Abstract
The brain is the ultimate adaptive system, a complex network organized across multiple levels of spatial and temporal resolution that is sculpted over several decades via its interactions with the environment. This review sets out to examine how fundamental biological processes in early and late neurodevelopment, in interaction with environmental inputs, guide the formation of the brain's network and its ongoing reorganization throughout the course of development. Moreover, we explore how disruptions in these processes could lead to abnormal brain network architecture and organization and thereby give rise to schizophrenia. Arguing that the neurodevelopmental trajectory leading up to the manifestation of psychosis may best be understood from the sequential trajectory of connectome formation and maturation, we propose a novel extension to the neurodevelopmental model of the illness that posits that schizophrenia is a disorder of connectome development.
Keywords: brain network, connectome, connectivity, critical window development, illness trajectory, neurodevelopmental mode, schizophrenia
Abstract
El cerebro es el útimo sistema de adaptación, una red compleja organizada en múltiples niveles de resolución espacial y temporal que se ha esculpido durante varias décadas a través de sus interacciones con el medio ambiente. Esta revisión se propone examinar cómo los procesos biológicos fundamentales en el neurodesarrollo precoz y tardío, al interactuar con los estímulos ambientales, guían la formación de la red cerebral y su reorganización permanente a lo largo del curso del desarrollo. Además, se explora la forma cómo las alteraciones en estos procesos podrían llevar a una anomalía en la arquitectura y en la organización de la red cerebral, y así facilitar la aparición de la esquizofrenia. Se argumenta que la trayectoria del neurodesarrollo que conduce a la manifestación de la psicosis se puede entender mejor a partir de la trayectoria secuencial de la formación y maduración del conectoma, y se propone una nueva extensión para el modelo del neurodesarrollo de la enferme-dad, que postula que la esquizofrenia es un trastorno del desarrollo del conectoma.
Abstract
Le cerveau est le système adaptatif par excellence, un réseau complexe organisé en plusieurs niveaux de résolution temporelle et spatiale, sculpté au fil des décennies par ses interactions avec l'environnement. Cet article examine comment les processus biologiques fondamentaux guident la formation du réseau cérébral et sa réorganisation continue au cours de l'évolution, dans le développement neuronal précoce et tardif, en interaction avec des aspects environnementaux. De plus, nous analysons comment des perturbations de ces processus peuvent conduire à une organisation et une architecture anormales du réseau cérébral et ainsi conduire à la schizophrénie. Nous proposons une extension nouvelle au modèle neurodéveloppemental de la maladie qui stipule que la schizophrénie est un trouble de développement du connectome, en soutenant que la trajectoire neurodéveloppementale conduisant à la manifestation de la psychose peut être mieux comprise à partir de la trajectoire séquentielle de formation et de maturation du connectome.
Introduction
Human brain development involves a protracted sequence of events that starts in utero and extends into adulthood. During this time, the brain develops from a simple tubular structure to arguably the most complex system in biology; an intricately folded organ comprising a vast network of interconnected neurons known as the human connectome.1 The connectome is sculpted over many years by genetically mediated processes and environmental inputs that exert an especially potent influence over the developing brain network during circuit-specific windows of developmental plasticity.2,3 Abnormalities at any stage of this process—from the initial formation of the brain's network to its ongoing reorganization over the course of development—may underlie the evolution of neurodevelopmental disorders such as schizophrenia.4,5
Schizophrenia is a psychiatric disorder that manifests early in life and derails social, cognitive, and academic development. The first psychotic episode marks the formal onset of the illness, but converging evidence suggests that psychosis is a relatively late stage in illness development.6,7 The first episode is typically preceded by a prodromal phase characterized by subthreshold psychotic symptoms, social withdrawal, cognitive difficulties, and functional decline.8,9 Preceding even this phase, children that will develop schizophrenia later in life have been noted to show subtle and nonspecific deviations from normal neuromotor, language, and socioemotional development.10,11 The slow progression from early risk markers to the first psychotic episode represents both an extended window of vulnerability for schizophrenia and an opportunity for preventive or therapeutic intervention.6
This review examines connectome development in relation to the neurodevelopmental trajectory of schizophrenia. Following an overview of key features of brain network architecture, the first section of our review focuses on connectome formation and examines how fundamental properties of connectome organization may stem from biological processes in early brain development. The second section addresses mechanisms driving connectome maturation in childhood and adolescence. In the third section, we discuss connectome maldevelopment, and ask how this could give rise to the neurodevelopmental trajectory of schizophrenia. Drawing from developmental neurobiology, neuroimaging, and brain network analysis, we put forward a novel extension to the neurodevelopmental model of schizophrenia. Arguing that schizophrenia is ultimately a disorder of connectome development, we propose that the sequential trajectory in which the illness develops may best be understood from the neurodevelopmental mechanisms guiding connectome formation and maturation.
