Abstract
Hormesis is a new concept in dose–response relationship. Despite of traditional dose–response curves, there is a low-dose stimulation and a high-dose inhibition in this case. Hormesis effect in apoptosis induction/inhibition by natural compounds is reported previously. Here, we searched this effect for myeloid cell leukemia type-1 (Mcl-1) gene expression by phytochemicals 7-isopenthenyloxycoumarin (7-IP), arctigenin (Arg), and hesperidin (Hsp). For this purpose, first we tested the cytotoxicity of various doses of these compounds against K562 leukemia cell lines for different times by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide method. After that we explored the effect of various doses of these phytochemicals on Mcl-1 gene expression for different times by real-time polymerase chain reaction method. We found that these phytochemicals have cytotoxicity against K562 cell line. Hesperidin is the most cytotoxic agent. We also found that these natural compounds have hormetic effect on Mcl-1 gene expression. The hormetic model in Mcl-1 gene expression is overcompensation stimulation. This phenomenon is reported for the first time. We conclude that 7-IP, Arg, and Hsp are cytotoxic against K562 cancerous cells and induce/inhibit Mcl-1 gene expression by hormesis dose–response relationship.
Keywords: 7-isopenthenyloxycoumarin, arctigenin, hesperidin, myeloid cell leukemia type-1 (Mcl-1), hormesis
Introduction
Cancer is the uncontrolled growth of body cells. Cancer of the body’s blood forming cells is named leukemia. One of the leukemia types is chronic myelogenous leukemia (CML). Chronic myelogenous leukemia accounts for approximately 10% to 15% of all leukemias. The worldwide annual incidence of CML is approximately 1 to 1.5 in 100 000 persons.1 The survival rate ≥5 years from 2006 through 2012 is approximately 65% of patients.2
Apoptosis means a physiological cell suicide program and is essential for the regulation of development, the maintenance of homeostasis and the prevention of tumorigenesis. Disruption the apoptotic program is one of the hallmarks of cancer.3 So induction of apoptosis in cancerous cells is one of the main strategies in chemotherapy. Apoptotic pathways consist of intrinsic and extrinsic pathway. The intrinsic pathway (also known as the mitochondrial pathway) integrates various intracellular signals at the mitochondrial membrane and is regulated by Bcl-2 family proteins.3 Bcl-2 family proteins include 3 subfamilies. One of them is antiapoptotic proteins include Bcl-2, Bcl-xL, Bcl-w, myeloid cell leukemia type-1 (Mcl-1), and A1. Antiapoptotic proteins are controlled by another Bcl-2 subfamily named BH3-only members (Bim, Puma, Bid, Bad, Noxa, etc).
The constitutive active fusion tyrosine kinase Bcr-Abl is responsible for deregulated apoptosis and resistance to cytotoxic insults in CML cells.4 In CML pathophysiology, Bcr-Abl protein inhibits Bim, and Bim inhibits all antiapoptotic Bcl-2 proteins include Mcl-1.4 So inhibition of Mcl-1 gene and protein expression could be a strategic plan for CML chemotherapy.
One of the main concepts in dose–response relationships is hormesis. Hormetic effects are seen when the dose–response curve is biphasic, with 2 types of biological responses when an organism is exposed to different doses of a stress agent. One of them is stimulatory and the other is inhibitory.5 Based on Calabrese’s report, many toxic substances induce hormesis.6,7 The hermetic-like effect in apoptosis induction/inhibition by phytochemicals reported previously.8,9
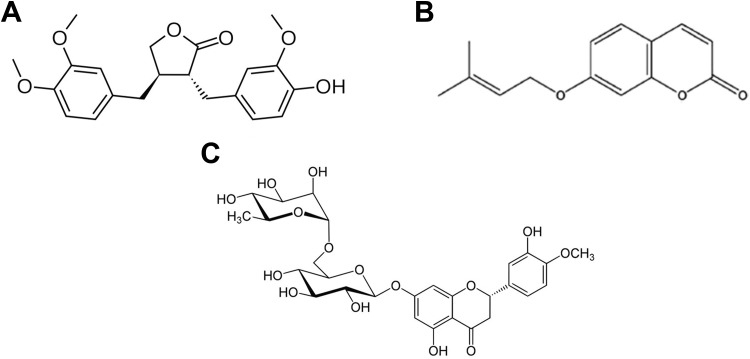
Herbal plants and their constituents have a long history in folk medicine. One of them is the genus Cousinia. The genus comprises of 210 species in Iran from which 172 species are endemic to the country.10 Arctigenin (Arg; Figure 1A) is a bioactive lignin isolated from Cousinia concolor.10 Moreover, it has been isolated from Bardanae fructus, Arctium lappa, Saussurea medusa, Torreya nucifera, and Ipomea cairica.11 Arctigenin has different pharmacological effects including anticancer.12–15
Figure 1.
