SUMMARY
The treatment of spinal metastasis has considerably improved with the advent of stereotactic body radiotherapy. Technological advances have enabled the precise delivery of high-dose radiation that may supplant surgery and standard fractionation postoperative radiation as a treatment for spinal metastasis without cord compression. Unfortunately, the higher biologically equivalent doses conferred by stereotactic body radiotherapy can also result in radiation toxicity, notably myelitis and vertebral body fracture. These are toxicities that the radiation oncologist must be able to anticipate, mitigate and manage. Although myelitis can be prevented largely by instituting dose constraints, it is less clear what the fracture risk of a structurally compromised vertebra is, and what should be done in terms of stabilization and dosimetry to mitigate this risk. This review answers these questions by defining the appropriate patient for stereotactic body radiotherapy, and what dose, fractionation and spinal stabilization should be used for potentially unstable spines.
Practice Points.
Potentially unstable spines (according to Spine Instability Neoplastic Score [SINS]) should be evaluated in a multidisciplinary setting.
Pre-existing fractures with lytic involvement >50% should have pre-stereotactic body radiotherapy (SBRT) stabilization with kyphoplasty or vertebroplasty.
Unstable spines should have stabilization pre- or immediately post-SBRT.
Pre-SBRT stabilization interventions that require significant hardware that may confound dosimetry with artifacts should be considered in the immediate post-SBRT setting.
Dosimetry and patient follow-up should be rigorously recorded and archived for multi-institutional evaluation to further define parameters for at-risk patients and proper dosimetric limits.
Epidemiology of spinal metastases
The treatment of spinal metastases to both alleviate pain and relieve or prevent spinal cord compression poses a common, yet challenging situation. Bone metastasis is the third most common site of metastases [1], with the spine being the most common subsite [2]. Between 30 and 36% of cancer patients have spinal involvement at death [3,4]. In addition to pain, 10–20% of patients with spinal metastasis will develop metastatic epidural spinal cord compression necessitating treatment [2], resulting in 20,000–25,000 cases per year [2,5].
▪ Treatment options & outcomes
Treatment of spinal metastasis has evolved from radiation or surgery alone into a new paradigm of high-dose radiation alone, delivered by stereotactic body radiotherapy (SBRT). The wide range of treatment options means that a patient with metastatic spine disease must be evaluated in a multidisciplinary setting. In order to evaluate the role of SBRT in spinal metastasis, the benefits and risks, consisting of local control (LC) and toxicity, of the alternatives must be understood.
Surgery (laminectomy) or conventionally fractionated radiotherapy alone
Surgical intervention has a long-standing role in treating spinal metastasis. Early surgical intervention consisted of a laminectomy to relieve spinal cord compression [6], with 64–88% of patients experiencing improvement in motor and pain symptoms [1]. In the non-cord compression setting, surgery enables histopathological confirmation of the tumor and an opportunity for spine stabilization. Studies comparing the role of conventionally fractionated radiotherapy (CFRT) to laminectomy showed no benefit for laminectomy [2,7,8], largely because the tumor is often in the vertebral body and, therefore, not removed. Owing to this equivalency, patients can be treated with CFRT alone [9].
The outcomes with CFRT alone are poor. With regards to functional improvement, a review of 4155 patients showed that 81% of patients remained ambulatory, but only 32% regained the ability to walk [9]. These numbers dropped to 60–74% and 19–33%, respectively, when only level 1 evidence was considered. The toxicity of CFRT alone was minimal (mucositis, diarrhea and fatigue) [9] and generally transient.
Surgery (corpectomy) with radiation
The poor results of laminectomy and CFRT alone prompted the development of advanced surgical techniques that removed the vertebral body, improving outcomes for both motor function and pain control [1,10,11]. Surgery, however, is not without risks: laminectomy and corpectomy have 6 and 10% mortality, respectively [1], with complications ranging from 0 to 10% and 10 to 54%, respectively [11,12].
With the improved functional outcomes of corpectomy over laminectomy [1,13], surgery was re-evaluated in concert with radiation. A landmark trial randomized patients with metastatic epidural spinal cord compression caused by a single contiguous lesion and exhibiting at least one neurological sign, to either decompressive surgery followed by CFRT or CFRT alone [14]. The surgical arm had improved functional outcomes and this became the standard of care.