Connectome architecture: key features
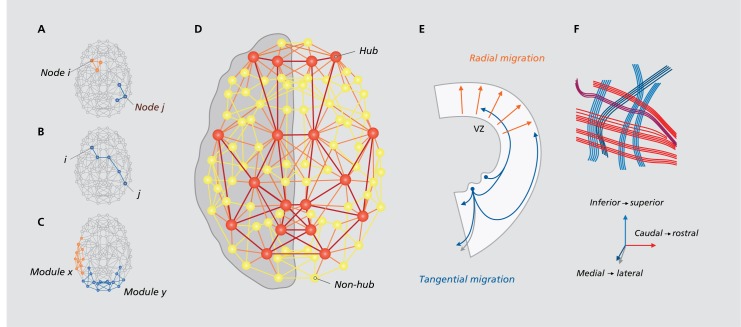
Graph theoretical analyses of brain connectivity data have demonstrated a number of key organizational properties of the connectome (for review, see ref 1). One central feature of brain network architecture is that it combines a high level of clustering with short average path length (Figure 1A, B). In other words, most connections link nearby neighboring neurons or neuronal populations, while a small number of long-distance connections make it easy to traverse the whole network in just a few steps. In addition to this “small-world” topology, the connectome has a modular organization, meaning that it can be decomposed into modules or communities (Figure 1C). Modules are thought to allow for specialized processing by limiting interference from brain regions processing other types of information.12 Another characteristic feature of connectome topology is that it contains a small subset of brain regions with a disproportionately high level of connectivity. These highly connected “hub” regions (Figure 1D) have been identified across the heteromodal association cortex.13 Hubs are especially tightly connected to other hubs, forming a “rich club” system that spans different modules (Figure 1D).14,15 This feature of connectome organization may thus enable tasks requiring the collaboration of multiple specialized systems such as the integration of multisensory information or higher-order cognitive tasks.
Figure 1. Connectome organization and formation. (A) The neighbors of node i are mutually connected, reflecting local clustering. In contrast, the neighbors of node j are not connected. A network with many triangular motifs (in orange) is highly clustered. (B) The shortest path from node i to node j is four steps. A network's average path length is the average number of steps along the shortest path between each node pair. Lower path length is consistent with a more efficient network. (C) shows connectome modules, with module x reflecting a left lateralized language module, and y posterior visual module. (D) The brain's network contains hubs that are tightly interconnected into a rich club system (in red). (E) illustrates the two main forms of neuronal translocation: radical and tangential migration (VZ = Ventricular Zone). (F) depicts the potentiel grid-like microstructure of the brain's fiber pathways consistent with three primordial gradients of early embryogenesis. (F) reproduced from ref 26. Wedeen VJ, Rosene DL, Wang R, et al. The geometric structure of the brain fiber pathways. Science. 2012;337:1628-1634. Copyright © Amercian Associaion for the Advancement of Science, 2012.
Connectome formation
The establishment of these key features of connectome organization —ie, high-clustering, short path length, a modular organization, and the existence of highly connected hubs—may depend on fundamental processes in early neurodevelopment.
Clustering and path length
Early in prenatal life, neuronal precursor cells migrate into the cortical plate by one of two main forms of neuronal migration: radial migration and tangential migration (Figure 1E).16,17 Radially migrating neurons move towards the pial surface of the cortex,18 while tangential migration occurs in a parallel direction, allowing neurons from distant origins to intermingle in a common destination.19,20 To connect to other neurons, neurons extend axons that navigate the developing brain, guided by molecules emitted by target cells or intermediate “guidepost” cells that serve as stepping stones along axonal trajectories.21,22 Radial migration contributes to this process by providing patterning information that guides the appropriate arrangement of axon guidance cues, while tangential migration results in the formation of “permissive corridors,” which are used by growing axons to bypass regions that they could otherwise not traverse.20 The emergence of high local clustering and short global path length in the brain's network may relate to these processes. As radial migration is the primary form of neuronal translocation, axons connect primarily to nearby neurons of similar origins, thereby promoting local clustering. Tangential migration is less frequent, and may thus drive the formation of a smaller number of long-distance connections between spatially distributed neuronal populations as a result of parallel migration patterns and permissive corridor formation. As cross-level studies of brain connectivity indicate that microscale architectonic features of a brain region relate to its network profile on the macroscale23 the resulting connectivity patterns on the cellular scale may translate into concurrent patterns of connectome wiring on the whole-brain level.
Modular topology
The formation of modules of spatially distributed neuronal populations or brain regions may also relate to fundamental neurodevelopmental processes. The cortex is a patchwork of anatomically and functionally distinct regions, but these areas originate from a single neuroepithelial sheath through gradients in extracellular signals and transcription factors that operate across the field of cortical stem cells.24 These stem cells use the spatial information encoded by gene expression gradients together with temporal information to generate differentially patterned neuronal progeny at different times in development.24 Considering findings in Drosophila that neurons within the same module tend to be born around the same time,25 differentially patterned generations of neurons may contribute to the formation of different brain network modules at different times in human brain development. There may be an additional mechanism by which primordial gradients could contribute to module formation, as these gradients are also thought to contribute to pathway formation in the brain. Indeed, recent diffusion spectrum magnetic resonance imaging (MRI) findings indicate that cerebral fiber pathways form a highly curved three-dimensional grid continuous with the three principal axes of development (Figure 1F).26 This grid-like connectivity structure may serve as a scaffold for early functional collaboration in the brain and only develop into a more modular system over time in order to tune brain wiring in accordance with functional communication patterns. A synthesis of these mechanisms may also be in place, with the cortex itself having an early modular organization conferred by cortical stem cells, and the early “proto-network” refining into a modular system with ongoing development.