Chemical structure of arctigenin (A), 7-isopenthenyloxycoumarin (B), and hesperidin (C).
Natural coumarins are another class of herbal constituents that have different pharmacological effects. 7-isopenthenyloxycoumarin (7-IP; Figure 1B) is one of the natural coumarins that first isolated in 1966 from the fruit of Libanotis intermedia and widely found in edible vegetables and fruits.16 Anticancer effect of 7-IP is shown in different studies.16–19
Flavonoids are abundantly found in fruits and vegetables including grains. Hesperidin (Hsp; Figure 1C) is an active flavanone glycoside found in the citrus juice, whole fruit, and peel.20 Anticancer effect of Hsp is found in some articles.20–23 Antileukemic effect of Hsp has been shown in some articles in vitro.22,24–26
In our study, we examined the cytotoxicity of different concentrations of Arg, 7-IP, and Hsp on K562 cell line for different times of incubation by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method and compared those together. We also tested and compared their effect on Mcl-1 gene expression in K562 cells for different concentrations and times of incubation by real-time polymerase chain reaction (PCR) method.
Materials and Methods
Cell Culture
K562 (human myelogenous leukemia cell line) cells were prepared from National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). Cells were cultured in RPMI 1640 (Gibco, Massachusetts, U.S.A. Cat. Number: 11875-093) and incubated at 95% humidity, with 5% CO2 at 37°C. Culture medium containing 10% fetal bovine serum (FBS; Gibco, Massachusetts, U.S.A. Cat. Number: 10082-147) and 50 U/mL penicillin/streptomycin (Sigma-Aldrich, St. Louis, Missouri, U.S.A. Cat. Number: 4458). After that cells were frozen in FBS containing 10% dimethyl sulfoxide (DMSO) and stored in liquid nitrogen (5 × 106 cells/vial). The viability of cryopreserved cells was determined by trypan blue staining, immediately upon thawing. Only cells whose viability exceeded 93% (range: 93.4%-99%) were used in this study.
Preparation of Phytochemicals
7-isopenthenyloxycoumarin (CAS Number 10387-50-5) was prepared from ALB Technology Company, Henderson, U.S.A. Hesperidin (CAS Number 520-26-3) were prepared from Golexir Pars Company (Mashhad, Iran). Arctigenin (CAS Number 7770-78-7) was prepared from Sigma-Aldrich Company.
3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay
The effects of 7-IP, Arg, and Hsp on the growth of cell line were examined using the MTT assay. Cells were subcultured in 96-well plates at a density of 5 × 104 cells per well with or without phytochemicals (10, 20, 40 μg/mL) for 24 and 48 hours in a final volume of 100 μL. Compounds were diluted in DMSO. Immediately before use, it was diluted in the culture medium to obtain a final DMSO concentration of 0.5% (vol/vol). Then, the medium was changed with fresh medium supplemented with 20 μL of MTT (5 mg/mL in PBS). Plates were incubated at 37°C for 2 hours. After addition of 100 μL DMSO to each well, plates were agitated for 1 minute. Spectrophotometric absorbance at 570 nm was measured. For calculating cell viability, we applied following formula: (viable cells) % = (OD of drug-treated sample/OD of untreated sample) × 100.
RNA Isolation and Real-Time Quantitative PCR Analysis
K562 cells were incubated by 7-IP, Arg, and Hsp (10, 20, and 40 μg/mL) in 37°C and 5% CO2 for 1, 2, and 3 hours. Total RNA was isolated using RNX-Plus kit in accordance with the manufacturer’s instructions (Sinagene, Tehran, Iran). The quantity and quality of the total RNA was verified with the PicoDrop spectrophotometer (Alpha Biotech, Cambridge, United Kingdom) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 2 μg total RNA treated with DNaseI using TaKaRa kit (PrimeScript First Strand cDNA Synthesis Kit).
