Abstract
The frontoparietal network is critical for our ability to coordinate behavior in a rapid, accurate, and flexible goal-driven manner. In this review, we outline support for the framing of the frontoparietal network as a distinct control network, in part functioning to flexibly interact with and alter other functional brain networks. This network coordination likely occurs in a 4 Hz to 73 Hz θ/α rhythm, both during resting state and task state. Precision mapping of individual human brains has revealed that the functional topography of the frontoparietal network is variable between individuals, underscoring the notion that group-average studies of the frontoparietal network may be obscuring important typical and atypical features. Many forms of psychopathology implicate the frontoparietal network, such as schizophrenia and attention-deficit/hyperactivity disorder. Given the interindividual variability in frontoparietal network organization, clinical studies will likely benefit greatly from acquiring more individual subject data to accurately characterize resting-state networks compromised in psychopathology.
Keywords: cognitive control, cognitive flexibility, frontoparietal, lateral prefrontal cortex, intraparietal sulcus, psychopathology
Abstract
La red fronto-parietal es fundamental para nuestra capacidad de coordinar la conducta orientada hacia un objetivo de una manera rápida, precisa y flexible. En esta revisión, se describe el soporte para la formación de la red fronto-parietal, como una red de control diferente, que funciona en parte interactuando o alterando otras redes cerebrales funcionales de manera flexible. Esta coordinación de red ocurre probablemente a un ritmo theta/alfa de 4 Hz a 13 Hz, tanto durante el estado de reposo como durante una tarea. El mapeo de precisión de cerebros humanos individuales ha revelado que la topografía funcional de la red fronto-parietal varía entre los sujetos, lo que subraya la noción de que los estudios de promedio de grupo de la red fronto-parietal pueden ocultar importantes características típicas y atípicas. Muchas formas de psicopatología, como la esquizofrenia y el trastorno por déficit de atención/hiperactividad, involucran a la red fronto-parietal. Dada la variabilidad interindividual en la organización de la red fronto-parietal, es probable que los estudios clínicos tengan un gran beneficio, a partir de la adquisición de más datos de sujetos individuales, para la caracterización más precisa de las redes (en estado de reposo) que están alteradas en la psicopatología.
Abstract
Le réseau frontopariétal est essentiel pour organiser notre comportement de manière rapide, précise et centrée sur l'objectif de façon flexible. Dans cet article, nous soutenons le cadre du réseau frontopariétal comme réseau de contrôle distinct, fonctionnant en partie pour communiquer et modifier d'autres réseaux cérébraux fonctionnels de façon flexible. Cette association en réseau intervient vraisemblablement avec un rythme θ/α de 4 Hz à 13 Hz, à la fois pendant le repos et l'activité. Une modélisation précise des cerveaux individuels humains montre que la topographie fonctionnelle du réseau frontopariétal est variable entre les individus, soulignant le fait que des études de moyennes de groupes du réseau frontopariétal peuvent occulter d'importantes caractéristiques typiques et atypiques. De nombreuses formes de psychopathologie impliquent le réseau frontopariétal, comme la schizophrénie et les troubles du déficit de l'attention/hyperactivité. Compte tenu de la variabilité interindividuelle dans l'organisation du réseau frontopariétal, des études cliniques bénéficieront probablement grandement de l'apport de données individuelles de sujets pour caractériser de façon précise des réseaux au repos compromis en psychopathologie.
Introduction
The human brain is unique among other species in its ability to accurately and rapidly learn new concepts and switch between states, while maintaining complex rule sets. We engage in countless goal-directed tasks throughout a given day, adopting task sets that flexibly configure information processing in response to changing task demands. In cognitive psychology and neuroscience, this process of volitional goal-driven behavior is referred to as cognitive control. Cognitive control is not executed by a single brain region or single brain network, but rather by several largely non-overlapping brain networks, each consisting of a relatively large set of anatomically distributed regions, including the frontoparietal, cingulo-opercular, and salience networks. There is now abundant evidence that these networks are anatomically separate from downstream processing or attention networks, both during task states and the resting state. Each network plays a unique role in cognitive control, including its implementation, maintenance, and updating. Networks related to attention vs cognitive control map onto those outlined by Petersen and Posner,1,2 with the dorsal and ventral attention networks supporting orienting and the frontoparietal and cingulo-opercular networks supporting cognitive control. For the duration of this review, we will be focused on the control networks, with emphasis on the frontoparietal control network.