Hub and rich club organization
Insights into potential mechanisms supporting hub formation in brain development come from the field of brain evolution. Specifically, studies on the origins of evolutionarily recent additions to the brain suggest that new cortical areas evolved from older regions via a process of “descent with modification.” This implies that as a new area develops from an evolutionary older region, it inherits a large degree of the parent structure's design and organization.27 If something similar occurs in ontogenetic neurodevelopment—ie, that later-developing brain regions develop via separation from earlier developing areas—this could mean that late-developing regions largely inherit connectivity patterns of earlier developing areas.28 Such a mechanism would give rise to increasingly well-connected regions with ongoing development and may thus explain the hub-role of late-developing heteromodal regions. This hypothesis fits with observations that both in evolution and in brain development, expansion is non-uniform across the cortex. Prefrontal, parietal, and temporal association cortices, which house the brain's most highly connected hubs, expand about twice as much as, for example, primary visual cortex.29
A competing theory on hub formation from the field of network science is the preferential attachment or “rich-gets-richer” hypothesis.30 This theory states that newly developing nodes in a network preferentially attach to nodes that already have high levels of connectivity. In terms of connectome development, this implies that early developing neurons and cortical regions would tend to accumulate more connections and thus become hubs. Indeed, such a mechanism has been shown for the 302-neuron nervous system of C. elegans,31 but whether this contributes to hub formation in the human brain is currently unknown. If a similar mechanism is indeed in place, it could potentially explain the hub-role of early developing regions such as hippocampus and insula14 in lieu of the mechanism described above.
Connectome maturation
Neuroimaging studies in infants suggest that the gross connectivity and organization of the structural connectome is largely established by the time of term birth.32,33 The neonatal cortex displays an adult-like gyrification pattern, major connection pathways are in place, and the connectome shows many of the fundamental properties characterizing the adult connectome, including a small-world, modular topology with hubs and a central rich club system.34,35 Despite this adult-like connectivity backbone, early postnatal neurodevelopment is known to involve a process of exuberant development, with an initial overproduction of connections that is gradually pruned back into a sparser, more efficient connectivity pattern36-39 and with ongoing changes in the diameter and myelination of axonal connections underlying wide-spread changes in structural connectivity beyond the early postnatal phase.39,40
Molecular mechanisms
Developmental changes in brain connectivity and network organization are shaped by a combination of genes, environment, and their interaction. The extent to which experience—in the broadest sense, including sensory information, social interaction, and exposure to stress—is able to shape brain circuits changes greatly across the lifespan.3 There are a number of highly regulated intervals known as critical periods, during which specific neural systems are intensely attuned to the outside world. A comprehensive review on critical periods41 states that sensitive periods for seeing, hearing, speech production, and higher cognitive functions have been documented in developing children.42 However, as the authors note, “the best understood critical periods are those controlling specific attributes of primary sensory modalities in animals, such as the representation of different tones in auditory cortex or of left versus right eye inputs in the visual cortex.”41 During these windows of high developmental plasticity, incoming information serves as a cue to establish appropriate patterns of connections, strengthening synapses between neurons that fire together and weakening those between neurons with “out-of-sync” firing patterns.43 Over time, this process results in a connectivity pattern that is optimally attuned to the surroundings in which we grow up.
The field of critical window biology is starting to offer a glimpse into the molecular machinery governing this process.2,44 Early in life, neurotransmission is mainly excitatory, but as the brain matures, inhibitory transmission strengthens. When this inhibition reaches a certain threshold, it triggers a period of heightened developmental plasticity.45 Crucial to this process are a subset of γ-aminobutyric acid (GABA)-ergic inhibitory interneurons known as parvalbumin (PV) positive large basket cells. These cells are thought to quiet down haphazardly firing excitatory neurons and promote excitatory/inhibitory (E/I) balance, allowing the best neural representation to be selected from many inputs bombarding the maturing nervous system.2 With ongoing development, the neural circuits that were sculpted by experience are stabilized by perineuronal nets, extracellular matrix structures that put a molecular brake on plasticity.44 These processes have been noted to occur across increasingly more complex aspects of sensorimotor experience, suggesting a developmental sequence of critical periods from lower- to higher-order circuits.46
Systems-level findings
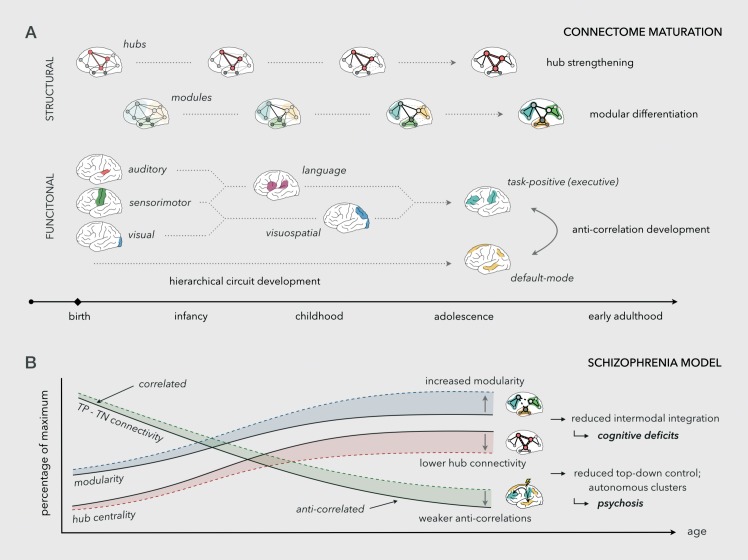
Developmental processes on the cellular and molecular level as referenced above may underlie findings of a systems-level reorganization of the connectome over the course of development. While the broad topology of the structural connectome appears to be largely established by the time of birth, the prominence of existing network features evolves throughout childhood and adolescence.33 Recent findings from network analyses of diffusion-weighted MRI data indicate that from late childhood to early adulthood, the structural connectome becomes both more modular and more integrated.47 This development is thought to stem from ongoing modular differentiation on one side and the strengthening of hub connections through the maturation of longdistance fiber pathways on the other (Figure 2A).47,48 Consistent with these findings, brain hubs become increasingly closely integrated and overall network efficiency goes up, while local clustering decreases with age from early childhood to young adulthood.48-51
