Abstract
A case of tenofovir-induced Fanconi syndrome in a patient receiving antiretroviral therapy for HIV infection, with resolution of the related electrolyte abnormalities upon switch from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate, is reported. Tenofovir alafenamide fumarate, a novel prodrug of tenofovir containing significantly lower doses of tenofovir than tenofovir disoproxil fumarate, has been associated with a favourable renal profile compared to tenofovir disoproxil fumarate. Generally, the rare complication of tenofovir disoproxil fumarate–induced Fanconi syndrome is managed by cessation of tenofovir. There are limited reports of the impact of a switch strategy from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate, which may be necessary in patients unable to discontinue tenofovir.
Keywords: Fanconi syndrome, HIV, tenofovir, tenofovir alafenamide fumarate
Case
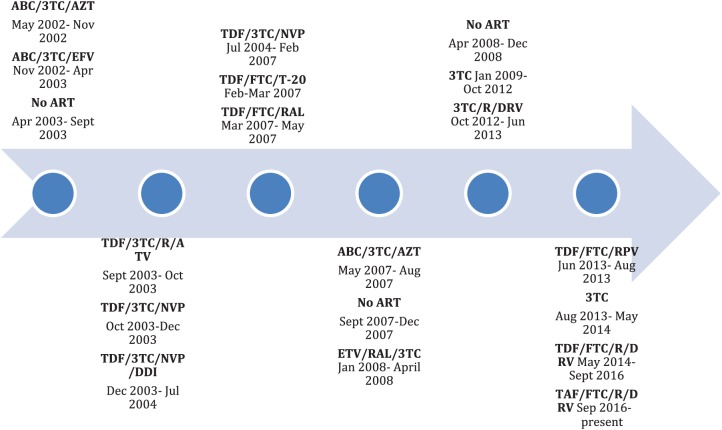
A 56-year-old man with chronic HIV infection initially diagnosed in 1994 with a nadir CD4 count of 180 was admitted to hospital following a seizure in September 2016. Combination antiretroviral therapy was initiated in 2002 and he had been treated with several different regimens (Figure 1). At the time of admission, the patient was virally suppressed with a CD4 count of 365, while receiving an antiretroviral regimen consisting of tenofovir disoproxil fumarate (TDF) 300 mg and emtricitabine 200 mg in a once daily combination tablet, ritonavir 100 mg daily and darunavir 800 mg daily for 28 months. He had previously been treated with TDF between 2003 and 2007 and for a brief period in 2013, without any abnormalities in renal function or electrolytes during these periods.
Figure 1.
Antiretroviral therapy (ART) timeline.
3TC, lamivudine; ABC, abacavir; ATV, atazanavir; AZT, zidovudine; DDI, didanosine; DRV, darunavir; EFV, efavirenz; ETV, etravirine; FTC, emtricitabine; NVP, nevirapine; R, ritonavir; RAL, raltegravir; RPV, rilpivirine; T-20, enfuvirtide; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil.
Comorbidities included HIV-associated neurocognitive disorder and epilepsy which was difficult to control but stabilized on carbamazepine, levetiracetam, sodium valproate and gabapentin. Hepatitis C co-infection (genotype 3a with mild fibrosis) remained untreated at the time of admission. Long-standing osteoporosis in the context of multiple risk factors, including HIV, earlier TDF-based antiretroviral therapy, anti-epileptic medications and recurrent falls, was diagnosed following a minimal trauma ankle fracture in 2006 and was complicated by several fractures and chronic pain requiring regular opioids. Other comorbidities included peptic ulcer disease, paroxysmal atrial fibrillation and hypogonadism. Additional medications on admission were cholecalciferol, digoxin, pantoprazole, potassium chloride and thiamine.