We begin our review by summarizing evidence for the frontoparietal network as distinct from other control and attention networks, including its privileged role as a flexible hub of cognitive control. We then move into a discussion of the oscillations underlying frontoparietal network interactions, during both resting and task states. Following this, we will discuss the importance of densely sampling individual subjects. There is compelling evidence that while core regions of the frontoparietal network are present across individuals, critical variants in this network's topography exist. We conclude by briefly reviewing evidence for frontoparietal dysfunction in several forms of psychopathology that emerge during adolescence, a time when the frontoparietal network is refining many of its interactions with other brain networks. Given the anatomical heterogeneity of the frontoparietal network across individuals, we argue a complimentary shift towards densely sampling individual subjects in both normative and diseased states is of paramount importance to understanding the frontoparietal network in typical and atypical cohorts.
Evidence for parallel, segregated control networks
The original focus on the anatomical substrate of cognitive control was within the anterior cingulate cortex and to a lesser extent the anterior insula. This is because the anterior cingulate demonstrates reliable activation in response to many forms of control, including, but not limited to, task switching, novelty detection, focal attention, and error commission.3-6 This early work led Botvinick and colleagues to conclude that the anterior cingulate facilitates outcome monitoring by evaluating the result of an individual's actions, and facilitating the resolution of conflict during task (ie, conflict monitoring).7 Thus, it was proposed that the anterior cingulate acts to alert regulatory regions, such as the dorsolateral prefrontal cortex, which in turn exert top-down control. Since this time, the conflict monitoring hypothesis has evolved, prescribing a role of dorsal anterior cingulate in signaling the expected value of control.8 Working closely in conjunction with this region and comprising the core of the brain's salience network, the anterior insula is thought to detect salient features for additional processing and is thought to act as a switchboard to direct other brain networks.9-11
Although the conflict-monitoring hypothesis ascribes the anterior cingulate a central role in control, a separate dual network's view holds that cognitive control is supported by multiple, anatomically distributed brain networks.12 This model is the result of studies specifically aiming to delineate distinct control signals. Though the detection of salient stimuli and conflict resolution are essential features of cognitive control, humans also need to maintain and adapt control. Thus, there are three main signals related to cognitive control: (i) a transient signal resulting from the realization of the need to instantiate control; (ii) a sustained signal supporting the maintenance of control; and (iii) a transient signal supporting performance feedback. An early attempt to disentangle brain regions supporting different modes of control was executed by Braver and colleagues using a mixed block/event related design in functional magnetic resonance imaging (fMRI).13 Results from this study concluded that the anterior prefrontal cortex was most reliably activated during the maintenance of control, while the superior parietal lobes were involved in transient control. Several years later, Dosenbach and colleagues executed a cross-studies analysis on 10 mixed block/event-related fMRI studies to tease apart regions contributing to the main signals contributing to cognitive control.12 These tasks included visual and auditory stimuli, with many different decision criteria, such as semantic, timing, and similarity judgments. They discovered a set of regions including the anterior prefrontal, anterior insular, and anterior cingulate cortices that showed preferable activation for the maintenance of control, whereas the bilateral intraparietal sulcus and lateral prefrontal cortex showed preferable activation for task-set initiation. Lastly, performance feedback seemed to be supported by the inferior parietal lobe, dorsolateral prefrontal cortex, and lateral cerebellum.12
During the mid-2000s, fMRI analysis was shifting from a focus on regional contributions to brain function to a broader network-level focus. With respect to this network-level approach, a key observation is that co-fluctuations during the resting-state largely recapitulate patterns of activation during task.14 Capitalizing on this observation, Dosenbach and colleagues implemented resting-state fMRI to delineate a whole brain network's view of the brain's control architecture.15,16 During the resting state, two largely parallel control networks emerged. These two distinct networks were coined the frontoparietal and cingulo-opercular networks. The original putative role of the cingulo-opercular network was in the flexible control of goal-directed behavior through the stable implementation of task sets in downstream sensorimotor processors across trials, while control needed to be maintained. Conversely, the frontoparietal network was prescribed the role of supporting control initiation and provide flexibility by adjusting control in response to feedback.