Real-time PCR was performed in 3 replicates in 20 μL reaction volumes using 0.2 μL cDNA, 10 μL SYBR Green master mix (Yekta Tajhiz Azma, (Tehran, Iran) SYBR Green qPCR MasterMix 2×), and 0.4 μL of each primer on a Bio-Rad Real-Time PCR System (Hercules, California, U.S.A.). Polymerase chain reaction was performed using Mcl-1 primers (5′-CCA AGA AAG CTG CAT CGA ACC AT-3′ and 5′-CAG CAC ATT CCT GAT GCC ACC T-3′) and GAPDH primers (5′-GGA AGG TGA AGG TCG GAG T-3′ and 5′-GTC ATT GAT GGC AAC AAT ACC ACT-3′) as internal control.
Statistical Analysis
One-way analysis of variance test was used for statistical analysis by SPSS16 software. The P value was considered significant when it was less than .05. Error bars indicate standard deviations. Number of readings (N) for MTT assay and real-time PCR were 3 replicate. Calculations were done with GraphPad Prism 5 (GraphPad Software, San Diego, California).
Results
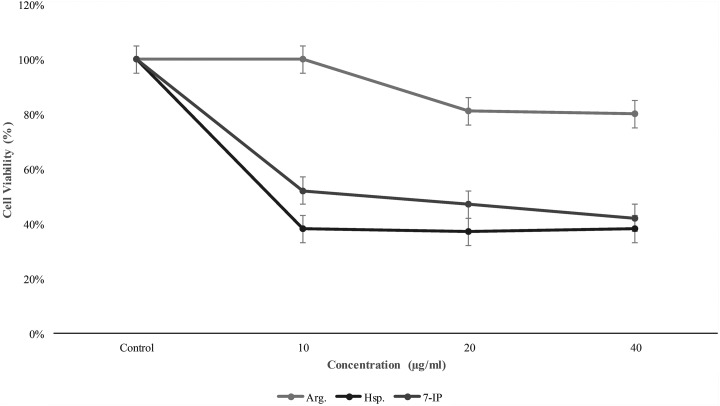
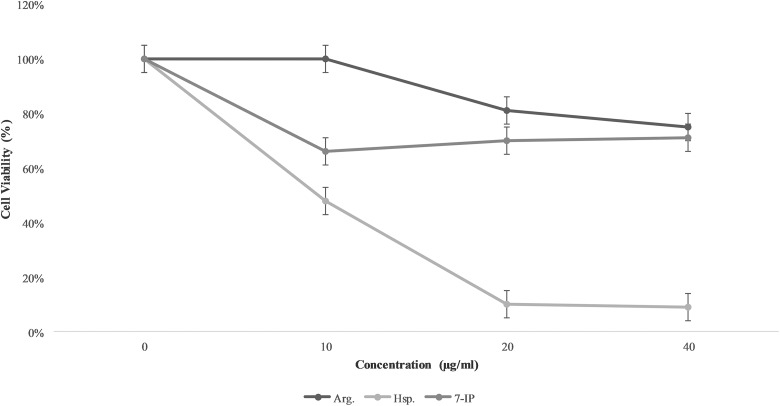
For comparison of cytotoxicity of Arg, Hsp, and 7-IP, we incubated various concentrations (10, 20, and 40 µg/mL) of these compounds with K562 cells for 24 and 48 hours. After these times, we tested the cytotoxicity by MTT assay. Hesperidin was the most cytotoxic compound against these cell lines (Figures 2 and 3).
Figure 2.
Cell viability of K562 cells after 24 hours incubation with 7-isopenthenyloxycoumarin (7-IP), arctigenin (Arg), and hesperidin (Hsp; 0, 10, 20, and 40 µg/mL) for 24 hours. Data are shown as mean (standard deviation).
Figure 3.
Cell viability of K562 cells after 48 hours incubation with 7-isopenthenyloxycoumarin (7-IP), arctigenin (Arg), and hesperidin (Hsp; 0, 10, 20, and 40 µg/mL) for 48 hours. Data are shown as mean (standard deviation).