Stereotactic body radiotherapy
Although immediate surgical decompression is indicated for cord compression, worsening neurologic compromise due to metastatic epidural spinal cord compression or bony impingement, patients not meeting these selection criteria (or who are not surgical candidates) often want to avoid the significant morbidity, mortality and recovery time associated with surgery. With the intention of balancing goals of care, patient preference and prognosis – including the opportunity for effective systemic therapy – there has been considerable interest in using radiation alone to achieve LC and functional outcomes comparable with surgery and CFRT. This has led to an increase in the total dose and dose per fraction, known as hypofractionation, resulting in a higher bioequivalent dose (BED).
Early attempts at hypofractionation were hindered by the inability to precisely deliver radiation, making the spinal cord the dose limiting structure. Consequently, hypofractionation trials could only utilize a moderate dose, and 8 Gy in a single fraction (1 × 8 Gy) to spinal metastases did not show significant differences from CFRT [15].
Significant technological advances in the field of radiosurgery, along with better understanding of cord tolerance in the hypofractionated setting, has enabled the accurate delivery of significantly greater BED to spinal metastases. Extreme care must be taken during planning, as the traditional paradigm of normal tissue repair with CFRT is markedly reduced in the hypofractionated setting; knowledge of dose constraints to critical structures is essential. Techniques such as real-time image guidance, modern immobilization techniques, advanced treatment planning and highly conformal delivery have enabled shrinking of margins, reducing the needed to treat adjacent vertebral bodies, lowering the chance of radiation toxicity.
The doses used in radiosurgery are guided by delivering the maximal dose to the tumor, providing excellent LC and pain relief, while respecting critical structure tolerance. For patients not previously radiated, clinicians often use single-fraction doses of 18–24 Gy [16–18]. Single-fraction doses of 24 Gy are considered curative for local tumors [19], and this dose, or 24–27 Gy in three fractions, more than doubles the BED of CFRT [20]. Dose coverage, and in particular minimum dose coverage, may be a predictor of LC [21]. Currently, RTOG 0631 is examining pain control with 1 × 16–18 Gy, but increased doses may be optimal for increased LC, particularly for unfavorable histologies. Additional sparing of critical structures can also be obtained by additional fractionation, such as 5 × 6 Gy [22]. These higher doses have led to higher LC rates for SBRT, typically upwards of 90%. A recent Phase II trial showed excellent pain control and LC with SBRT in three fractions of 9–10 Gy with minimal toxicity [23]. Patterns of failure are consistent with this high rate of LC [24], as tumors do not recur in-field. Additionally, certain histologies have been traditionally classified as ‘radio resistant’, such as sarcoma, melanoma, renal cell and non-small-cell lung carcinoma [9], and have been ineffectively treated using CFRT. Radiobiologically, these tumors are assumed to have a low α:β ratio [25] and are theoretically more responsive to hypofractionated radiation. Not surprisingly, early studies using CFRT showed differing responses in motor function for various tumor histologies, with breast, myeloma, prostate and lymphoma being among those with a better duration of response (favorable histologies), and lung, liver, kidney and bladder (unfavorable) having a poorer and shorter duration of response [26]. Case series using single-session SBRT show that 87, 100, 100 and 75% of patients with metastatic renal [16], breast [17] and lung [27] cancer, and melanoma [28], respectively, were radiographically controlled.
▪ SBRT toxicity
The benefits of SBRT on LC and pain reduction are not without risks. The high BED of SBRT introduces a side-effect profile not commonly seen with CFRT: radiation toxicity in the form of radiation myelitis, esophageal ulceration or vertebral body fracture. The risk of each of these toxicities can vary based on the location of the metastatic disease, particularly if epidural extension abuts a critical structure. For the purpose of this review, we will emphasize the effect of SBRT on vertebral fractures.
Myelopathy
The tolerance of the spinal cord often represents the dose-limiting critical structure. In CFRT, a dose of 45–50 Gy gives a 5% chance of myelopathy [29]; although 60 Gy has been suggested as a more accurate figure [18]. In the hypofractionated setting, dose constraints of the spinal cord range from a maximum point dose of 10–12 Gy [18] to volume [V]8Gy <1 cc [30], V10Gy <10% [31] or V12Gy <0.15 cc [32]. A recent comprehensive review showed that a 5% risk of myelopathy occurred with fractions and doses of 1 × 12.4 Gy, 2 × 8.5 Gy, 3 × 6.7 Gy, 4 × 5.75 Gy and 5 × 5.06 Gy [33].
Esophagus
The esophagus represents another organ at risk when the cervical and thoracic spines are involved. SBRT trials have limited the esophagus to a point dose of 3 × 9 Gy (RTOG 0618) with dose received by 5 cc of tissue (D5cc) <7 Gy per fraction [34] or 1 × 14 Gy [35] with dose received by 50% of the structure volume (D50%) <10 Gy [36]. A retrospective study of 1 × 24 Gy to the spine found that doses of Dmax = 22 Gy and V2.5 cc <14 Gy to the esophagus confer a relative risk of 6–13% for grade ≥3 toxicity and an average risk of 3% [37].