Currently, there is an abundance of evidence for both a unified framework of control (conflict monitoring) and for parallel control networks. However, we and others1 argue that the latter is more likely. First, investigations of lesions in lateral PFC have shown these patients have deficits in the ability to switch tasks; however, they retain the ability to maintain a task set.17 Conversely, lesions of midline prefrontal cortex, including the anterior cingulate, have resulted in the ability to switch tasks, but not maintain a set. Second, there is little to no evidence for any temporal lag between the anterior cingulate cortex and lateral prefrontal cortex. In a study by Ploran and colleagues,18 noisy images were slowly revealed to track the rate of sensory evidence accumulation. Activity in the frontoparietal network slowly increased as evidence was accumulated, but the cingulo-opercular network was activated in the periresponse period. These data suggested that the cingulo-opercular network has a more prominent role in motor control, rather than in higher-order control. Third, electrophysiological studies also point to segregated control networks, using a working memory paradigm in which cues were presented either before the memory array or during the maintenance period to assess prospective and retrospective control of working memory.19 The frontoparietal network showed increased activity for both prospective and retrospective cues, while the cingulo-opercular network only showed increases in activity for retrospective cues during working memory maintenance. Furthermore, a cross-correlation analysis revealed frontoparietal network activity modulated α-band activity in downstream visual association cortices, whereas there was no evidence for top-down modulation of the visual network by the cingulo-opercular network, supporting a role for the frontoparietal network in bias sensory information in processing networks.20,21
Role of the frontoparietal network: a flexible hub for cognitive control
Humans are unique and quite remarkable in their degree of flexibility and speed when instantiating cognitive control. How the human brain is capable of doing this given its rigid anatomical backbone is an area of ongoing research. Given its role in task adaptation and implementation, a reasonable hypothesis is that the frontoparietal network, or at least a subset of it, is a functional hub (ie, it engages in strong co-fluctuations with many other brain networks). Indeed, not only does the frontoparietal network share a high degree of functional connectivity without considering functional network organization,22,23 but it also demonstrates a large degree of connectivity to many diverse brain networks, meaning that the frontoparietal network is a functional hub both globally, and specifically in terms of distributed connectivity.23,25 Moreover, fluid intelligence is positively correlated with the degree to which the frontoparietal network's coupling is distributed to other brain networks26; in particular, greater connectivity between the frontoparietal and default mode networks during resting state was correlated with higher intelligence scores.27 Furthermore, there is a significant positive correlation between functional integration of the frontoparietal network and overall cognitive ability, indicating that the strength of functional integration of the frontoparietal network and the rest of the brain is crucial for supporting superior cognitive functioning.28 Given previous evidence for its role in task adaptation and implementation, the frontoparietal network was hypothesized to play a role in instantiating and flexibly modulating cognitive control.
To test this hypothesis, Cole and colleagues used a rapid instructed task learning paradigm,29 which refers to the ability to immediately perform novel instructed procedures accurately after the first instance a new instruction (rule) is given.30 Twelve task rules were randomly permuted to achieve 64 different task “states.” The 12 task rules were created to assess three distinct cognitive domains (logical decision, sensory semantics, and motor response) with four rules per domain. The frontoparietal network's pattern of coupling shifted significantly more throughout the rapid switching of tasks than any other network, including other control networks, providing evidence that the frontoparietal network is a functional hub for influencing brain-wide communication to meet task demands. Moreover, the pattern of functional connectivity was specific, such that the individual task being completed was predicted by the pattern of connectivity of the frontoparietal network to other networks. Lastly, these predictive patterns held even when the tasks were practiced. Thus, the frontoparietal network (and not the cingulo-opercular network) is a flexible hub amongst other brain networks for the flexible coordination of cognitive control,29 providing further evidence in favor of dissociable and parallel control networks. Taken together, the frontoparietal network is highly integrated with other brain networks, providing a functional backbone for rapid and flexible modulation of other brain networks.