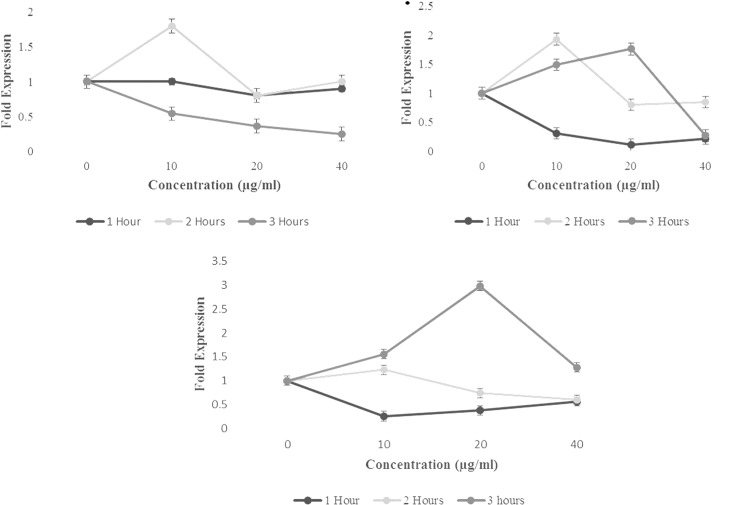
In the next step, we tested the effect of 7-IP, Arg, and Hsp on Mcl-1 gene expression. We incubated K562 cells with 10, 20, and 40 μg/mL of the compounds for 1, 2, and 3 hours, respectively. After that this effect was examined by real-time PCR method. As it shown in Figure 4, all the phytochemicals showed the hormetic effect in Mcl-1 gene expression for different concentrations and times of incubation.
Figure 4.
Effect of 7-isopenthenyloxycoumarin (7-IP), arctigenin (Arg), and hesperidin (Hsp) on myeloid cell leukemia type-1 (Mcl-1) gene expression after 1, 2, or 3 hours incubation in K562 cells. Data are shown as mean (standard deviation).
Discussion
In our study, we compared the cytotoxicity of 7-IP, Arg, and Hsp. By MTT method, we found that our phytochemicals are cytotoxic against K562 myelogenous leukemia cells. Based on our data, Hsp is the most cytotoxic agent among them. This cytotoxicity is time and dose dependent. LC50 for Hsp against K562 cells is 13.51 µg/mL for 24 hours incubation and 10.41 µg/mL for 48 hours incubation. To the best of our knowledge, this is the first report about the LC50 of Hsp on K562 cells.
Dose–response relationship is the most fundamental concept in toxicology. For most of the years, it has been widely accepted that the dose–response relationship follows a sigmoidal or S-shaped pattern of response. In this case, the tails of the lower and upper ends of the distribution asymptotically approaching 0 and 100%, respectively.27 Despite its long-term acceptance in toxicology and pharmacology, over the past 2 decades it has been suggested that alternative dose–response models like biphasic hormetic model may better account for observed dose–responses in the low-dose zone.27 Hormetic dose–response curves can be an inverted U-shaped or a J-shaped. Nowadays, hormesis becomes an essential concept to the biomedical sciences due to its important implications in therapeutics, in drug discovery, in the clinical trial, risk assessment for chemicals, radiation, and pharmaceutical agents.8
Hormetic effect in apoptosis induction/inhibition by phytochemicals is reported in previous studies.8,9 Zhang et al found that berberine induced a typical hormetic response in PC12 cells, that is, low-dose berberine significantly increased the cell viability, while high-dose berberine inhibited the cell viability. The hormetic and neuroprotective effects of berberine were confirmed to be mediated by upregulated PI3K/AKT/Bcl-2 cell survival and Nrf2/HO-1 antioxidative signaling pathways.8 Gholami showed that umbelliprenin induces and inhibits apoptosis by hormetic effect. In low doses, umbelliprenin increases the percentage of apoptosis, and in high doses, it decreases the percentage of apoptosis.9
Modification in Mcl-1 gene expression by phytochemicals was shown in other studies.28,29 In this study, we compared the effect of our phytochemicals on Mcl-1 gene expression by real-time PCR. Based on our findings, Mcl-1 gene expression is affected by 7-IP, Arg, and Hsp by hormetic dose responses. This kind of dose–response relationship was seen for umbelliprenin and auraptene in our previous studies.28,29 These findings bear potential significant clinical relevance. Our phytochemicals inhibit Mcl-1 dose dependently in CML cells. This finding could be a guidance for dose selection in future clinical studies.