At the time of initiation of the current antiretroviral regimen, the patient’s renal function, electrolytes and urinalysis were normal (Table 1). Creatinine and estimated glomerular filtration rate (eGFR) remained stable; however, hypophosphataemia, proteinuria and glycosuria initially occurred approximately 12 months into therapy (Table 1). The electrolyte abnormalities, noted during a number of admissions to hospital, were attributed to various acute deteriorations in the patient’s health and resolved with electrolyte replacement. The diagnosis of Fanconi syndrome was not specifically considered or investigated until the most recent admission, although it is likely that he had already developed Fanconi syndrome 16 months earlier.
Table 1.
Serum biochemistry and urinalysis from TDF initiation to 14 months post switch to TAF.
| Initiation of TDF | 12 months post TDF | Admission (28 months post TDF) | 10 months post TAF | 14 months post TAF | Normal values | |
|---|---|---|---|---|---|---|
| Sodium, mmol/l | 132 | 130 | 137 | 138 | 138 | 137–146 |
| Potassium, mmol/l | 4.7 | 3.7 | 2.9 | 3.7 | 4.8 | 3.5–5.2 |
| Bicarbonate, mmol/l | 26 | 23 | 17 | 26 | 26 | 22–32 |
| Creatinine, μmol/l | 42 | 66 | 161 | 108 | 106 | 60–120 |
| eGFR, ml/min/1.73 m2 | >90 | >90 | 41 | 65 | 67 | >60 |
| Phosphate, mmol/l | 1.40 | 0.37 | 0.29 | 0.72 | 1.08 | 0.70–1.40 |
| Random glucose, mmol/l | – | – | 5.2 | 4.6 | 4.6 | 3.0–7.8 |
| Urine glucose | Nil | 4+ | 4+ | – | Nil | Nil |
| Urine protein | Nil | 3+ | 3+ | – | 1+ | Nil |
| Urine casts | Nil | Nil | 1+ | – | Nil | Nil |
| Urine phosphate concentration, mmol/l | – | – | 32.3 | – | 9.4 | – |
| Urine protein concentration, g/l | – | – | 2.04 | – | 0.32 | 0.00–0.10 |
eGFR, estimated glomerular filtration rate; TAF, tenofovir alafenamide fumarate; TDF, tenofovir disoproxil fumarate. (Bold text- abnormal result)
On this occasion, the patient presented to the emergency department following a seizure at home. He reported diarrhoea following a course of antibiotics for a respiratory tract infection, and a polymerase chain reaction (PCR) test for detection of faecal pathogens demonstrated Clostridium difficile. Blood concentrations of anti-epileptic drugs were therapeutic, and an electroencephalogram (EEG) was performed and was unchanged.
On admission, the white cell count was 7.5 × 109/l (reference range, 4–11 × 109/l) and C-reactive protein (CRP) 51.5 mg/l (reference range, <5 mg/l). The electrolytes were significantly deranged with potassium of 2.9 mmol/l (reference range, 3.5–5.2 mmol/l), phosphate 0.29 mmol/l (reference range, 0.70–1.40 mmol/l) and bicarbonate 17 mmol/l (reference range, 22–32 mmol/l) suggestive of metabolic acidosis (Table 1). The sodium, chloride, magnesium and calcium levels were normal. He also had acute kidney injury (AKI) with a creatinine of 161 μmol/l (reference range, 60–120 μmol/l) and eGFR of 41 ml/min/1.73 m2 (reference range > 60 ml/min/1.73 m2) with normal urea. Urine dipstick chemistry identified 4+ glycosuria (despite normal serum glucose) and 3+ proteinuria (Table 1).
The patient was commenced on oral metronidazole for Clostridium difficile diarrhoea and his usual anti-epileptic agents continued. The electrolyte derangement was initially thought to be caused by diarrhoea and he received intravenous fluids with electrolyte replacement. The hypophosphataemia and metabolic acidosis, however, persisted despite the resolution of diarrhoea and AKI. Urinalysis confirmed phosphaturia, proteinuria and glycosuria, and granular casts were observed on microscopic examination (Table 1). Based on these biochemical parameters, a diagnosis of tenofovir-induced Fanconi syndrome was made.