Electrophysiology of the frontoparietal network
fMRI has been the primary tool used to understand the role of the frontoparietal network, which is only sensitive to slow oscillations (0.005 Hz to 0.1 Hz; ie, approximately 1 cycle per minute). However, the cognitive constructs that the frontoparietal network supports, including flexible integration of other networks supporting cognitive control, occur at much faster timescales6 (ie, 1-100 Hz). For example, consider a simple task in which you are instructed to make a left finger motor response to a green crosshair and a right finger motor response to a blue crosshair as quickly and accurately as possible. In the task, the presentation of blue and green cues is mixed, such that the sequence is random. At each switch in cue color (eg, from blue to green), the frontoparietal network must signal the instantiation of control and recruit downstream networks, such as the motor network, for a correct response. The reaction time on any given switch trial would be less than 1 second, faster than one full cycle of a blood oxygen level-dependent (BOLD) oscillation. Thus, there is great interest as to how control networks recruit other networks for rapid accurate responses to task switching, including temporal precedence.
Much of the work characterizing specific contributions of neural oscillations to brain function in this faster range (1Hz to 100 Hz) has been done in task-state analyses. The correlation between electrophysiology and BOLD has been studied in both human and nonhuman primates, with a consistent finding of correlations between modalities in broadband y activity (40 Hz to 100 Hz).31,32 Oscillations in this frequency range play a critical role in enabling local neuronal synchronization, whereas slower θ/α (4Hz to 14Hz) band oscillations have been shown to be critical for long-distance integration.33,34 Inter-areal synchronization of θ/α band oscillations within the frontoparietal network are associated with cognitive control, and have been shown to improve behavioral performance on control tasks, most prominently when switching rule sets.35,36 Additionally, θ/α power has been shown to intensify when control demands are increased.37 Hence, slow-frequency oscillations across control regions may underlie top-down modulation of sensory networks.6,38,39 For example, longrange frontoparietal interactions during working memory retention and mental imagery evolved most strongly in the θ and α (4 Hz to 14Hz) frequency range,40 and the prefrontal cortex has been shown to lead the posterior parietal cortex in sustained visual attention tasks in theta band oscillations.19 Slower frequency oscillations, often in the θ band (4 Hz to 10 Hz) have been shown to organize local neural activity in the y band, such that neurons tend to have greater firing rates in the trough of an ongoing slow-frequency oscillation.41 As such, the phase of slower-frequency oscillations may be critical for coordination of neural activity over long distances, perhaps acting as an organizing mechanism for downstream sensorimotor function.39,41,42
In contrast to task states, less is known about the electrophysiological correlates of control networks defined by BOLD fMRI during the resting state, There is some evidence that resting-state BOLD networks correlate to the α and β band, as measured with magnetoencephalography.43 However, there is evidence that correlations with BOLD may be greater at even slower frequencies (4 Hz to 13 Hz).44 More recently, Hacker and colleagues characterized the spatial correspondence in humans of resting state BOLD fMRI and band-limited power using electrocorticographic recordings.45 They found that γ band correlation was high throughout the brain. In addition to this, they uncovered a dissociation between the frontoparietal control network and dorsal attention network, such that the frontoparietal network demonstrated greater coupling of θ band power (3 Hz to 8 Hz) to BOLD, whereas the dorsal attention network had greater coupling between band-limited power and BOLD in the α band (8 Hz to 12 Hz). In sum, the frontoparietal network seems to map onto slower-frequency oscillations (4 Hz to 14 Hz), critical for supporting its role as a flexible hub for coordinating the activity of other brain networks.
Precision mapping and its implication for the frontoparietal network
fMRI data has a notoriously low signal-to-noise ratio. To overcome this issue, the standard paradigm of fMRI imaging in humans has been to collect small quantities of data (5 to 10 mins of resting state) per subject and to then average them over tens, hundreds, or sometimes thousands of individuals to identify central tendencies of both healthy and diseased cohorts. This paradigm has been fruitful in helping investigators understand regional and network-level brain organization and function. While group averaging has revealed many basic principles of functional brain organization, it has been understood for centuries that individual brains differ in their functional neuroanatomy. The current lack of emphasis on understanding individuals limits the utility of fMRI to characterize and understand normative and atypical cohorts.
