Abstract
Background:
While mortality of HIV-related cryptococcal meningitis (CM) in developed countries is relatively low, in developing countries over half of patients die within 10 weeks. Current recommended therapies are often not suitable for resource-poor settings, and new shorter regimens are urgently needed. Intrathecal administration of liposomal amphotericin B (lAmB) has shown promising results in animal models. However, the safety and tolerability of intrathecal lAmB in humans are not well known.
Methods:
In this retrospective observational study, we report the tolerability and safety of intrathecal lAmB in patients with CM from an HIV cohort study in India.
Results:
In all, 18 patients were included in the analysis. Six were female and the median age was 40 years [interquartile range (IQR): 35–45]. The median CD4 count was 42 cells/µl (IQR: 19–127). Compared with a historical control group, the hazard ratio for mortality was 0.59 (95% confidence interval: 0.26–1.29). Two patients complained of transient lumbar pain in single occasion. One patient had a skin reaction to chlorhexidine, which was used as skin disinfectant. After initial improvement, one patient requested to stop lumbar punctures for the last 2 days of treatment.
Conclusion:
Intrathecal lAmB was safe and well tolerated in HIV-infected patients with CM.
Keywords: AIDS-related opportunistic infections, antifungal agents, injections, patient safety, poverty, spinal
Introduction
Despite the dramatic expansion of antiretroviral treatment in developing countries, cryptococcal meningitis (CM) is still a frequent cause of death among HIV-infected patients. It is estimated that 223,100 (95% confidence interval (CI): 150,600–282,400) HIV- infected patients had CM in 2014 and 181,100 (95% CI: 119,400–234,300) died.1 While over half of patients with CM from low- and middle-income countries die within 10 weeks of diagnosis, the mortality in developed countries is nearly 10%.2
Amphotericin B (AmB) has a strong fungicidal activity against Cryptococcus, but it has poor penetration into the cerebrospinal fluid (CSF).3,4 To achieve higher concentration in CSF, intrathecal administration of AmB deoxycholate (AmBd) has been used for the treatment of CM, and observational studies from Asia suggest that it could be associated with improved survival.5 However, AmB deoxycholate has a direct irritant effect and intrathecal administration is poorly tolerated.6,7 Lipid formulations of AmB are less irritant8–10 and have shown promising results in animal models of CM.11,12 In a previous study from our cohort, we reported our experience with a CM regimen using intrathecal AmB lipid emulsion (Amphomul®).13 However, this lipid form of AmB is not available in many parts of the world. In this retrospective study, we aimed to describe the safety and tolerability of the intrathecal administration of liposomal AmB (lAmB) for the treatment of CM in HIV-infected patients.14
Methods
The Vicente Ferrer HIV Cohort Study (VFHCS) is a prospective cohort study of HIV-infected patients who have attended the Rural Development Trust Hospital in Bathalapalli, Anantapur District, AP, India. The VFHCS is registered at clinicaltrials.gov (No. NCT02454569). The hospital belongs to a nongovernmental organization and provides medical care to HIV-infected people free of charge. In our setting, 72% of the population live in rural areas,15 and the HIV epidemic is largely driven by heterosexual transmission and it is characterized by low CD4 cell counts at presentation, poor socioeconomic conditions and high levels of illiteracy.16–18
The diagnosis of CM was based on the presence of Cryptococcus antigens in CSF by latex agglutination.19 Before 15 June 2013, patients were treated with the standard 2-week induction treatment with intravenous AmBd 0.7–1 mg/kg and oral fluconazole 1200 mg once daily followed by 600 mg once daily for 8 weeks (consolidation phase) and 200 mg once daily thereafter (maintenance phase).20 From 15 June 2013 to 31 October 2016, the induction phase was changed to AmBd 0.7 mg/kg once daily for 7 days, intrathecal AmB lipid emulsion (Amphomul®; Bharat Serums and Vaccines, India) once daily for 7 days, and oral fluconazole 600 mg twice daily for 14 days. Although the use of the short regimen with intrathecal Amphomul® was associated with a significant reduction in mortality,13 the supply of Amphomul® to the hospital stopped on 31 October 2016, and it was replaced by lAmB (AmBisome®). In this study, we describe our experience with patients who received intrathecal lAmB.