Vertebral body fracture
Radiation to bone invaded by a tumor can lead to increased risk of fracture [38,39], poorer bone healing [40] and weakening of adjacent bone [41]. A review of palliative radiotherapy regimens for bone metastases showed a fracture rate of 4–5% with conventional doses (1 × 8 Gy to 10 × 3 Gy) [42]. These effects are exacerbated by the high BED delivered with SBRT and, therefore, vertebral body fracture should be anticipated in susceptible patients. The challenge lies in identifying these patients. Retrospective studies have identified several factors predisposing patients to fracture. In a study of 123 vertebral bodies involved with metastatic disease (93 patients), with a median follow-up of 15 months and SBRT doses of 1 × 18 Gy, 3 × 9 Gy or 5 × 6 Gy (BED: α:β = 3, or BED3: 126, 108 and 90, respectively), 20% of vertebral bodies exhibited new or progressing fractures with a median time to progression of 3 months (Figure 1) [43]. As expected, a pre-existing fracture was a significant predictor of fracture progression (hazard ratio [HR]: 6), implying that prophylactic stabilization prior to SBRT should be considered. Other risk factors included age >55 years (HR: 6) and existing pain (HR: 1.4), as well as large, lytic lesions (HR: 4.5 for >80% involvement). Another study noted a 39% chance of fracture progression after SBRT with a median time to fracture of 25 months (19 for lytic, 32 for mixed and sclerotic) when single-fraction doses of 18–24 Gy (BED3: 124–216) were used [44]. No dose response was seen in this range of doses. Risk factors included lytic lesions (HR: 3.8) and vertebral body involvement (>40%; HR: 3.9). Location caudal to T10 resulted in a median time to fracture of 20 months versus higher lesions at 35 months, probably due to increased weight-bearing mechanical stress. An analysis of 167 vertebra bodies [45] with metastatic cancer treated with SBRT (dose range: 5 × 5 Gy to 1 × 24 Gy) showed a fracture rate of 11% (two out of three de novo and one out of three progression of an existing fracture). They found that histology (lung and hepatocellular), type of lesion (lytic, mixed/sclerotic or blastic) and dose per fraction of >20 Gy (BED3: 153) were predictive of fracture. Other retrospective series reported only a few instances of fractures after SBRT in their cohorts (Table 1). This is largely because unstable spines are typically excluded. Yamada reported two fractures out of 103 treated lesions when treated in a single fraction of 24 Gy (BED3: 216) [19]. A review of a series of 200 patients (274 vertebral bodies) noted two patients that developed fractures after resection and SBRT to 21–24 Gy in three fractions (BED3: 70–88) and to 37.5 Gy in five fractions (BED3: 131) [46]. They postulate that these fractures are likely to have been caused by placing the hardware on osteoporotic bone and recommended reinforcement of osteoportic vertebrae with polymethylmethacrylate.
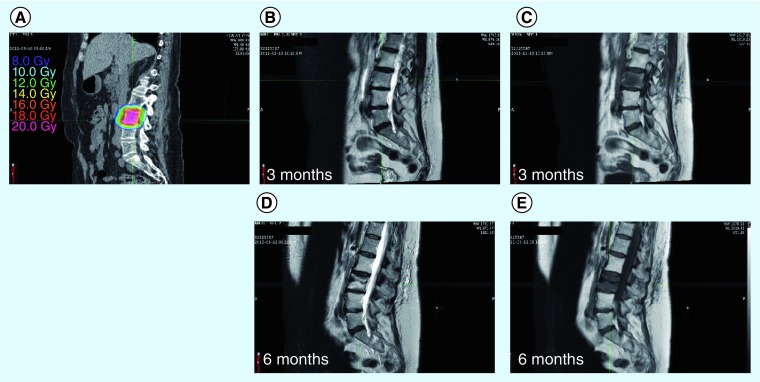
Figure 1. A 57-year-old female patient with a metastatic neuroendocrine tumor who was treated for L3 vertebral metastasis to a dose of 16 Gy in a single session, prescribed to a 71% isodose line.
Approximately 6 months after treatment, the patient returned for follow-up and was found to have an asymptomatic vertebral body fracture at the site of original treatment. (A) The delivered treatment. (B & D) T2-weighted magnetic resonance sequences at 3 and 6 months, respectively, and (C & E) T1-weighted sequences at 3 and 6 months, respectively. (D & E) Loss of L3 vertebral body height at 6 months.