The stimulatory aspect of the hormetic dose response can be derived from either a direct stimulation or an overcompensation response following a disruption in homeostasis.30 Professor Calabrese stated that in overcompensation stimulation hormesis, the dose response is a series of time-based snap shots (Figure 2 in the study of Calabrese27). Based on Professor Calabrese findings and our data, we could propose that Mcl-1 gene expression modification by our phytochemicals followed by overcompensation stimulation model.
Hormetic dose–responses can be mediated by 2 mechanisms: cellular receptors and/or cell signaling pathways.31 Since the Mcl-1 gene and protein have a role in intrinsic pathway of apoptosis, we can conclude that our phytochemicals at least make this hormetic effect by cell signaling pathway. Elucidation of this should be done in future studies. Moreover, in this study, the hormetic effect of our phytochemicals on Mcl-1 protein and other apoptotic proteins could be the subject of future studies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Deputy of Research and Technology, Sabzevar University of Medical Sciences.
References
- 1. Emole J, Talabi T, Pinilla-Ibarz J. Update on the management of Philadelphia chromosome positive chronic myelogenous leukemia: role of nilotinib. Biologics. 2016;10:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Talpaz M, Saglio G, Atallah E, Rousselot P. Dasatinib dose management for the treatment of chronic myeloid leukemia. Cancer. 2018;124(8):1660–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Billard C. Apoptosis inducers in chronic lymphocytic leukemia. Oncotarget. 2014;5(2):309–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuroda J, Yamamoto M, Nagoshi H, et al. Targeting activating transcription factor 3 by Galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol Cancer Res. 2010;8(7):994–1001. [DOI] [PubMed] [Google Scholar]
- 5. Brito IP, Tropaldi L, Carbonari CA, Velini ED. Hormetic effects of glyphosate on plants. Pest Manag Sci. 2018;74(5):1064–1070. [DOI] [PubMed] [Google Scholar]
- 6. Cook R, Calabrese EJ. The importance of hormesis to public health. Environ Health Perspect. 2006;114(11):1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calabrese EJ. Paradigm lost, paradigm found: the re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut. 2005;138(3):379–411. [DOI] [PubMed] [Google Scholar]
- 8. Zhang C, Li C, Chen S, et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gholami O. Umbelliprenin mediates its apoptotic effect by hormesis: a commentary. Dose Response. 2017;15(2):1559325817710035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iranshahy M, Tayarani-Najaran Z, Kasaian J, et al. Highly oxygenated sesquiterpene lactones from cousinia aitchisonii and their cytotoxic properties: rhaserolide induces apoptosis in human T lymphocyte (Jurkat) cells via the activation of c-jun n-terminal kinase phosphorylation. Phytother Res. 2016;30(2):222–226. [DOI] [PubMed] [Google Scholar]
- 11. Yao X, Zhu F, Zhao Z, Liu C, Luo L, Yin Z. Arctigenin enhances chemosensitivity of cancer cells to cisplatin through inhibition of the STAT3 signaling pathway. J Cell Biochem. 2011;112(10):2837–2849. [DOI] [PubMed] [Google Scholar]
- 12. Brecht K, Riebel V, Couttet P, et al. Mechanistic insights into selective killing of OXPHOS-dependent cancer cells by arctigenin. Toxicol In Vitro. 2017;40:55–65. [DOI] [PubMed] [Google Scholar]