For the preparation of the intrathecal administration of lAmB, a vial containing 50 mg of AmBisome® powder was diluted with 10 ml of sterile water to achieve a final concentration of 5 mg/ml in the vial. Lumbar punctures (LPs) were performed daily during the first week of treatment. After removing at least 20 ml of CSF (typically 30–40 ml), 2 ml of the lAmB solution (10 mg) was extracted and mixed with 3 ml of 5% dextrose in a 5-ml syringe and administered intrathecally slowly over 2 min. After the LP, patients were asked to lay flat on the bed for 1 h. The rest of the AmBisome® vial was kept in a fridge at 2–8°C and used as required for a maximum of 7 days, according to the stability information given by the manufacturer. Intravenous AmBd was diluted in 500 ml of 5% dextrose and administered slowly over 4 h.
Medical records of all HIV-infected patients with CM who received lAmB until 21 May 2017 were reviewed searching for side effects secondary to the intrathecal administration of lAmB. Patients’ attitudes towards repeated LPs were also collected. To study the mortality of the treatment compared with the standard of care, patients who received the standard 2-week induction treatment with AmBd (but no intrathecal administration of any form of AmB) were included in the control group.
Statistical analysis was performed using Stata Statistical Software (Stata Corporation. Release 14.1; College Station, TX, USA). The log-rank test was used to compare mortality during the first 10 weeks after the initiation of treatment. The study was approved by the Ethics Committee of the Rural Development Trust Hospital.
Results
We identified 18 patients who received intrathecal lAmB. Six were female and the median age was 40 years [interquartile range (IQR) = 35–45]. The median CD4 count 42 cells/µl (IQR = 19–127). Two patients complained of transient lumbar pain after the intrathecal administration of lAmB in single occasions. One patient had a skin reaction to chlorhexidine, which was used as skin disinfectant before LPs. After initial improvement, one patient requested to stop LPs for the last 2 days of treatment.
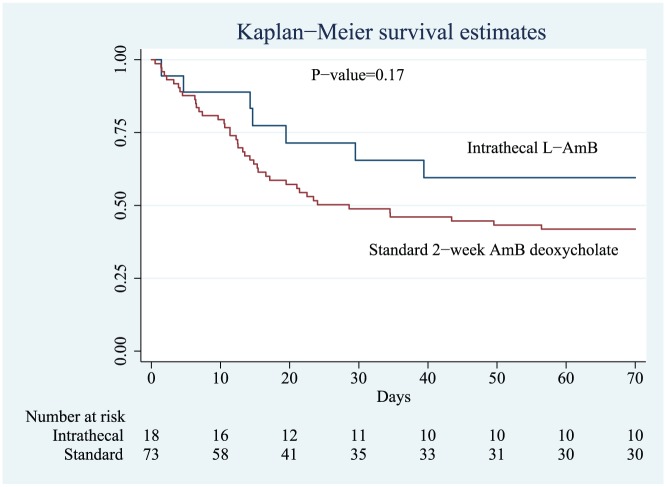
Compared with a control group of 73 patients who received the standard 2-week induction phase regimen with AmBd, there was a statistically nonsignificant trend towards improved survival during the first 10 weeks after initiation of CM treatment (hazard ratio 0.59; 95% CI: 0.26–1.29) in the intrathecal lAmB group (Figure 1). In the control group, the median CD4 count 62.5 cells/µl (IQR = 28.5–142.5). Two patients, one in each group, did not complete the 10-week follow-up period.
Figure 1.
Kaplan–Meier survival estimates of patients receiving 1-week induction treatment with intrathecal liposomal amphotericin B (lAmB) or the standard 2-week regimen with AmB deoxycholate.