Table 1. . Large series of spinal stereotactic body radiotherapy and reported fracture incidence.
| Study (year) | FU (months) | Dose; Gy (mean) | Isodose (%) | BED3 (Gy) | Patients (n) | Lesions (n) | Fractures; n (%) | Notes | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Boehling et al.(2012) | 14.9 | 1 × 18 3 × 9 5 × 6 |
– | 126 108 90 |
93 | 123 | 25 (20%) | – | [43] |
| Cunha et al. (2012) | 7.4 | 1 × 24 to 5 × 7 | – | 216 116 |
90 | 167 | 19 (11%) | Median time to fracture: 2 months; unstable patients were excluded | [45] |
| Rose et al. (2009) | 13 | 1 × 18 to 1 × 24 | 100 | 124–216 | 62 | 71 | 27 (39%) | Lytic lesions and 40% involvement were risk factors | [44] |
| Gagnon et al. (2009) | 12 | 3 × 7–8 5 × 7.5 |
75 | 88 131 |
200 | 274 | 2 | – | [46] |
| Yamada et al. (2008) | 15 | 1 × 18–24 (24) | 100 | – | 93 | 103 | 2 | – | [19] |
| Chang et al. (2007) | 21.3 | 5 × 6 3 × 9 |
– | 90 108 |
63 | 74 | NR | Excluded unstable spine | [62] |
| Degen et al. (2005) | 12 | (3.6) × (6.45) | 70 | 73 | 51 | 72 | NR | – | [63] |
| Dodd et al. (2006) | 23 | 1 × (19.6) | 80 | 148 | 51 | 55 | NR | – | [64] |
| Gertszen et al. (2007) | 21 | 12.5–25 | 80 | – | 393 | 500 | NR | Excluded unstable spine | [27] |
| Gertszen et al. (2004) | 18 | 1 × 12–20 (14) | – | 80 | 115 | 125 | NR | – | [65] |
BED3: Bioequivalent dose; FU: Follow-up; NR: Not reported.
SBRT & fracture: the lung experience
Due to the paucity of information on fracture incidence with SBRT to the spine, we draw from the experiences with lung SBRT and rib fractures to assess dose tolerance. Since the rib typically lacks direct tumor involvement and is not weight bearing, doses of radiation that cause rib fractures should represent an upper limit of radiation for the vertebra. A recent series noted that 23% of patients had a fracture after receiving a BED3 of 240 Gy, while those without fractures received 146 Gy [47]. Another study found that nine out of 42 patients (21%) of patients had rib fractures when the ribs received between 46 and 50 Gy in three fractions (BED3: 281–327) [48]. Dunlap et al. noted that >30 Gy in three to five (BED3: 90–130) fractions to >30 cc resulted in an increased risk of rib fractures [49]. The volume effect may explain the relatively low, observed BED3 threshold values. An analysis using a smaller volume constraint noted that the 5 and 50% chances of causing a rib fracture occurred when 2 cc was irradiated to 3 × 9.1 Gy (BED3: 110) and 3 × 16.6 Gy (BED3: 325) [50], while others showed the mean maximum dose to 2 cc of patients (57 out of 500) who had rib fractures was 55 Gy in five fractions (BED3: 257) [51].
Taken together, the BED3 data from the SBRT lung and spine experiences are consistent. The majority of rib fractures are above 250 Gy BED3 and the risk of fracture for SBRT of the spine begins to appear at a lower BED3 of 150 Gy, as we would expect.
Evaluation of the spine: Spine Instability Neoplastic Score
To provide a metric to consistently assess spinal stability in the setting of neoplastic disease, the Spine Oncology Study Group has defined criteria to predict fracture in vertebral bodies with neoplastic disease: the Spine Instability Neoplastic Score (SINS) (Table 2) [52,53]. Previous efforts to categorize risk had centered on weight bearing bones of the extremities [54,55]. The complete scoring system can be found elsewhere [52], but briefly, there are six categories each with a potential of 3 points each: location, pain, bone lesion and radiographic spinal alignment (4 points); vertebral body collapse (with percentage involvement); and posterolateral involvement of spinal elements. These concur with the factors predisposing patients to vertebral fracture in the retrospective series discussed above: lytic lesions, vertebral body collapse (implying significant neoplastic involvement) and mechanical stress. Scores of 0–6, 7–12 and 13–18 are considered stable, potentially unstable, and unstable, respectively. The sensitivity and specificity for potentially unstable, and unstable lesions was found to be 95.7 and 79.5%, respectively [52]. It should be noted that >50% involvement of a vertebral body contributes only one component to the SINS scale, but probably has a disproportionately higher contribution to fracture rates post-SBRT than the SINS scale suggests. Patients meeting selection criteria for SBRT, with potentially unstable, or unstable SINS lesions, particularly those with lytic histology and significant vertebral involvement, should be evaluated for surgical management for stabilization prior to SBRT.
