Abstract
Aim
The aim of this article is to determine whether a combination of noncontrast CT (NCCT), three-dimensional-phase contrast magnetic resonance venography (3D PC-MRV), T1- and T2-weighted MRI sequences can help to identify acute and subacute dural venous sinus thrombosis (DVST) with greater accuracy.
Methods
A total of 147 patients with DVST (n = 30) and a control group (n = 117) underwent NCCT, T1- and T2-weighted MRI sequences, and 3D PC-MRV from 2012 to 2016. Two experienced observers interpreted the images retrospectively for the presence of DVST. Nonvisualization of the dural venous sinuses on 3D PC-MRV and signal changes supporting acute or subacute thrombus on T2- and T1-weighted images were considered a direct sign of DVST. Also, using circle region of interest (ROI) techniques, attenuation measurement from each sinus was obtained on NCCT. Sensitivity and specificity were computed for these modalities separately and in combination for diagnosis of DVST using digital subtraction angiography as the reference standard.
Results
Nonvisualization of venous sinuses on 3D PC-MRV (sensitivity 100%, specificity 71%) in combination with both applying Hounsfield unit (HU) threshold values of greater than 60 on NCCT (sensitivity 70%, specificity 94%) and acquiring signal changes supporting DVST on T2- and T1-weighted images (sensitivity 83%, specificity 96%), were found to have 100% sensitivity and 100% specificity in the identification of acute or subacute DVST.
Conclusion
The combination of NCCT, T1- and T2-weighted MRI and 3D PC-MRV may allow the diagnosis of acute or subacute DVST and may obviate the need for contrast usage in patients with renal impairment or contrast allergies.
Keywords: Dural venous sinus thrombosis (DVST), noncontrast computed tomography (NCCT), 3D phase-contrast magnetic resonance venography
Introduction
Dural venous sinus thrombosis (DVST) is an entity characterized by thrombosis of one or more dural sinuses with an incidence of two per 100,000, although the true incidence is presumably underestimated.1,2 DVST may occur spontaneously and it may be caused by a wide variety of underlying conditions including hormone replacement therapy, oral contraceptive medication, dehydration, hematological disorders, and pregnancy.3,4
The recognition of DVST is challenging because of the variety of predisposing factors and the absence of uniform imaging findings. Early diagnosis of DVST is necessary to institute prompt therapy.5,6 At present, digital subtraction angiography (DSA), an invasive imaging method, is accepted as a standard of reference in the diagnosis of venous thrombosis and the patency of dural sinuses. Consequently, a less invasive and an effective diagnostic technique is required for the early diagnosis of DVST in the emergency department.7
Noncontrast computed tomography (NCCT) is frequently the first examination to evaluate DVST because it is rapid and widely available. Although it helps to rule out other common pathologies, it nevertheless fails to consistently provide an unequivocal diagnosis of sinus thrombosis. The most important finding of acute DVST on NCCT is a hyperattenuating dural sinus, reflecting an occluded vein consisting of a newly formed thrombus.8,9 Buyck et al. proposed the measurement of dural sinus attenuation in patients having a suspicion for DVST as it may increase the diagnostic value of the CT examination.10
Magnetic resonance imaging (MRI) is mostly requested to exclude or confirm DVST regardless of the results obtained from NCCT. It enables direct visualization of clots as well as associated cerebral lesions and is not invasive. Over the last decade, MRI has proved to be an effective method with use of MR angiography techniques and it has become the modality of choice for the evaluation of DVST.11,12
Nonetheless, NCCT, conventional MRI sequences and phase-contrast MR venography (PC-MRV) are of incomplete accuracy for the diagnosis. The MRI has two main limitations: flow artifacts and the lack of apparent signal intensity on T1-weighted images (WI) at the acute stage of thrombosis. The thrombus is isointense or hypointense on T1-weighted and hypointense on T2-weighted MRI sequences in the first five days of DVST; therefore it is highly difficult to distinguish it from normal venous sinuses. PC-MRV may not distinguish thrombosis from hypoplasia, which is a common diagnostic problem for the lateral sinuses.13,14 Although contrast-enhanced MRV is known as one of the most reliable methods for the diagnosis of DVST, it poses the risk of allergic reactions or nephrogenic systemic fibrosis due to gadolinium-based contrast administration and is widely contraindicated in various patient populations, such as pregnant patients.15
We hypothesized whether the combination of NCCT, T1- and T2-weighted MRI sequences and three-dimensional (3D) PC-MRV can help to identify DVST with a greater accuracy in the emergency department.