Discussion
CM is still responsible for 15% of deaths in patients with AIDS.1 The recommended therapy for CM comprises 2-week induction treatment with AmBd accompanied by flucytosine or fluconazole.20 However, flucytosine is rarely available in developing countries, and AmBd is underused in resource-poor settings because long hospital admissions and close renal and electrolyte monitoring are required.21 Shortening treatment to 1 week could improve substantially the feasibility of the treatment for resource-poor settings because the nephrotoxicity of AmBd occurs mainly during the second week of therapy, and severe renal toxicity is rare when the treatment is shortened to 7 days or less.22–24 Our results suggest that intrathecal administration of lAmB could help shorten AmBd treatment to 1 week without observing an increase in mortality.
Previous studies have reported that the use of intrathecal AmBd is poorly tolerated and adverse events such as leg pain, vomiting, prostration or altered mental status were common.6,7 However, in our study, adverse events in patients who received intrathecal lAmB were mild and transient.
The study has important limitations. We observed a clinically important reduction of mortality in the intrathecal lAmB group similar to the one observed in a previous study from our cohort using another lipid form of AmB.13 However, differences were nonstatistically significant and patients were not randomized. Historical confounders could have influenced in the results. Moreover, the number of patients treated with intrathecal lipid forms of AmB was relatively small and from a single centre. Clinical trials are needed to confirm the survival benefits of the intrathecal administration of lAmB in CM.
Conclusion
Current pharmaceutical forms of AmB have poor penetration into CSF. The results from our cohort indicate that intrathecal administration of lAmB could help reduce the duration of the intravenous AmBd to 7 days, making the treatment more feasible for resource-poor settings. Intrathecal lAmB was safe and well tolerated by patients, and it was not associated with an increased mortality compared with the standard of care.
Footnotes
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Presentation of research: Oral presentation at the first joint meeting of ISAAR (International Symposium on Antimicrobial Agents and Resistance) & ICIC (International Interscience Conference on Infection and Chemotherapy) in Busan, South Korea, 14–16 September 2017.
ORCID iD: Gerardo Alvarez-Uria  https://orcid.org/0000-0003-1847-9614
https://orcid.org/0000-0003-1847-9614
Contributor Information
Gerardo Alvarez-Uria, Department of Infectious Diseases, Bathalapalli Rural Development Trust Hospital, Kadiri Road, Bathalapalli 515661, Anantapur District, Andhra Pradesh, India.
Manoranjan Midde, Department of Infectious Diseases, Bathalapalli Rural Development Trust Hospital, Bathalapalli, India.
Jayaram Battula, Department of Infectious Diseases, Bathalapalli Rural Development Trust Hospital, Bathalapalli, India.
Himachandra N.B. Pujari, Department of Infectious Diseases, Bathalapalli Rural Development Trust Hospital, Bathalapalli, India
References
- 1. Rajasingham R, Smith RM, Park BJ, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis 2017; 17: 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tenforde MW, Wake R, Leeme T, et al. HIV-associated cryptococcal meningitis: bridging the gap between developed and resource-limited settings. Curr Clin Microbiol Rep 2016; 3: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strenger V, Meinitzer A, Donnerer J, et al. Amphotericin B transfer to CSF following intravenous administration of liposomal amphotericin B. J Antimicrob Chemother 2014; 69: 2522–2526. [DOI] [PubMed] [Google Scholar]
- 4. Kethireddy S, Andes D. CNS pharmacokinetics of antifungal agents. Expert Opin Drug Metab Toxicol 2007; 3: 573–581. [DOI] [PubMed] [Google Scholar]
- 5. Fang W, Fa Z, Liao W. Epidemiology of cryptococcus and cryptococcosis in China. Fungal Genet Biol. Epub ahead of print 7 November 2014. DOI: 10.1016/j.fgb.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 6. Yuchong C, Jianghan C, Hai W, et al. Lumbar puncture drainage with intrathecal injection of amphotericin B for control of cryptococcal meningitis. Mycoses 2011; 54: e248–e251. [DOI] [PubMed] [Google Scholar]
