoma. Acta Neurochir. (Wien) 2006;148:67–71; discussion 71. doi: 10.1007/s00701-005-0549-8. [DOI] [PubMed] [Google Scholar]
- 53.Marton E, Feletti A, Orvieto E, Longatti P. Malignant progression in pleomorphic xanthoastrocytoma: personal experience and review of the literature. J. Neurol. Sci. 2007;252:144–153. doi: 10.1016/j.jns.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Macaulay RJ, Jay V, Hoffman HJ, Becker LE. Increased mitotic activity as a negative prognostic indicator in pleomorphic xanthoastrocytoma. Case report. J. Neurosurg. 1993;79:761–768. doi: 10.3171/jns.1993.79.5.0761. [DOI] [PubMed] [Google Scholar]
- 55.Leonard N, Alcutt DA, Farrell MA. Fatal pleomorphic xanthoastrocytoma with meningeal gliomatosis. Histopathology. 1998;32:375–378. doi: 10.1046/j.1365-2559.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 56.Charbel FT. Pleomorphic xanthoastrocytoma with malignant progression. Surg. Neurol. 1998;50:385–386. doi: 10.1016/s0090-3019(97)00311-x. [DOI] [PubMed] [Google Scholar]
- 57.Allegranza A, Ferraresi S, Bruzzone M, Giombini S. Cerebromeningeal pleomorphic xanthoastrocytoma. Report on four cases: clinical, radiologic and pathological features. (Including a case with malignant evolution) Neurosurg. Rev. 1991;14:43–49. doi: 10.1007/BF00338191. [DOI] [PubMed] [Google Scholar]
- 58.de Tella OI, Jr, Herculano MA, Prandini MN, Stavale JN, Aguiar PH. Malignant transformation of pleomorphic xanthoastrocytoma: case report. Arq. Neuropsiquiatr. 2003;61:104–106. doi: 10.1590/s0004-282x2003000100020. [DOI] [PubMed] [Google Scholar]
- 59.Bayindir C, Balak N, Karasu A, Kasaroglu D. Anaplastic pleomorphic xanthoastrocytoma. Childs Nerv. Syst. 1997;13:50–56. doi: 10.1007/s003810050040. [DOI] [PubMed] [Google Scholar]
- 60.Fu YJ, Miyahara H, Uzuka T, et al. Intraventricular pleomorphic xanthoastrocytoma with anaplastic features. Neuropathology. 2010;30:443–448. doi: 10.1111/j.1440-1789.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 61.Kepes JJ. Glioblastoma multiforme masquerading as a pleomorphic xanthoastrocytoma. Childs Nerv. Syst. 1989;5:127. doi: 10.1007/BF00272110. [DOI] [PubMed] [Google Scholar]
- 62.Pahapill PA, Ramsay DA, Del Maestro RF. Pleomorphic xanthoastrocytoma: case report and analysis of the literature concerning the efficacy of resection and the significance of necrosis. Neurosurgery. 1996;38:822–828; discussion 8–9. [PubMed] [Google Scholar]
- 63.Chacko G, Chacko AG, Dunham CP, Judkins AR, Biegel JA, Perry A. Atypical teratoid/rhabdoid tumor arising in the setting of a pleomorphic xanthoastrocytoma. J. Neurooncol. 2007;84:217–222. doi: 10.1007/s11060-007-9361-z. [DOI] [PubMed] [Google Scholar]
- 64.Asano K, Miyamoto S, Kubo O, Kikkukawa T, Yagihashi A, Ohkuma H. A case of anaplastic pleomorphic xanthoastrocytoma presenting with tumor bleeding and cerebrospinal fluid dissemination. Brain Tumor Pathol. 2006;23:55–63. doi: 10.1007/s10014-006-0197-6. [DOI] [PubMed] [Google Scholar]
- 65.Gelpi E, Popovic M, Preusser M, Budka H, Hainfellner J. Pleomorphic xanthoastrocytoma with anaplastic features presenting without GFAP immunoreactivity: implications for differential diagnosis. Neuropathology. 2005;25:241–246. doi: 10.1111/j.1440-1789.2005.00612.x. [DOI] [PubMed] [Google Scholar]
- 66.Tsutsumi S, Abe Y, Yasumoto Y, Ito M. Anaplastic pleomorphic xanthoastrocytoma with a component of anaplastic astrocytoma presenting as skull base tumor followed by downward extracranial extension. Case report. Neurol. Med. Chir. (Tokyo) 2010;50:1108–1112. doi: 10.2176/nmc.50.1108. [DOI] [PubMed] [Google Scholar]