Materials and methods
Participants
This retrospective study was approved by our hospital’s institutional review board and conducted in compliance with Health Insurance Portability and Accountability Act guidelines.
We evaluated a total of 147 patients (100 women, 47 men; mean age, 46 years; age range, 20–91 years) who were admitted to the emergency department between January 2012 and September 2016 with clinical symptoms compatible with DVST. Patients were included in the study only if NCCT was performed on admission and MRI including 3D PC-MRV was gathered within 24 hours of admission. Patients having signs and symptoms of DVST related to intracranial infection, brain tumor, skull fracture or neurosurgical interventions and isolated cortical vein thrombosis without dural sinus involvement were not included. Also, cases were excluded from the study if they presented with symptoms older than 14 days before the NCCT and MRI examinations.
The following standard of reference was applied for the diagnosis of DVST: (a) history and clinical findings suggestive of DVST and (b) presence of a partial or complete venous sinus occlusion on DSA.
Thirty patients (24 women, six men; mean age, 44.4 years; age range, 22–91 years) were found to have clinical and radiological findings compatible with acute or subacute DVST. In addition, the remaining 117 consecutive patients were accepted as a control group (76 women, 41 men; mean age, 46.1 years; age range, 20–85 years) who initially demonstrated clinical symptoms of DVST and had undergone NCCT and MRI examinations but in whom imaging modalities demonstrated no pathologic findings and follow-up was unremarkable. The patients from the control group also had to meet the above-mentioned inclusion criteria except that DSA was available for 22 of 117 patients. In the remaining 95 control cases, clinical follow-up and CT, MRI findings were considered sufficient to exclude the diagnosis of DVST.
Proved DVST were categorized regarding the interval between initiation of clinical findings and MR examination. Fourteen out of 30 (47%) DVST were imaged at the acute stage (mean interval, 2.4 days; range, 1–5) and 16 out of 30 (53%) at the subacute stage (mean interval, 9.3 days; range, 6–14).
Image acquisition
CT scans were obtained with a commercially available multislice scanner equipped with 64 detector rows (Somatom Definition, Siemens Healthcare, Forchheim, Germany). From the raw data of each acquisition, contiguous 5-mm-thick axial slices were obtained from the skull base to the vertex using reconstruction algorithms for the soft tissue window. The following scanning parameters were used: 120 kV, 300 mA.
Images were acquired on a 3T MRI scanner (Achieva; Philips Healthcare, Best, the Netherlands) by using a 16-channel head coil. The sequence parameters for conventional images were as follows: T1-WI (repetition time (TR), 600 ms; echo time (TE), 12 ms; 20 slices, axial slice; 5 mm thickness; matrix size, 288 × 224; field of view, 24 × 24 cm; acquisition time, 1.44 minutes; one excitation), T2-WI (TR, 5400 ms; TE, 105 ms; 20 slices; axial and sagittal slices; slices thickness, 5 mm; matrix size, 288 × 288, field of view, 24 × 24 cm; acquisition time, 2.30 minutes; two excitations).
MRV was utilized (always following MRI examination) with a 3D phase-contrast technique using a gradient-echo sequence (TR 23, TE 7.1, flip angle 30 degrees) with a slice thickness of 1.6, slice spacing of 0.8, and velocity encoding value of 6–15 cm/s. The 3D PC-MRV source images were transferred to a GE Advantage Windows workstation for postprocessing to create a projection venogram with maximum intensity projection (MIP) algorithm.