- 7. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis. Clin Infect Dis 2005; 41: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 8. Clemons KV, Sobel RA, Williams PL, et al. Comparative toxicities and pharmacokinetics of intrathecal lipid (amphotericin B colloidal dispersion) and conventional deoxycholate formulations of amphotericin B in rabbits. Antimicrob Agents Chemother 2001; 45: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mete B, Saltoglu N, Vanli E, et al. Simultaneous cryptococcal and tuberculous meningitis in a patient with systemic lupus erythematosus. J Microbiol Immunol Infect. Epub ahead of print 7 June 2013. DOI: 10.1016/j.jmii.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 10. Grannan BL, Yanamadala V, Venteicher AS, et al. Use of external ventriculostomy and intrathecal anti-fungal treatment in cerebral mucormycotic abscess. J Clin Neurosci 2014; 21: 1819–1821. [DOI] [PubMed] [Google Scholar]
- 11. Capilla J, Flavia A, Mayayo E, et al. Efficacy of intrathecal liposomal amphotericin B plus oral posaconazole in the treatment of acute meningeal cryptococcosis in a murine model. Int J Antimicrob Agents 2013; 42: 282–283. [DOI] [PubMed] [Google Scholar]
- 12. Gazzoni AF, Capilla J, Mayayo E, et al. Efficacy of intrathecal administration of liposomal amphotericin B combined with voriconazole in a murine model of cryptococcal meningitis. Int J Antimicrob Agents 2012; 39: 223–227. [DOI] [PubMed] [Google Scholar]
- 13. Alvarez-Uria G, Midde M, Pakam R, et al. Short-course induction treatment with intrathecal amphotericin B lipid emulsion for HIV infected patients with cryptococcal meningitis. J Trop Med 2015; 2015: 864271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adler-Moore JP, Gangneux JP, Pappas PG. Comparison between liposomal formulations of amphotericin B. Med Mycol 2016; 54: 223–231. [DOI] [PubMed] [Google Scholar]
- 15. Census of India. Office of The Registrar General & Census Commissioner India, 2011. http://censusindia.gov.in/ [Google Scholar]
- 16. Alvarez-Uria G, Midde M, Pakam R, et al. Gender differences, routes of transmission, socio-demographic characteristics and prevalence of HIV related infections of adults and children in an HIV cohort from a rural district of India. Infect Dis Rep 2012; 4: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alvarez-Uria G, Pakam R, Midde M, et al. Entry retention virological suppression in an HIV cohort study in India: description of the cascade of care and implications for reducing HIV-related mortality in low- and middle-income countries. Interdiscip Perspect Infect Dis 2013; 2013: 384805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alvarez-Uria G, Naik PK, Pakam R, et al. Natural history and factors associated with early and delayed mortality in HIV infected patients treated of tuberculosis under directly observed treatment short course (DOTS) strategy: a prospective cohort study in India. Interdiscip Perspect Infect Dis 2012; 2012: 502012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol 2014; 6: 169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Available at: http://www.who.int/hiv/pub/cryptococcal_disease2011/en/ (2011, accessed 12 February 2015). [PubMed]
- 21. Rajasingham R, Rolfes MA, Birkenkamp KE, et al. Cryptococcal meningitis treatment strategies in resource-limited settings: a cost-effectiveness analysis. PLoS Med 2012; 9: e1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bicanic T, Wood R, Meintjes G, et al. High-dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV-infected patients: a randomized trial. Clin Infect Dis 2008; 47: 123–130. [DOI] [PubMed] [Google Scholar]
- 23. Bicanic T, Meintjes G, Wood R, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis 2007; 45: 76–80. [DOI] [PubMed] [Google Scholar]
- 24. Jackson AT, Nussbaum JC, Phulusa J, et al. A phase II randomized controlled trial adding oral flucytosine to high-dose fluconazole, with short-course amphotericin B, for cryptococcal meningitis. AIDS 2012; 26: 1363–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]