Figure 2. Connectome maturation and connectomic model of schizophrenia. (A) Structural and functional connectome maturation. The topology of the structural connectome is largely established at birth, but modular differentiation and the strengthening of hubs increase levels of modularity and integration. Functional circuits develop in hierarchical sequence; anti-correlations between task-positive and default-mode networks evolve. (B) Proposed connectomic model of schizophrenia. Reduced structural connectivity between hubs reduces inter-modular integration invoking cognitive deficits. Lower hub connectivity and weaker anti-correlations between task-positive (TP) and task-negative (TN) systems lead to reduced top-down control and autonomous 'runaway' activity of self-oriented (TN) networks giving rise to psychosis.
Resting-state functional MRI (fMRI) studies are indicative of an immature functional organization of the connectome in infants. At this stage, the functional connectome is dominated by visual, auditory, and sensorimotor networks, consistent with an emphasis on primary functions.52,53 With age, higher-order networks develop and take on an increasingly central role in the brain's functional organization.54,55 One of the most widely studied functional systems is the default-mode network (DMN), a set of regions in medial prefrontal and parietal cortex that is highly functionally connected during rest and that largely overlaps with the hubs of the structural connectome. DMN activity has been linked to self-referential processing, and its deactivation during goal-directed tasks is thought to be an important aspect of healthy DMN functioning. The extent to which the DMN is able to disengage from task-positive networks (TPN) subserving attention-demanding tasks goes up in childhood and adolescence, which may underlie improvements in cognitive control and working memory.56 Moreover, network studies indicate changes in the modular organization of the functional connectome during development, with for example cingulo-opercular and frontoparietal networks evolving from one single network in childhood to the known separate systems in adulthood.54,57,58
Linking maturational mechanisms across levels of resolution
The link between neural circuit development on the molecular level and connectome maturation on the whole-brain scale is poorly understood, but findings from neurophysiological studies offer an intermediate model of brain circuit development that spans the two extremes of spatial and temporal resolution.59 These studies indicate modifications in the synchrony and amplitude of neural oscillations across frequency bands (including γ-oscillations) during childhood and adolescence. With ongoing development, high-frequency oscillations increase and long-range synchronization—eg, between frontal and parietal circuits—strengthens, suggesting a reorganization of the functional connectome during the transition from adolescence to early adulthood.60 Importantly, developmental changes in synchronous oscillations have been related to modifications in neurotransmitter systems and the interaction of excitatory glutamatergic and dopaminergic systems with inhibitory GABA-ergic interneurons.61 This observation suggests that some of the molecular mechanism-controlling brain circuit maturation at the level of individual synapses during circuit-specific developmental windows are also important to the generation of high-frequency oscillations. That play a crucial role in the activity-dependent self-organization of developing neural networks and large-scale functional reorganization of the brain network during late neurodevelopment.62
Connectome maldevelopment in schizophrenia
Studies of brain connectivity and network organization in schizophrenia have largely focused on adult patients with established illness (for review see ref 63). Well-replicated findings from structural connectome studies include increased path length and disruptions in brain hubs and the rich club system, reflecting a less efficient and less well-integrated network.64 Among the most replicated functional findings are DMN abnormalities, including increased activity and connectivity within the DMN and weaker anti-correlations between DMN and TPNs reflecting reduced task-related DMN suppression.65-67
The development of anti-correlations between self- and task-oriented networks may support the emergence of executive functions68 and the failure of such maturation could lead to cognitive deficits in schizophrenia. Moreover, impaired control by late-developing task-positive networks over earlier developing circuits including DMN and primary sensory networks may cause them to function more autonomously,69 leading to internal preoccupation, psychotic symptoms, and social withdrawal (Figure 2B). Longitudinal connectomic studies in young individuals at risk for psychotic disorders may shed light on this hypothesis. Abnormal task-related DMN disengagement may also reflect difficulties in dynamically switching between functional connectivity patterns as a result of an affected central rich club structure, which fits observations in schizophrenia patients and offspring that rich club disruptions are accompanied by increased coherence between structural and functional connectivity.64,70 An important open question is when, in the course of brain development, connectome disruptions arise and how this relates to the timeline of illness development in schizophrenia. In the next paragraphs, we discuss three broad ways in which connectome maldevelopment and the neurodevelopmental trajectory of schizophrenia may be related.