DSA was performed with selective catheterizations of both internal carotid arteries and the dominant vertebral artery on a biplanar DSA unit (AXIOM Artis Biplane Angiosuite, Siemens) including late venous phases, and oblique projections to better analyze the venous structures.
Image interpretation
The studies were reviewed on a Picture Archiving and Communication System (PACS; GE Medical Systems) workstation by a staff neuroradiologist with more than 20 years’ experience and a general radiologist. The images were magnified, and window width and window level parameters were optimized manually to visualize dural venous sinuses. Although the observers knew the purpose of the study, they were blinded to clinical information and any prior or follow-up imaging finding of the patients.
Readings were randomized and the following structures were evaluated: superior sagittal sinus (SSS), sinus rectus (SR), right and left internal jugular vein (R-IJV, L-IJV), and right and left sigmoid sinuses (R-SIGMS, L-SIGMS). Attenuation values of the dural venous sinuses were estimated on NCCT that could be reliably distinguished from surrounding brain parenchyma. The calculated attenuation values were documented in Hounsfield units (HU) for all 147 patients.
The dural sinus attenuation values were calculated using the circle regions of interest (ROI) method. The ROI area was set to a limit of 10–20 mm2 and a circular ROI area was gathered as large as possible that could be measured at three or more points. Cases for which the ROI could not be measured in at least three points because of significant artifacts such as beam hardening or partial volume or small dural sinuses <10 mm2 were excluded. The mean value of venous sinus attenuation at three or four points was calculated.
T1- and T2-weighted sequences gathered at MRI examination were interpreted retrospectively for every patient by two observers. First, the localization of venous occlusion was evaluated. The appearance of the venous sinuses on T1-WI and T2-WI were classified as hyperintense, isointense or hypointense in comparison with gray matter. Diagnosis of venous sinus thrombosis on 3D PC-MRV was based on lack of flow signal in a dural sinus. In interpreting the 3D PC-MRV, the source images and MIP images were all included. To rule out the possibility of an anaplastic or hypoplastic sinus, source images were analyzed.
The observers performed a consensus reading to achieve a result regarding the DVST after having interpreted the T1- and T2-weighted MRI sequences, 3D PC-MRV and NCCT datasets. They also cooperatively evaluated clinical records and all available imaging modalities including follow-ups of any patient. They subsequently determined (1) the presence of an acute or subacute DVST; (2) the presence of an intracerebral hemorrhage or edema and any hemorrhagic transformation of a venous infarction. If there was any conflicting findings between the modalities, the reference standard was based on the results of the DSA.
Statistical analysis
All statistical comparisons between the DVST and nonthrombosed dural venous sinuses were performed by using Statistical Package for the Social Sciences software (SPSS, version 23.0; SPSS, Chicago, IL, USA). Receiver operating characteristic (ROC) analysis was performed by using an analysis program (MedCalc for Windows, Ostend, Belgium). Descriptive statistics were given as mean ± standard deviation for continuous variables and count as a percentage for categorical variables. Gender was compared by using Pearson’s chi-square test, and the Student t test was used for patient ages.
We measured and compared the mean attenuation values (HU) of the dural venous sinuses using the Student t test on NCCT.
The signal changes occurring in venous clots were investigated, and MRI results at the sites of dural venous sinuses were considered positive if the signal intensity of the sinuses were iso- or hyperintense, and accepted as negative if the signal intensity was hypointense with regard to the diagnosis of DVST. Nonvisualization of the venous sinuses on PC-MRV were accepted as DVST thereafter ruling out the possibility of an anaplastic or hypoplastic sinus from source images.
Based on collected data, the specificity and sensitivity of NCCT, T1- and T2-weighted MRI sequences and 3D PC-MRV separately as well as in combination with the diagnosis of DVST were estimated. Patients in whom the diagnosis of DVST was excluded were used as a control group.
ROC analysis was utilized to assess the diagnostic performance of these parameters. A p value of less than 0.05 was considered as the statistically significant difference.