To begin understanding individual differences in functional neuroanatomy, a single individual was scanned for a total of 200 minutes of resting-state data across 10 different sessions, known as precision mapping.46 Several crucial observations were made. First, high reliability of resting-state correlations can be achieved with long enough data acquisition (~45 minutes), overcoming the low signal-to-noise nature of fMRI in a single individual subject. Second, individuals exhibit measurable variants in functional network organization compared with a group average. Third, in the individual brain, part of the lateral prefrontal cortex bilaterally contained a variant belonging to the cingulo-opercular network, which belongs to the frontoparietal network in group studies.
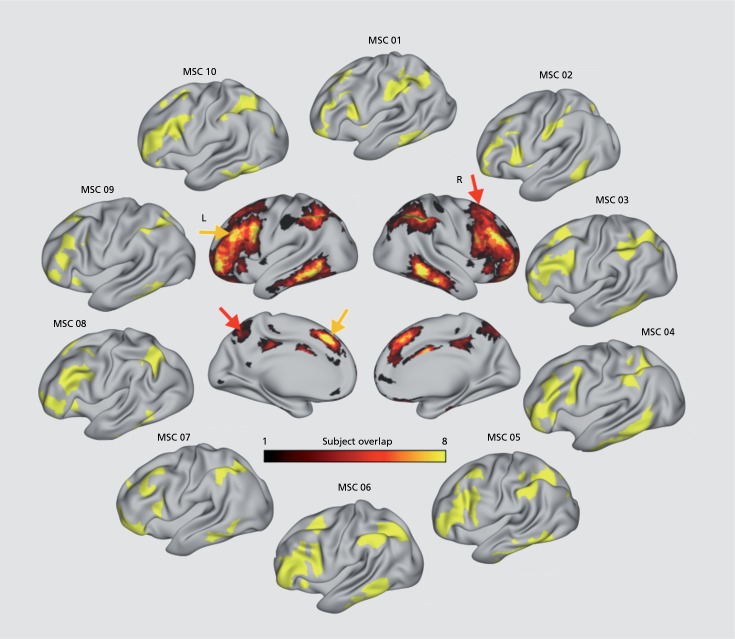
As an extension of densely mapping a single individual, 10 individuals were scanned for a total of 300 minutes of resting state data over 10 sessions, referred to as the Midnight Scan Club (MSC).47 In addition to the motor, visual, and cingulo-opercular variants observed in a single individual,46 several new types of spatial and organizational variability in brain networks emerged. These included unique network features and topologies that corresponded with structural and task-derived brain features. For example, even a well-defined network, such as the somatomotor hand network, demonstrated measurable variability between subjects, especially in the degree of their task/rest overlap on a block design motor task. Moreover, there was significant heterogeneity in network assignments in frontal and parietal association cortices. Specifically, with respect to the frontoparietal network, areas of high overlap between subjects were in the intraparietal sulcus, ventral inferior temporal lobe, and localized regions of the lateral prefrontal cortex. However, across the prefrontal cortex, there were substantial deviations between subjects, with variants of other control and attention networks located in regions of frontal cortex affiliated with the frontoparietal network in other subjects (Figure 1). Thus, there is substantial individual variation in the precise anatomical distribution of the frontoparietal network.
Figure 1. Individual frontoparietal network assignments (yellow patches) displayed on the left hemisphere cortical surface from the Midnight Scan Club (outer ring). The central montage depicts the number of subjects having a frontoparietal network assignment on the left and right lateral and medial cortical surface. Yellow arrows indicate exemplar patches where there is a high degree of overlap in frontoparietal assignment across subjects. Conversely, red arrows show exemplar areas where a minority of subjects contains frontoparietal network patches, highlighting the relatively large degree of heterogeneity in frontoparietal network topography. Only 52 vertices out of the 19 074 (0.3%) vertices had overlap across all 10 subjects, and 1171 of 19 074 vertices (6.1%) had overlap across eight subjects. This high degree of heterogeneity is especially prominent across large swaths of the lateral prefrontal cortex.