- 13. Cai E, Song X, Han M, et al. Experimental study of the anti-tumour activity and pharmacokinetics of arctigenin and its valine ester derivative. Sci Rep. 2018;8(1):3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang P, Solorzano W, Diaz T, Magyar CE, Henning SM, Vadgama JV. Arctigenin inhibits prostate tumor cell growth in vitro and in vivo. Clin Nutr Exp. 2017;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu Y, Lou Z, Lee SH. Arctigenin represses TGF-beta-induced epithelial mesenchymal transition in human lung cancer cells. Biochem Biophys Res Commun. 2017;493(2):934–939. [DOI] [PubMed] [Google Scholar]
- 16. Bisi A, Cappadone C, Rampa A, et al. Coumarin derivatives as potential antitumor agents: growth inhibition, apoptosis induction and multidrug resistance reverting activity. Eur J Med Chem. 2017;127:577–585. [DOI] [PubMed] [Google Scholar]
- 17. Baba M, Jin Y, Mizuno A, et al. Studies on cancer chemoprevention by traditional folk medicines XXIV. Inhibitory effect of a coumarin derivative, 7-isopentenyloxycoumarin, against tumor-promotion. Biol Pharm Bull. 2002;25(2):244–246. [DOI] [PubMed] [Google Scholar]
- 18. Haghighi F, Matin MM, Bahrami AR, Iranshahi M, Rassouli FB, Haghighitalab A. The cytotoxic activities of 7-isopentenyloxycoumarin on 5637 cells via induction of apoptosis and cell cycle arrest in G2/M stage. Daru. 2014;22(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valiahdi SM, Iranshahi M, Sahebkar A. Cytotoxic activities of phytochemicals from Ferula species. Daru. 2013;21(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banjerdpongchai R, Wudtiwai B, Khaw-On P, Rachakhom W, Duangnil N, Kongtawelert P. Hesperidin from citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol. 2016;37(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao J, Li Y, Gao J, De Y. Hesperidin inhibits ovarian cancer cell viability through endoplasmic reticulum stress signaling pathways. Oncol Lett. 2017;14(5):5569–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desai UN, Shah KP, Mirza SH, Panchal DK, Parikh SK, Rawal RM. Enhancement of the cytotoxic effects of cytarabine in synergism with hesperidine and silibinin in acute myeloid leukemia: an in-vitro approach. J Cancer Res Ther. 2015;11(2):352–357. [DOI] [PubMed] [Google Scholar]
- 23. Birsu Cincin Z, Unlu M, Kiran B, Sinem Bireller E, Baran Y, Cakmakoglu B. Anti-proliferative, apoptotic and signal transduction effects of hesperidin in non-small cell lung cancer cells. Cell Oncol (Dordr). 2015;38(3):195–204. [DOI] [PubMed] [Google Scholar]
- 24. Lewin G, Maciuk A, Thoret S, Aubert G, Dubois J, Cresteil T. Semisynthesis of natural flavones inhibiting tubulin polymerization, from hesperidin. J Nat Prod. 2010;73(4):702–706. [DOI] [PubMed] [Google Scholar]
- 25. El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from citrus species in human colon and leukaemia cell lines. Eur J Pharmacol. 2010;626(2-3):139–145. [DOI] [PubMed] [Google Scholar]
- 26. Chen YC, Shen SC, Lin HY. Rutinoside at C7 attenuates the apoptosis-inducing activity of flavonoids. Biochem Pharmacol. 2003;66(7):1139–1150. [DOI] [PubMed] [Google Scholar]
- 27. Calabrese EJ. Hormesis: principles and applications. Homeopathy. 2015;104(2):69–82. [DOI] [PubMed] [Google Scholar]
- 28. Motlagh FM, Gholami O. Comparison of Umbelliprenin and Auraptene in Cytotoxic Effects and Myeloid Cell Leukaemia Type-1 (Mcl-1) Gene Expression. Indian J Pharm Sci. 2016;78(6):827–833. [Google Scholar]
- 29. Gholami O, Jeddi-Tehrani M, Iranshahi M, Zarnani AH, Ziai SA. Mcl-1 is up regulated by prenylated coumarin, umbelliprenin in jurkat cells. Iran J Pharm Res. 2014;13(4):1387–1392. [PMC free article] [PubMed] [Google Scholar]
- 30. Calabrese EJ. Hormesis within a mechanistic context. Homeopathy. 2015;104(2):90–96. [DOI] [PubMed] [Google Scholar]
- 31. Calabrese EJ. Hormetic mechanisms. Crit Rev Toxicol. 2013;43(7):580–606. [DOI] [PubMed] [Google Scholar]