Table 2. . Spine Instability Neoplastic Score.
| SINS component | Score |
|---|---|
| Location | |
| Junctional (occiput–C2, C7–T2, T11–L1, L5–S1) | 3 |
| Mobile spine (C3–C6, L2–L4) | 2 |
| Semirigid (T3–T10) | 1 |
| Rigid (S2–S5) | 0 |
| Pain† | |
| Yes | 3 |
| Occasional pain but not mechanical | 1 |
| Pain-free lesion | 0 |
| Bone lesion | |
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Sublaxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse with >50% of body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement of spinal elements‡ | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
†Pain improvement with recumbency and/or pain with movement/loading of spine.
‡Facet, pedicle or costovertebral joint fracture or replacement with tumor.
SINS: Spinal Instability Neoplastic Score.
Adapted with permission from [52].
Identifying the patient at risk for fracture
Determining a patient's risk for developing a spine fracture is of paramount importance given the variety of treatment options and toxicity profiles mentioned above. Three major considerations include: the risk for vertebral facture (either pre-existing or post-SBRT); who would benefit from a preoperative stabilization procedure; and optimal dose and fractionation.
Patients with a pre-existing small fracture or compression fracture are at nearly a sixfold increased risk for fracture progression after radiation [43]; these patients should be considered for preradiation vertebroplasty or kyphoplasty, respectively. Recent data show that this is a safe and efficacious practice, with minimal morbidity and delay in treating the patient [56].
In the absence of an existing fracture, characteristics of the lesion and spine should be considered. Lesions that are lytic or that occupy greater than 40–50% of the vertebral body volume have a higher propensity to fracture and preradiation vertebroplasty or kyphoplasty should be considered. Spines that are unstable or potentially unstable by the SINS criteria should be considered for prophylactic stabilization using a minimally invasive surgical (MIS) procedure in a multidisciplinary setting.
The primary goal of radiotherapy is to relieve pain, achieve LC and improve neurological function. These factors should not be compromised as salvage is difficult. In the single-fraction setting, doses often range from 18 to 24 Gy (or 3 × 8–9 Gy or 5 × 6 Gy if fractionation is desired) with a high degree of LC, with lower and higher BED for favorable and unfavorable histologies, respectively.
▪ Surgical management for pre-SBRT spine stabilization
There are various minimally invasive options for spinal stabilization: vertebroplasty, kyphoplasty and MIS techniques [57]. Vertebral body fractures can present as small fractures or larger compression fractures that cause a substantial change in the size of the vertebral body. Small fractures are often treated with vertebroplasty. Larger compression fractures are treated with kyphoplasty, which has shown significant, persistent pain reduction, with a lower rate of cement extravasation than vertebroplasty [58,59].
Treatment of the patient who is at risk for fracture, but does not have one, is controversial. Vertebrae can be structurally weakened by an invading tumor, and it is known that both location (due to weight-bearing stress) and percentage vertebral body involvement can increase the likelihood of fracture [60,61]. In a more proactive approach to patients with pre-existing vertebral body compression [56], balloon kyphoplasty was performed on 26 patients with known compression fractures from metastatic disease, followed by single-fraction SBRT to a mean of 18 Gy. Although patients were treated with SBRT, no toxicity was noted and 90% of patients experienced a reduction in pain a mean 12 days after kyphoplasty. Only a single case of progressive kyphosis was noted.
For patients with significant vertebral body involvement (>50%) with lytic lesions, stabilization with MIS should be considered in advance of radiosurgery with BED3: >150 Gy. MIS can consist of percutaneous posterior pedicle screw-based stabilization, limited open decompressive procedures and percutaneous decompression (extracavitary/costotransversectomy or direct lateral approach) [57]. These procedures can add spine stability and provide some decompression if needed, without the morbidity associated with a corpectomy. These procedures are contraindicated for vascular tumors, such as renal cell, and for patients who lack anterior column support as the materials for percutaneous implants are not as structurally strong as those for open procedures. For patients with unstable SINS scores (particularly >50% lytic lesion involvement or multilevel disease), efforts should be made to stabilize the spine prior to SBRT. For patients that will require significant hardware for stabilization, stabilization should be considered immediately post-SBRT, as the artifacts introduced by hardware can make accurate calculation of dosimetry challenging. For patients with epidural extension, which may be exacerbated by radiation, MIS procedures to decompress the cord should be considered. Neurologic deficits or frank cord compression should be dealt with by immediate surgical intervention as noted above.