The patient had previously experienced several drug intolerances and drug interactions with his other medications while on alternative antiretroviral agents thereby limiting the therapeutic options; thus, TDF was switched to tenofovir alafenamide fumarate (TAF) 10 mg daily to minimize adjustment to the regimen while hoping to reduce tenofovir-induced renal toxicity.
The patient’s admission was further complicated by methicillin-resistant Staphylococcus aureus sepsis, recurrence of Clostridium difficile diarrhoea, Enterobacter cloacae urosepsis and a fall resulting in a fractured left neck of femur. He completed antibiotic therapy and underwent a left hip hemiarthroplasty and was able to be discharged home after a period in rehabilitation while continuing the new antiretroviral regimen. Just prior to discharge from rehabilitation, after 6 weeks on TAF, his phosphate had normalized. The bicarbonate remained slightly low, and a reduction in proteinuria and glycosuria was noted (Table 1).
Following discharge, the electrolyte replacement was weaned with close blood test monitoring. Eight weeks after the change from TDF to TAF, the patient remained virologically suppressed. Phosphate replacement was ceased after 3 months on TAF and sodium bicarbonate after 9 months. Serum biochemistry 10 months after commencing TAF demonstrated normal electrolytes, and urinalysis at 14 months revealed no glycosuria, minimal residual proteinuria and a significant reduction in urine phosphate concentration (Table 1).
Discussion
TDF, a prodrug of tenofovir, is a potent antiretroviral agent that has been in clinical use for treatment of HIV and hepatitis B since 2001. However, TDF has been associated with nephrotoxicity including Fanconi syndrome.
Drug-induced Fanconi syndrome results from the breakdown of solute transport in the proximal tubule and tenofovir is one of the commonly implicated drugs. It is characterized by hypophosphataemia (the most important aspect), normoglycaemic glycosuria, metabolic acidosis (usually mild, with bicarbonate levels ⩾ 15 mmol/l), low-molecular-weight proteinuria and aminoaciduria.1 The eGFR and urine albumin/creatinine ratio, which are frequently used to monitor AKI and chronic kidney disease, are not sensitive markers of proximal tubular function.1 The mainstay of therapy is withdrawal of the offending drug, which results in regeneration of the proximal tubule, although this can take months and residual defects may remain.1
Fanconi syndrome due to TDF was first reported in the literature in 2002.2 Further reports have followed, but TDF-associated Fanconi syndrome remains a rare complication of TDF therapy, occurring in <0.1% of TDF-treated HIV patients.3 Risk factors for renal tubular dysfunction include older age, reduced body mass, pre-existing renal impairment and simultaneous use of other nephrotoxic medications and onset is frequently after several months of TDF-based therapy.4 A recent cohort study found that the risk of Fanconi syndrome was increased approximately fivefold with concurrent ritonavir administration.5 Renal dysfunction due to TDF including Fanconi syndrome is usually reversible with cessation of the drug. Tenofovir dose reduction as a strategy to recover renal function has not been adequately investigated in clinical studies and is not recommended in current clinical guidelines.6 There is a lack of data to support this recommendation.