Development and clinical implications of the frontoparietal network
Both cognitive control and the functional brain networks that support it show a protracted development through adolescence and early adulthood. Children and adolescents are able to exert cognitive control. Thus, development is not characterized by the emergence of cognitive control, but rather the refinement of it. Paralleling this notion, there is evidence that the brain's control networks are apparent by 2 years of age,48,49 Control networks are observable in infants younger than 2 years of age, and are thought to be immature forms of control networks identifiable later in development. Throughout childhood and adolescence, the brain's control networks become more integrated with other brain networks, potentially laying the early groundwork for greater flexible engagement later in development.50 For example, the increased integration of the cingulo-opercular and salience networks supports the maturation of inhibitory control engagement.25 Recently, Chai and colleagues showed that the expression of the frontoparietal network increased in both strength and flexibility throughout development.51 As such, the developmental trajectory of the control networks parallels advances in cognitive control abilities. Cognitive control is commonly comprised in many forms of psychopathology, many of which emerge during adolescence while control is being refined. It is likely that there exist shared mechanisms in the dysfunction of neural networks resulting in these different forms of psychopathology.52 The abnormal developmental of a flexible brain network, such as the frontoparietal network, may be a common feature across many diseases, including schizophrenia, depression, and anxiety.53-55 For example, patients with schizophrenia consistently exhibit relatively low levels of cognitive control,56 often apparent as early as childhood. Patients with schizophrenia demonstrate reduced BOLD activity and connectivity within and between regions of both the frontoparietal and cingulo-opercular networks across many cognitive tasks.28,57-59 These findings underscore the notion that schizophrenia may be characterized by a generalized cognitive deficit implicating similar neurobiological mechanisms across cognitive domains.28
Disorders involving cognitive control can be broadly broken into primary and secondary control disorders.60 Primary control disorders directly impact control networks,60 such as schizophrenia, in which substantial cellular and molecular alterations occur within the lateral prefrontal cortex,61 possibly underlying changes in global connectivity of the lateral prefrontal cortex observed in humans.57 Secondary control disorders, such as anxiety and depression, are those that manifest in such a way as to not directly impact control networks.60 For these disorders, cognitive control is thought to act as a buffer, such that high control abilities assuage symptoms, whereas lower control abilities cannot compensate for downstream abnormalities. As such, lower cognitive control capacity evident early in development may be a risk factor for schizophrenia and other forms of psychopathology.62 It has been proposed that frontoparietal connectivity could be augmented through cognitive training, such as in psychotherapy.60,63 Future research should focus on the contributions of modulations within frontoparietal interactions through cognitive training to ameliorating symptoms of psychopathology. Moreover, accurately characterizing frontoparietal network topography in individual subjects may be critical for treatments targeting these regions, as is often done using transcranial magnetic stimulation.
Conclusion
The frontoparietal network is a control network, distinct from the salience and cingulo-opercular networks, serving to rapidly and instantiate new task states by flexibly interacting with other control and processing networks. The slow-frequency BOLD components that define it are correlated with relatively slow oscillations in the frequency range sensitive to electrophysiological recordings (θ/α band), likely supporting its role in coordination of whole-brain network activity. Along with the other control networks, the frontoparietal network demonstrates a protracted development, perhaps lending it to vulnerability to various forms of psychopathologies linked to cognitive control deficits, such as schizophrenia. Due to its heterogeneity in anatomical location within individual subjects, future studies should seek to densely sample individuals, mapping frontoparietal networks individually, and subsequently comparing and contrasting normal and diseased states to further our understanding of the neural basis of psychopathology.
Acknowledgments
This work was supported by National Institutes of Health grants 5T32 MH100019-02 (S.M.) NS088590, TR000448 (N.U.F.D.), 1P30NS098577 (to the Neuroimaging Informatics and Analysis Center), and HD087011 (to the Intellectual and Developmental Disabilities Research Center at Washington University); the Jacobs Foundation 2016121703 (N.U.F.D.); the Child Neurology Foundation (N.U.F.D); the McDonnell Center for Systems Neuroscience (N.U.F.D.) the Mallinckrodt Institute of Radiology grant 14-011 (N.U.F.D.); the Hope Center for Neurological Disorders (N.U.F.D.). The authors declare no conflicts of interest.
Contributor Information
Scott Marek, Department of Neurology, Washington University School of Medicine, St Louis, Missouri, USA.
Nico U. F. Dosenbach, Department of Neurology, Washington University School of Medicine, St Louis, Missouri, USA; Program in Occupational Therapy, Washington University School of Medicine, St Louis, Missouri, USA; Department of Pediatrics, Washington University School of Medicine, St Louis, Missouri, USA.