Conclusion & future perspective
Metastatic disease to the spine represents a common, yet difficult treatment paradigm. Treatment options have improved dramatically from laminectomy or CFRT alone. Although patients who present with cord compression or recent-onset neurological symptoms should be considered for urgent surgical decompression, it involves significant morbidity, mortality and recovery time, despite being palliative in nature. For patients with spinal metastases without neurological compromise, advances in radiotherapy enable highly conformal delivery of radiation to the vertebral body, while sparing critical structures. In addition to improved LC and neurological function, SBRT has the added benefit of patient convenience, with treatments often completed in a single outpatient session. For patients with a life expectancy <1 month, 1 × 8 Gy may be appropriate to avoid the delay caused by simulation and planning for SBRT.
Due to the high BED delivered, SBRT does come with the potential for vertebral body fracture. This drawback is challenging, but not insurmountable thanks to the variety of treatment options available. For the patient with an unstable or potentially unstable spine, particularly with a pre-existing fracture or extensive lytic involvement, thorough pretreatment evaluation and a multidisciplinary approach may achieve the goal of locally curative radiation and improved quality of life.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest
- 1.Witham TF, Khavkin YA, Gallia GL, Wolinsky JP, Gokaslan ZL. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nat. Clin. Pract. Neurol. 2006;2(2):87–94. doi: 10.1038/ncpneuro0116. [DOI] [PubMed] [Google Scholar]
- 2.Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist. 2004;9(2):188–196. doi: 10.1634/theoncologist.9-2-188. [DOI] [PubMed] [Google Scholar]
- 3.Ortiz Gomez JA. The incidence of vertebral body metastases. Int. Orthop. 1995;19(5):309–311. doi: 10.1007/BF00181116. [DOI] [PubMed] [Google Scholar]
- 4.Wong DA, Fornasier VL, Macnab I. Spinal metastases: the obvious, the occult, and the impostors. Spine (Phila. Pa 1976) 1990;15(1):1–4. [PubMed] [Google Scholar]
- 5.Byrne TN. Spinal cord compression from epidural metastases. N. Engl. J. Med. 1992;327(9):614–619. doi: 10.1056/NEJM199208273270907. [DOI] [PubMed] [Google Scholar]
- 6.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir. (Wien) 1998;140(9):957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann. Neurol. 1978;3(1):40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 8.Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J. Neurosurg. 1980;53(6):741–748. doi: 10.3171/jns.1980.53.6.0741. [DOI] [PubMed] [Google Scholar]
- 9.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila. Pa 1976) 2009;34(22 Suppl.):S78–S92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]; ▪ Comprehensive review of the stereotactic body radiotherapy (SBRT) and conventionally fractionated radiotherapy spine literature and outcomes.
- 10.Moulding HD, Elder JB, Lis E, et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J. Neurosurg. Spine. 2010;13(1):87–93. doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 11.Wang JC, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. J. Neurosurg. Spine. 2004;1(3):287–298. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 12.Loblaw DA, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: the Cancer Care Ontario Practice Guidelines Initiative's Neuro-Oncology Disease Site Group. J. Clin. Oncol. 2005;23(9):2028–2037. doi: 10.1200/JCO.2005.00.067. [DOI] [PubMed] [Google Scholar]
- 13.Klimo P, Thompson CJ, Kestle JR, Schmidt MH. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro Oncol. 2005;7(1):64–76. doi: 10.1215/S1152851704000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 15.Tombolini V, Zurlo A, Montagna A, et al. Radiation therapy of spinal metastases: results with different fractionations. Tumori. 1994;80(5):353–356. doi: 10.1177/030089169408000508. [DOI] [PubMed] [Google Scholar]
- 16.Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J. Neurosurg. Spine. 2005;3(4):288–295. doi: 10.3171/spi.2005.3.4.0288. [DOI] [PubMed] [Google Scholar]
- 17.Gerszten PC, Burton SA, Welch WC, et al. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104(10):2244–2254. doi: 10.1002/cncr.21467. [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Lovelock DM, Bilsky MH. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61(2):226–235. doi: 10.1227/01.NEU.0000279970.10309.B5. [DOI] [PubMed] [Google Scholar]