TAF is a novel prodrug of tenofovir which undergoes phosphorylation to the active metabolite of tenofovir, tenofovir diphosphate, after cellular uptake. Therefore, administration results in higher intracellular levels of the active metabolite at HIV target cells with significantly lower doses of tenofovir.7 Since it is cleared by renal elimination, reducing the dose of tenofovir administered, and therefore circulating levels of the drug, reduces the exposure of the kidneys to tenofovir, thereby reducing renal toxicity.7
Clinical trials have shown improved renal parameters in patients treated with TAF compared with TDF.8–10 There is minimal data regarding the impact of switching from TDF to TAF on pre-existing renal dysfunction. One phase 3 study suggested that there was limited impact on eGFR, but improvement in proteinuria and albuminuria in HIV patients with mild to moderate renal impairment switched to a TAF-based regimen from various regimens, 65% of which were TDF-based.11 Limited cases of resolution of tenofovir-induced Fanconi syndrome with switch from TDF to TAF have been reported in the literature.12,13
Our case illustrates the reversal of tubular dysfunction resulting from late-onset tenofovir-induced Fanconi syndrome with a switch from TDF to TAF. This supports the existing data that have reported reduced renal toxicity with the use of TAF compared with TDF. This may also be a future strategy for patients similar to ours with TDF-related renal tubular toxicity who are unable to tolerate other non-tenofovir-based antiretroviral drugs for the treatment of HIV infection. Further data are required before this can be a recommended strategy, particularly into the possibility of subclinical toxicity with long-term effects in the presence of low doses of tenofovir.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Informed consent: The patient provided written informed consent for the publication of this case.
Contributor Information
Nomvuyo Z. Mothobi, Department of HIV, Immunology and Infectious Diseases; Department of Microbiology, St. Vincent’s Hospital, NSW, Australia.
Jeffrey Masters, Department of HIV, Immunology and Infectious Diseases, St. Vincent’s Hospital, Darlinghurst, NSW, Australia.
Deborah J. Marriott, Department of HIV, Immunology and Infectious Diseases; Department of Microbiology, St. Vincent’s Hospital, Darlinghurst, NSW, Australia
References
- 1. Hall A, Bass P, Unwin R. Drug-induced renal Fanconi syndrome. QJM 2013; 107: 261–269. [DOI] [PubMed] [Google Scholar]
- 2. Verhelst D, Monge M, Meynard J, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis 2002; 40: 1331–1333. [DOI] [PubMed] [Google Scholar]
- 3. Nelson M, Katlama C, Montaner J, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS 2007; 21: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 4. Hall A, Hendry B, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis 2011; 57: 773–780. [DOI] [PubMed] [Google Scholar]
- 5. Medland NA, Chow EP, Walker RG, et al. Incidence of renal Fanconi syndrome in patients taking antiretroviral therapy including tenofovir disoproxil fumarate. Int J STD AIDS 2018; 29: 227–236. [DOI] [PubMed] [Google Scholar]
- 6. Holt S, Gracey D, Levy M, et al. A consensus statement on the renal monitoring of Australian patients receiving tenofovir based antiviral therapy for HIV/HBV infection. AIDS Res Therapy 2014; 11: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ray AS, Fordyce MW, Hitchcock MJM. Tenofovir alafenamide: a novel prodrug of tenofovir for the treatment of human immunodeficiency virus. Antiviral Res 2016; 125: 63–70. [DOI] [PubMed] [Google Scholar]
- 8. Sax P, Wohl D, Yin M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385: 2606–2615. [DOI] [PubMed] [Google Scholar]
- 9. Sax P, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014; 67: 52–58. [DOI] [PubMed] [Google Scholar]
- 10. Mills A, Crofoot G, McDonald C, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2015; 69: 439–445. [DOI] [PubMed] [Google Scholar]
- 11. Pozniak A, Arribas J, Gathe J, et al. Switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected patients with renal impairment: 48-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr 2016; 71: 530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karris MY. Resolution of tenofovir disoproxil fumarate induced Fanconi syndrome with switch to tenofovir alafenamide fumarate in a HIV-1 and hepatitis B coinfected patient. AIDS Res Hum Retroviruses 2017; 33: 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Post F, Tebas P, Clarke A, et al. Brief report: switching to tenofovir alafenamide, coformulated with elvitegravir, cobicistat, and emtricitabine, in HIV-infected adults with renal impairment: 96-week results from a single-arm, multicenter, open-label phase 3 study. J Acquir Immune Defic Syndr 2017; 74: 180–184. [DOI] [PMC free article] [PubMed] [Google Scholar]