Affected connectome formation
First, schizophrenia may stem from abnormalities in the initial formation of the connectome leading to early deficits in brain network organization that become apparent in a sequential manner as affected functional systems come “online” throughout the course of development. Indeed, family and birth cohort studies indicate early developmental impairments in children that go on to develop schizophrenia, including delays in reaching developmental milestones such as the ability to sit, stand, and walk, and delays in speech and receptive language development (for review see ref 10). Developmental impairments in at-risk infants are consistent with an early deviation from normative connectome development.10 In this model, scaffolds of brain circuits that will come to support higher-order cognitive functions may already be affected, but this may only become apparent when socio-emotional and cognitive functions develop. Alternatively, if cognitive functions are understood to develop in a hierarchical manner (Box 1), it could be that higher-order “proto-circuits” are unaffected at this stage and that deficits in early-developing circuits in and of themselves are the foundation for abnormalities in higher-order circuits. In addition to developmental delays, biological support for early connectome malformation includes neuropathological findings of a maldistribution of interstitial neurons in subcortical white matter and neuroimaging findings of reduced intracranial volume and abnormal cortical gyrification in schizophrenia patients.71-73 These findings are all suggestive of disturbances in prenatal brain development, including abnormal neuronal migration and early connectivity deficits that may contribute to abnormal connectome formation.
Box 1. Hierarchical brain and cognitive development.
Understanding neurodevelopment as a hierarchical process implies that basic skills and associated low-level circuits need to be established before more advanced functions can evolve. Before learning to speak, a child needs to be able to hear and produce sounds. This allows him or her to imitate caregivers, producing nonspecific babble at first, then speech-like vocalizations, and finally recognizable words. Next, the child learns to interpret and attach meaning to words and combinations of words through interactions with caregivers, thereby developing vocabulary and syntax. Through this process, children acquire a code or system of rules, that allows them to capture abstract representations of objects and events in the outside world and communicate ideas. As a result, language in itself is a building block for the development of higher-order functions. In his book Seeing Voices: A Journey into the World of the Deaf Oliver Sacks argues that deafness is the most preventable cause of mental retardation. If deaf children do not learn sign language—as was common until the 18th century—other nonlingual cognitive abilities typically also fail to develop properly.74 Due to the timing of developmental windows for various skills, these deficits typically cannot be corrected when sign language is learned at a later age. This suggests that development not only follows a hierarchical sequential pattern for specific functional domains (eg, with simple motor functions developing before more complex motor behaviors), but that this hierarchy extends across domains, with lower-level sensorimotor skills forming the building blocks for the development of higher-order cognition.
Abnormal connectome maturation
Second, schizophrenia development may stem from a disruption in the neurobiological processes governing the maturation of functionally specialized brain circuits, giving rise to cognitive and behavioral impairments that show up in a sequential manner consistent with the timing of their respective developmental windows. An important pathophysiological model for schizophrenia, of N-methyl-D-aspartate receptor (NMDAR) hypofunction, could fit in with such a mechanism.75 NMDAR hypofunction has been hypothesized to disrupt PV-interneuron mediated inhibition, which is important to the opening and appropriate timing of developmental windows in infancy and childhood and the generation of high-frequency oscillations which are important to large-scale functional reorganization of the connectome during the transition from adolescence to early adulthood. NMDAR-hypofunction has been hypothesized to occur initially in cortical PV-cells during early postnatal development, with resulting maturational deficits causing GABAergic disinhibition and glutamate spillover, which may in turn elicit the dopamine dysregulation associated with psychotic development.75-77 This hypothesis may not explain all molecular and pathological findings in schizophrenia, but it offers some interesting conceptual advantages. For example, given the role of PV-interneurons in quieting down excitatory activity to allow the brain to focus on external input, abnormalities in PV-cell-mediated inhibitory control over pyramidal cells78 could result in neural circuits that remain overly attuned to internally generated activity. Such a mechanism could be consistent with findings that schizophrenia patients and relatives show reduced DMN disengagement during cognitive tasks65,66 and ties in with theories relating psychotic symptoms to internally generated cues that are misinterpreted as external signals. In all, schizophrenia may involve abnormalities in the biological mechanisms governing critical window development, leading to abnormal timing (ie, premature or delayed onset), duration, or progression of critical periods and contribute to brain circuits that remain overly attuned to internal processing, and thereby predispose to psychosis development.79
Abnormal connectome integration