- 19.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int. J. Radiat. Oncol. Biol. Phys. 2008;71(2):484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 20.Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J. Neurosurg. Spine. 2011;14(2):151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 21.Lo SS, Chang EL, Yamada Y, Sloan AE, Suh JH, Mendel E. Stereotactic radiosurgery and radiation therapy for spinal tumors. Expert Rev. Neurother. 2006;7(1):85–93. doi: 10.1586/14737175.7.1.85. [DOI] [PubMed] [Google Scholar]
- 22.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J. Neurosurg. Spine. 2007;7(2):151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 23.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a Phase 1–2 trial. Lancet Oncol. 2012;13(4):395–402. doi: 10.1016/S1470-2045(11)70384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu S, Rock J, Rosenblum M, Kim JH. Patterns of failure after single-dose radiosurgery for spinal metastasis. Special Suppl. 2004;101(Suppl. 3):402–405. [PubMed] [Google Scholar]
- 25.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989;62(740):679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 26.Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 1995;32(4):959–967. doi: 10.1016/0360-3016(95)00572-g. [DOI] [PubMed] [Google Scholar]
- 27.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 28.Gerszten PC, Burton SA, Quinn AE, Agarwala SS, Kirkwood JM. Radiosurgery for the treatment of spinal melanoma metastases. Stereotact. Funct. Neurosurg. 2005;83(5–6):213–221. doi: 10.1159/000091952. [DOI] [PubMed] [Google Scholar]
- 29.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs IC, Patil C, Gerszten PC, Adler JRJ, Burton SA. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64(2):A67–A72. doi: 10.1227/01.NEU.0000341628.98141.B6. [DOI] [PubMed] [Google Scholar]
- 31.Ryu S, Jin J-Y, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109(3):628–636. doi: 10.1002/cncr.22442. [DOI] [PubMed] [Google Scholar]
- 32.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother. Oncol. 2007;82(2):185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 33.Sahgal A, Weinberg V, Ma L, et al. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int. J. Radiat. Oncol. Biol. Phys. 2013;85(2):341–347. doi: 10.1016/j.ijrobp.2012.05.007. [DOI] [PubMed] [Google Scholar]; ▪ Comprehensive review of dose-limiting toxicities for the spinal cord with SBRT, representing a dose-limiting constraint for SBRT to vertebral metastasis.
- 34.Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: a single institution Phase I–II study. Acta Oncologica. 2006;45(7):831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 35.Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a Phase I/II trial. J. Clin. Oncol. 2001;19(1):164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 36.Whyte RI, Crownover R, Murphy MJ, et al. Stereotactic radiosurgery for lung tumors: preliminary report of a Phase I trial. Ann. Thorac. Surg. 2003;75(4):1097–1101. doi: 10.1016/s0003-4975(02)04681-7. [DOI] [PubMed] [Google Scholar]
- 37.Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(5):e661–e667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baxter N, Habermann E, Tepper J, Durham S, Virnig B. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294(20):2587–2593. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 39.Keene JS, Sellinger DS, McBeath AA, Engber WD. Metastatic breast cancer in the femur. A search for the lesion at risk of fracture. Clin. Orthop. Relat. Res. 1986;203:282–288. [PubMed] [Google Scholar]
- 40.Pelker RR, Friedlaender GE. The Nicolas Andry Award-1995 fracture healing; radiation induced alterations. Clin. Orthop. Relat. Res. 1997;341:267–282. [PubMed] [Google Scholar]
- 41.Healey JH, Brown HK. Complications of bone metastases. Cancer. 2000;88(S12):2940–2951. doi: 10.1002/1097-0142(20000615)88:12+<2940::aid-cncr10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 42.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J. Clin. Oncol. 2007;25(11):1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 43.Boehling NS, Grosshans DR, Allen PK, et al. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J. Neurosurg. Spine. 2012;16(4):379–386. doi: 10.3171/2011.11.SPINE116. [DOI] [PubMed] [Google Scholar]; ▪ Retrospective review of spinal fractures after SBRT that identifies risk factors.