REFERENCES
- 1.Petersen SE., Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Posner M., Petersen S. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 3.Carter CS., Botvinick MM., Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10(1):49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Barcelo F., Escera C., Corral MJ., Perianez JA. Task switching and novelty processing activate a common neural network for cognitive control. J Cogn Neurosci. 2006;18(10):1734–1748. doi: 10.1162/jocn.2006.18.10.1734. [DOI] [PubMed] [Google Scholar]
- 5.Barcelo F., Perianez JA., Knight RT. Think differently: a brain orienting response to task novelty. Neuroreport. 2002;13(15):1887–1892. doi: 10.1097/00001756-200210280-00011. [DOI] [PubMed] [Google Scholar]
- 6.Cavanagh JF., Frank MJ. Frontal theta as a mechanism for cognitive control. Trends Cogn Sci. 2014;18(8):414–421. doi: 10.1016/j.tics.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Botvinick MM., Cohen JD., Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Shenhav A., Botvinick MM., Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon V., Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Fund. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sridharan D., Levitin DJ., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- 12.Dosenbach NU, Visscher KM., Palmer ED., et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braver TS., Reynolds JR., Donaldson DI. Neural mechanisms of transient and sustained cognitive control during task switching. Neuron. 2003;39(4):713–726. doi: 10.1016/s0896-6273(03)00466-5. [DOI] [PubMed] [Google Scholar]
- 14.Cole MW., Bassett DS., Power JD., Braver TS., Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014;83(1):238–251. doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dosenbach NU., Fair DA., Cohen AL., Schlaggar BL., Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dosenbach NU., Fair DA., Miezin FM., et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi AF., Bichot NP., Desimone R., Ungerleider LG. Top down attentional deficits in macaques with lesions of lateral prefrontal cortex. J Neurosci. 2007;27(42):11306–11314. doi: 10.1523/JNEUROSCI.2939-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ploran EJ., Nelson SM., Velanova K., Donaldson DI., Petersen SE., Wheeler ME. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci. 2007;27(44):11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallis G., Stokes M., Cousijn H., Woolrich M., Nobre AC. Frontoparietal and cingulo-opercular networks play dissociable roles in control of working memory. J Cogn Neurosci. 2015;27(10):2019–2034. doi: 10.1162/jocn_a_00838. [DOI] [PubMed] [Google Scholar]
- 20.Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18(5):502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- 21.Gazzaley A., Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16(2):129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cole MW., Pathak S., Schneider W. Identifying the brain's most globally connected regions. Neuroimage. 2010;49(4):3132–3148. doi: 10.1016/j.neuroimage.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Power JD., Schlaggar BL., Lessov-Schlaggar CN., Petersen SE. Evidence for hubs in human functional brain networks. Neuron. 2013;79(4):798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power JD., Cohen AL., Nelson SM., et al. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marek S., Hwang K., Foran W., Hallquist MN., Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13(12):e1002328. doi: 10.1371/journal.pbio.1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole MW., Ito T., Braver TS. Lateral prefrontal cortex contributes to fluid intelligence through multinetwork connectivity. Brain Connect. 2015;5(8):497–504. doi: 10.1089/brain.2015.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hearne LJ., Mattingley JB., Cocchi L. Functional brain networks related to individual differences in human intelligence at rest. Sci Rep. 2016;6:32328. doi: 10.1038/srep32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheffield JM., Repovs G., Harms MP., et al. Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia. 2015;73:82–93. doi: 10.1016/j.neuropsychologia.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole MW., Reynolds JR., Power JD., Repovs G., Anticevic A., Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16(9):1348–1355. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole MW., Braver TS., Meiran N. The task novelty paradox: flexible control of inflexible neural pathways during rapid instructed task learning. Neurosci Biobehav Rev. 2017;81(Pt A):4–15. doi: 10.1016/j.neubiorev.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goense JB., Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18(9):631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 32.Koch SP., Werner P., Steinbrink J., Fries P., Obrig H. Stimulus-induced and state-dependent sustained gamma activity is tightly coupled to the hemodynamic response in humans. J Neurosci. 2009;29(44):13962–13970. doi: 10.1523/JNEUROSCI.1402-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donner TH., Siegel M. A framework for local cortical oscillation patterns. Trends Cogn Sci. 2011;15(5):191–199. doi: 10.1016/j.tics.