Third, schizophrenia development may relate to a failure to develop adequate integration between functional brain systems with ongoing development, due to deficits in anatomical connections linking connectome modules and associated abnormalities in synchronized oscillations. While cognitive and behavioral impairments in schizophrenia reach back as far as early childhood,10 the characteristic expression of (subthreshold) psychotic symptoms typically occurs in the period from adolescence to early adulthood; a phase that is characterized by the integration of early- and late-maturing functional systems and in which precise temporal coding between large-scale brain networks needs to be established.59,80 Although these processes are part of normal connectome maturation, we discuss them separately here to highlight the possibility that individual functional circuits develop normally, but that the integration between neural systems fails to develop properly. Such a mechanism may explain observations that hub-to-hub connections (ie, cross-linking modules) appear disproportionately affected in schizophrenia patients and at-risk relatives64,70,81 and is in line with hypothesized abnormalities in corollary discharge mechanisms that ought to inform the brain of self-generated activity, as possibly underlying the generation of psychotic phenomena such as auditory hallucinations and delusions of control.82
Conclusion
We have proposed a novel extension to the neurodevelopmental model of schizophrenia, which asserts that abnormal connectome formation and maturation, including the establishment of adequate intermodal integration, is central to the etiology of the illness. We discussed three broad ways in which connectome maldevelopment may give rise to the typical neurodevelopmental trajectory of schizophrenia. These mechanisms need not be mutually exclusive and may not be the same for individual patients, but abnormal anatomical architecture and functional organization of the connectome may be a final common pathway to the manifestation of schizophrenia symptoms. Our model leads to a set of testable hypotheses that could be addressed in different experimental approaches, including connectivity analyses in vitro using induced pluripotent stem cells or brain organoids, experimental perturbations of the molecular mechanisms guiding critical window biology in animal models, or longitudinal connectomic imaging studies of developing children at risk for schizophrenia.83,84 These studies may help elucidate how and when risk factors for schizophrenia dysregulate connectome development. As such, these investigations may clarify the timing and sequence of connectomic abnormalities leading up to the manifestation of psychosis, and thereby promote prognostic, preventative, and therapeutic advances for schizophrenia.
Acknowledgments
The preparation of this paper was overshadowed by the unexpected and untimely passing of Dr. Larry J. Seidman on September 7, 2017. We had intended to write this paper jointly and many of the ideas in the manuscript were thought out together. In honor of his life and work, we dedicate this paper to his memory. The authors have no conflict of interest to declare.
Contributor Information
Guusje Collin, Harvard Medical School at Beth Israel Deaconess Medical Center, Department of Psychiatry, Boston, Massachusetts, USA; Massachusetts Institute of Technology, Department of Brain and Cognitive Sciences, McGovern Institute for Brain Research, Cambridge, Massachusetts, USA.
Matcheri S. Keshavan, Massachusetts Institute of Technology, Department of Brain and Cognitive Sciences, McGovern Institute for Brain Research, Cambridge, Massachusetts, USA.
REFERENCES
- 1.Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- 2.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 3.Takesian AE., Hensch TK. Balancing plasticity/stability across brain development. Prog Brain Res. 2013;207:3–34. doi: 10.1016/B978-0-444-63327-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 4.Keshavan MS., Mehta UM., Padmanabhan JL., Shah J. Dysplasticity, metaplasticity, and schizophrenia: Implications for risk, illness, and novel interventions. Dev Psychopathol. 2015:615–635. doi: 10.1017/S095457941500019X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshavan MS., Paus T. Neurodevelopmental trajectories, disconnection, and schizophrenia risk. JAMA Psychiatry. 2015;72:943. doi: 10.1001/jamapsychiatry.2015.1119. [DOI] [PubMed] [Google Scholar]
- 6.Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- 7.Keshavan MS., Delisi LE., Seidman LJ. Early and broadly defined psychosis risk mental states. Schizophr Res. 2011;126:1–10. doi: 10.1016/j.schres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yung AR., McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22(2):353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- 9.Seidman LJ., Giuliano AJ., Meyer EC., et al. Neuropsychology of the prodrome to psychosis in the NAPLS Consortium. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu CH., Keshavan MS., Tronick E., Seidman LJ. Perinatal risks and childhood premorbid indicators of later psychosis: next steps for early psychosocial interventions. Schizophr Bull. 2015;41:801–816. doi: 10.1093/schbul/sbv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Serna E., Sugranyes G., Sanchez-Gistau V., et al. Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res. 2017;183:110–115. doi: 10.1016/j.schres.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Sporns O., Betzel RF. Modular brain networks. Annu Rev Psychol. 2016;67:613–640. doi: 10.1146/annurev-psych-122414-033634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Heuvel MP., Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2014;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 14.van den Heuvel MP., Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Reus MA., van den Heuvel MP. Rich club organization and intermodule communication in the cat connectome. J Neurosci. 