- 44.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J. Clin. Oncol. 2009;27(30):5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunha MVR, Al-Omair A, Atenafu EG, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(3):e343–e349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 46.Gagnon GJ, Nasr NM, Liao JJ, et al. Treatment of spinal tumors using cyberknife fractionated stereotactic radiosurgery: pain and quality-of-life assessment after treatment in 200 patients. Neurosurgery. 2009;64(2):297–307. doi: 10.1227/01.NEU.0000338072.30246.BD. [DOI] [PubMed] [Google Scholar]
- 47.Nambu A, Onishi H, Aoki S, et al. Rib fracture after stereotactic radiotherapy on follow-up thin-section computed tomography in 177 primary lung cancer patients. Rad. Oncol. 2011;6(1):137. doi: 10.1186/1748-717X-6-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voroney JP, Hope A, Dahele MR, et al. Chest wall pain and rib fracture after stereotactic radiotherapy for peripheral non-small cell lung cancer. J. Thorac. Oncol. 2009;4(8):1035–1037. doi: 10.1097/JTO.0b013e3181ae2962. [DOI] [PubMed] [Google Scholar]
- 49.Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving >30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010;76(3):796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 50.Pettersson N, Nyman J, Johansson KA. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: a dose- and volume-response analysis. Radiother. Oncol. 2009;91(3):360–368. doi: 10.1016/j.radonc.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 51.Bongers EM, Haasbeek CJA, Lagerwaard FJ, Slotman BJ, Senan S. Incidence and risk factors for chest wall toxicity after risk-adapted stereotactic radiotherapy for early-stage lung cancer. J. Thorac. Oncol. 2011;6(12):2052–2057. doi: 10.1097/JTO.0b013e3182307e74. [DOI] [PubMed] [Google Scholar]
- 52.Fourney DR, Frangou EM, Ryken TC, et al. Spinal instability neoplastic score: an analysis of reliability and validity from the Spine Oncology Study Group. J. Clin. Oncol. 2011;29(22):3072–3077. doi: 10.1200/JCO.2010.34.3897. [DOI] [PubMed] [Google Scholar]
- 53.Fisher CG, Dipaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila. Pa 1976) 2010;35(22):e1221–e1229. doi: 10.1097/BRS.0b013e3181e16ae2. [DOI] [PubMed] [Google Scholar]; ▪ Spinal Instability Neoplastic Score (SINS) classification system for spinal instability.
- 54.Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin. Orthop. Relat. Res. 1989;249:256–264. [PubMed] [Google Scholar]
- 55.Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr. Course Lect. 1986;35:357–381. [PubMed] [Google Scholar]
- 56.Gerszten PC, Germanwala A, Burton SA, Welch WC, Ozhasoglu C, Vogel WJ. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J. Neurosurg. Spine. 2005;3(4):296–301. doi: 10.3171/spi.2005.3.4.0296. [DOI] [PubMed] [Google Scholar]; ▪ Study of prophylactic kyphoplasty before SBRT.
- 57.Rose PS, Clarke MJ, Dekutoski MB. Minimally invasive treatment of spinal metastases: techniques. Int. J. Surg. Oncol. 2011;2011:494381. doi: 10.1155/2011/494381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coumans JV, Reinhardt MK, Lieberman IH. Kyphoplasty for vertebral compression fractures: 1-year clinical outcomes from a prospective study. J. Neurosurg. Spine. 2003;99(1):44–50. doi: 10.3171/spi.2003.99.1.0044. [DOI] [PubMed] [Google Scholar]
- 59.Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J. Neurosurg. 2003;98(1 Suppl.):21–30. doi: 10.3171/spi.2003.98.1.0021. [DOI] [PubMed] [Google Scholar]
- 60.Shah AN, Pietrobon R, Richardson WJ, Myers BS. Patterns of tumor spread and risk of fracture and epidural impingement in metastatic vertebrae. J. Spinal Disord. Techn. 2003;16(1):83–89. doi: 10.1097/00024720-200302000-00013. [DOI] [PubMed] [Google Scholar]
- 61.Taneichi H, Kaneda K, Takeda N, Abumi K, Satoh S. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine (Phila. Pa 1976) 1997;22(3):239–245. doi: 10.1097/00007632-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 62.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J. Neurosurg. Spine. 2007;7(2):151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 63.Degen JW, Gagnon GJ, Voyadzis J-M, et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J. Neurosurg. Spine. 2005;2(5):540–549. doi: 10.3171/spi.2005.2.5.0540. [DOI] [PubMed] [Google Scholar]
- 64.Dodd RL, Ryu MR, Kamnerdsupaphon P, Gibbs IC, Chang SD, Jr, Adler JR., Jr CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58(4):674–685. doi: 10.1227/01.NEU.0000204128.84742.8F. Discussion 674–685. [DOI] [PubMed] [Google Scholar]
- 65.Gerszten PC, Ozhasoglu C, Burton SA, et al. CyberKnife frameless stereotactic radiosurgery for spinal lesions: clinical experience in 125 cases. Neurosurgery. 2004;55(1):89–98. Discussion 98–89. [PubMed] [Google Scholar]