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 34.von Stein A., Sarnthein J. Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 35.Haegens S., Osipova D., Oostenveld R., Jensen O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum Brain Mapp. 2010;31(1):26–35. doi: 10.1002/hbm.20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Zanto TP., Rubens MT., Thangavel A., Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci. 2011;14(5):656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sadaghiani S., Scheeringa R., Lehongre K., et al. α-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J Neurosci. 2012;32(41):14305–14310. doi: 10.1523/JNEUROSCI.1358-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88(1):220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palva S., Palva JM. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front Psychol. 2011;2:204. doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Helfrich RF., Knight RT. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn Sci.. 2016;20(12):916–930. doi: 10.1016/j.tics.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hipp JF., Hawellek DJ., Corbetta M., Siegel M., Engel AK. Large-scale cortical correlation structure of spontaneous oscillatory activity. Nat Neurosci. 2012;15(6):884–890. doi: 10.1038/nn.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Saalmann YB., Pinsk MA., Arcaro MJ., Kastner S. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76(5):1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hacker CD., Snyder AZ., Pahwa M., Corbetta M., Leuthardt EC. Frequency-specific electrophysiologic correlates of resting state fMRI networks. Neuroimage. 2017;149:446–457. doi: 10.1016/j.neuroimage.2017.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laumann TO., Gordon EM., Adeyemo B., et al. Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87(3):657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gordon EM., Laumann TO., Gilmore AW., et al. Precision functional mapping of individual human brains. Neuron. 2017;95(4):791–807 e797. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb Cortex. 2011;21(1):145–154. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 49.Gao W., Alcauter S., Smith JK., Gilmore JH., Lin W. Development of human brain cortical network architecture during infancy. Brain Struct Funct. 2015;220(2):1173–1186. doi: 10.1007/s00429-014-0710-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grayson DS., Fair DA. Development of large-scale functional networks from birth to adulthood: A guide to the neuroimaging literature. Neuroimage. 2017;160:15–31. doi: 10.1016/j.neuroimage.2017.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chai LR., Khambhati AN., Ciric R., Moore TM. Evolution of brain network dynamics in neurodevelopment. Network Neuroscience. 2017;1(1):1430. doi: 10.1162/NETN_a_00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuthbert BN., Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sylvester CM., Barch DM., Corbetta M., Power JD., Schlaggar BL., Luby JL. Resting state functional connectivity of the ventral attention network in children with a history of depression or anxiety. J Am Acad Child Adolesc Psychiatry. 2013;52(12):1326–1336. doi: 10.1016/j.jaac.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinrichs RW., Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 55.Sheffield JM., Kandala S., Tamminga CA., et al. Transdiagnostic associations between functional brain network integrity and cognition. JAMA Psychiatry. 2017;74(6):605–613. doi: 10.1001/jamapsychiatry.2017.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lesh TA., Niendam TA., Minzenberg MJ., Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36(1):316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cole MW., Anticevic A., Repovs G., Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70(1):43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anticevic A., Repovs G., Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull. 2013;39(1):168–178. doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Repovs G., Csernansky JG., Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69(10):967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cole MW., Repovs G., Anticevic A. The frontoparietal control system: a central role in mental health. Neuroscientist. 2014;20(6):652–664. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewis DA., Curley AA., Glausier JR., Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.David AS., Malmberg A., Brandt L., Allebeck P., Lewis G. IQ and risk for schizophrenia: a population-based cohort study. Psychol Med. 1997;27(6):1311–1323. doi: 10.1017/s0033291797005680. [DOI] [PubMed] [Google Scholar]
- 63.Knekt P., Lindfors O., Sares-Jaske L., Virtala E., Harkanen T. Randomized trial on the effectiveness of long- and short-term psychotherapy on psychiatric symptoms and working ability during a 5-year follow-up. Nord J Psychiatry. 2013;6(1):59–68. doi: 10.3109/08039488.2012.680910. [DOI] [PubMed] [Google Scholar]