2013;33:12929–12939. doi: 10.1523/JNEUROSCI.1448-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiles J., Jernigan TL. The basics of brain development. Neuropsych Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bystron I., Rakic P., Molnar Z., Blakemore C. The first neurons of the human cerebral cortex. Nat Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- 18.Rakic P. Radial versus tangential migration of neuronal clones in the developing cerebral cortex. Proc Natl Acad Sci U S A. 1995;92:11323–11327. doi: 10.1073/pnas.92.25.11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marin O., Rubenstein JLR. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Bendito G., Cautinat A., Sanchez JA., et al. Tangential neuronal migration controls Axon Guidance: A role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tessier-Lavigne M., Goodman C. The molecular biology of axon guidance. Science. 1996;274(5290):1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 22.Squarzoni P., Thion MS., Garel S. Neuronal and microglial regulators of cortical wiring: usual and novel guideposts. Front Neurosci. 2015;9:1–16. doi: 10.3389/fnins.2015.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scholtens LH., Schmidt R., de Reus MA., van den Heuvel MP. Linking macroscale graph analytical organization to microscale neuroarchitectonics in the macaque connectome. J Neurosci. 2014;34(36):12192–12205. doi: 10.1523/JNEUROSCI.0752-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sansorn SN., Livesey FJ. Gradients in the brain: the control of the development of form and function in the cerebral cortex. Cold Spring Harb Perspect Biol. 2009;1(2):16. doi: 10.1101/cshperspect.a002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang AS., Lin CY., Chuang CC., et al. Three-dimensional reconstruction of brain-wide wiring networks in drosophila at single-cell resolution. Curr Biol. 2011;21(1):1–11. doi: 10.1016/j.cub.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 26.Wedeen VJ., Rosene DL., Wang R., et al. The geometric structure of the brain fiber pathways. Science. 2012;337:1628–1634. doi: 10.1126/science.1215280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett HC. A hierarchical model of the evolution of human brain specializations. Proc Natl Acad Sci U S A. 2012;109:10733–10740. doi: 10.1073/pnas.1201898109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaiser M. Mechanisms of connectome development. Trends Cogn Sci. 2017;21:703–717. doi: 10.1016/j.tics.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Hill J., Inder T., Neil J., Dierker D., Harwell J., Van Essen D. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci U S A. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman MEJ. Clustering and preferential attachment in growing networks. Phys Rev E. 2001;64:1–13. doi: 10.1103/PhysRevE.64.025102. [DOI] [PubMed] [Google Scholar]
- 31.Varier S., Kaiser M. Neural development features: spatio-temporal development of the Caenorhabditis elegans neuronal network. PLoS Comput Biol. 2011;7:e1001044. doi: 10.1371/journal.pcbi.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collin G., van den Heuvel MP. The ontogeny of the human connectome: development and dynamic changes of brain connectivity across the lifespan. Neuroscientist. 2013;19:616–628. doi: 10.1177/1073858413503712. [DOI] [PubMed] [Google Scholar]
- 33.Vértes PE., Bullmore ET. Annual research review: Growth connectomics - the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry. 2015;56:299–320. doi: 10.1111/jcpp.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Heuvel MP., Kersbergen KJ., De Reus MA., et al. The neonatal connectome during preterm brain development. Cereb Cortex. 2015;25:3000–3013. doi: 10.1093/cercor/bhu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ball G., Aljabar P., Zebari S., et al. Rich-club organization of the newborn human brain. Proc Natl Acad Sci U S A. 2014;111:7456–7461. doi: 10.1073/pnas.1324118111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaMantia AS., Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990;10:2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Innocenti GM., Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- 38.Luo L., O'Leary DDM. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 39.Haynes RL., Borenstein NS., Desilva TM., et al. Axonal development in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2005;484:156–167. doi: 10.1002/cne.20453. [DOI] [PubMed] [Google Scholar]
- 40.Hermoye L., Saint-Martin C., Cosnard G., et al. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage. 2006;29:493–504. doi: 10.1016/j.neuroimage.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 41.Hensch TK., Bilimoria PM. Re-opening windows: manipulating critical periods for brain development. Cerebrum. 2012:1–11. [PMC free article] [PubMed] [Google Scholar]
- 42.The timing and quality of early experiences combine to shape brain architecture: working paper #5. 2007. National Scientific Council on the Developing Child, Center of the Developing Child at Harvard University. [Google Scholar]
- 43.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 44.Bardin J. Neurodevelopment: Unlocking the brain. Nature. 2012;487:24–26. doi: 10.1038/487024a. [DOI] [PubMed] [Google Scholar]
- 45.Bavelier D., Levi DM., Li RW., Dan Y., Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Toyoizumi T., Miyamoto H., Yazaki-Sugiyama Y., Atapour N., Hensch TK., Miller KD. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron. 2013;80:51–63. doi: 10.1016/j.neuron.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baum GL., Ciric R., Roalf DR., et al. Modular segregation of structural brain networks supports the development of executive function in youth. Curr Biol. 2017;27(11):1561–1572e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagmann P., Sporns O., Madan N., et al. White matter maturation reshapes structural connectivity in the late developing human brain. Proc Natl Acad Sci U S A. 2010;107(44):19067–19072. doi: 10.1073/pnas.1009073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dennis EL., Jahanshad N., Toga AW., et al. Development of the “rich club” in brain connectivity networks from 438 adolescents & adults aged 12 to 30. Proc IEEE Int Symp Biomed Imaging. 2013:624–627. doi: 10.1109/ISBI.2013.6556552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dennis EL., Jahanshad N., McMahon KL., et al. Development of brain structural connectivity between ages 12 and 30: A 4-Tesla diffusion imaging study in 439 adolescents and adults. Neuroimage. 2013;64(1):161–684. doi: 10.1016/j.neuroimage.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim S., Han CE., Uhlhaas PJ., Kaiser M. Preferential detachment during human brain development: Age- and sex-specific structural connectivity in Diffusion Tensor Imaging (DTI) data. Cereb cortex. 2015;25(6):1477–1489. doi: 10.1093/cercor/bht333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fransson P., Skiöld B., Horsch S., et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao W., Gilmore JH., Giovanello KS., et al. Temporal and spatial evolution of brain network topology during the first two years of life. PLoS One. 2011;6:e25278. doi: 10.1371/journal.pone.0025278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Power JD., Fair DA., Schlaggar BL., Petersen SE. The development of human functional brain networks. Neuron. 2010;67:735–748. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Supekar K., Musen M., Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chai X., Ofen N., Gabrieli J., Whitfield-Gabrieli S. Selective development of anticorrelated networks in intrinsic functional organization of the human brain. J Cogn Neurosci. 2014;26(3):501–513. doi: 10.1162/jocn_a_00517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fair DA., Dosenbach NUF., Church JA., et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fair DA., Cohen AL., Power JD., et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uhlhaas PJ., Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37(3):514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Uhlhaas PJ., Roux F., Singer W., Haenschel C., Sireteanu R., Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. PNAS. 2009;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sohal VS., Zhang F., Yizhar O., Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uhlhaas PJ., Roux F., Rodriguez E., Rotarska-Jagiela A., Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 63.van den Heuvel MP., Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24(1):32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- 64.van den Heuvel MP., Sporns O., Collin G., et al. Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- 65.Whitfield-Gabrieli S., Thermenos HW., Milanovic S., et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. PNAS. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wotruba D., Michels L., Buechler R., et al. Aberrant coupling within and across the default mode, task-positive, and salience network in subjects at risk for psychosis. Schizophr Bull. 2014;40(5):1095–1104. doi: 10.1093/schbul/sbt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anticevic A., Cole MW., Murray JD., Corlett PR., Wang XJ., Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barber AD., Caffo BS., Pekar JJ., Mostofsky SH. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffman RE., Mcglashan TH. Corticocortical connectivity, autonomous networks, and schizophrenia. Schizophr Bull. 1994;20(2):257–261. doi: 10.1093/schbul/20.2.257. [DOI] [PubMed] [Google Scholar]
- 70.Collin G., Scholtens LH., Kahn RS., Hillegers MHJ., van den Heuvel MP. Affected anatomical rich club and structural-functional coupling in young offspring of schizophrenia and bipolar disorder patients. Biol Psychiatry. 2017:1–10. doi: 10.1016/j.biopsych.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 71.Akbarian S., Kim JJ., Pothin SG., Hetrick WP., Bunney WE., Jones EG. Maldistribution of interstitial. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- 72.Nanda P., Tandon N., Mathew IT., et al. Local gyrification index in Probands with psychotic disorders and their first-degree relatives. Biol Psychiatry. 2014;76:447–455. doi: 10.1016/j.biopsych.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haijma SV., Van Haren N., Cahn W., Koolschijn PCMP., Hulshoff Pol HE., Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39(5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sacks O. Seeing Voices: A Journey into the World of the Deaf. New York, NY: Vintage Books. 1989. [Google Scholar]
- 75.Gonzalez-Burgos G., Lewis DA. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull. 2012;38:950–957. doi: 10.1093/schbul/sbs010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakazawa K., Jeevakumar V., Nakao K. Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ Schizophr. 2017;3:1–11. doi: 10.1038/s41537-016-0003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Keshavan MS. Development, disease and degeneration in schizophrenia: a unitary pathophysiological model. J Psychiatr Res. 1999;33:513–521. doi: 10.1016/s0022-3956(99)00033-3. [DOI] [PubMed] [Google Scholar]
- 78.Lewis DA., Curley AA., Glausier JR., Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Do KQ., Cuenod M., Hensch TK. Targeting oxidative stress and aberrant critical period plasticity in the developmental trajectory to schizophrenia. Schizophr Bull. 2015;41:835–846. doi: 10.1093/schbul/sbv065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keshavan MS., Giedd J., Lau JYF., Lewis DA., Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1(7):549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- 81.Collin G., Kahn RS., de Reus MA., Cahn W., van den Heuvel MP. Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr Bull. 2014;40:438–448. doi: 10.1093/schbul/sbt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feinberg I. Corollary discharge, hallucinations, and dreaming. Schizophr Bull. 2011;37(1):1–3. doi: 10.1093/schbul/sbq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quadrato G., Brown J., Arlotta P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat Med. 2016;22:1220–1228. doi: 10.1038/nm.4214. [DOI] [PubMed] [Google Scholar]
- 84.Li M., Weinberger DR. Illuminating the dark road from schizophrenia genetic associations to disease mechanisms. Natl Sci Rev. 2017;4:240–251. [Google Scholar]