Results
Clinical findings
A total of 30 patients were proven to have an acute or subacute DVST according to the radiological and clinical findings. Most signs and symptoms were related to intracranial hypertension. Headache was the most common complaint (68%) often accompanied by nausea and vomiting (41%). At clinical examination, papilledema was present in 11 cases (37%). Other signs included sensory or motor deficit (27%), confusion or loss of consciousness (23%), diplopia related to cranial nerve palsy (17%), neck stiffness (10%) and language disturbance (7%).
Underlying conditions predisposing to DVST were hormonal treatment in 11 patients (oral contraceptives), hematological diseases in eight patients (protein C-S deficiency or thrombophilia in five, and hematological malignancy in three), mastoid infection in three patients, and the postpartum period in three patients. In five patients no predisposing factor could be identified.
Distribution of venous thrombosis
A total of 66 thrombosed venous segments were identified in 30 patients on the combination of NCCT, PC-MRV, and MRI. Thrombosed segments included: 15 SSS, 15 R-IJV, 13 L-IJV, nine R-SIGMS, 12 L-SIGMS, and two SR. None of the patients had isolated cortical vein thrombosis without dural sinus involvement in this study protocol. We found an occlusion of a deep cerebral vein together with the SR in one patient. Associated parenchymatous lesions corresponding to venous infarcts were found in 11 cases (37%). These included three unilateral and three bilateral hemorrhagic hemispheric lesions, three unilateral and two bilateral nonhemorrhagic hemispheric lesions, and one hemorrhagic bilateral thalamic infarct. Congestive cortical, epicranial, or transmedullary veins were found in 12 patients (40%) and a meningeal thickening in five (17%).
Venous sinus attenuation on NCCT
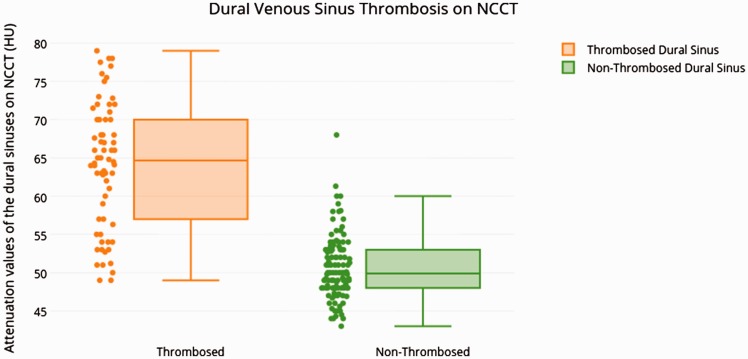
The mean venous HU value of the thrombosed venous sinuses was 64 (52–85) with a standard deviation of 11. The mean venous HU value in the control group was 48 (33–69) with a standard deviation of 8.6 (Table 1).
Table 1.
Venous sinus attenuation measurements on NCCT and 3D PC-MRV findings were demonstrated in patients with acute or subacute DVST.
| NCCT |
3D PC-MRV |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Phase of thrombosis | Thrombosed segment | SSS | R-IJV | L-IJV | R-SIGMS | L-SIGMS | SR | SSS | R-IJV | L-IJV | R-SIGMS | L-SIGMS | SR |
| 1a | 80 | Subacute | R-IJV | 47 | 51 | 49 | 49 | 48 | 50 | + | - | + | – | + | + |
| 2 | 34 | Subacute | L-IJV, L-SIGMS | 46 | 49 | 62 | 48 | 63 | 48 | + | + | – | + | – | + |
| 3 | 32 | Acute | SSS, R-IJV, R-SIGMS | 75 | 71 | 53 | 70 | 51 | 53 | – | – | + | – | + | + |
| 4a | 67 | Subacute | R-IJV | 58 | 49 | 60 | 50 | 61 | 54 | + | – | + | – | + | + |
| 5a | 31 | Subacute | R-IJV, R-SIGMS | 52 | 54 | 53 | 52 | 53 | 48 | + | – | – | – | – | + |
| 6a | 63 | Subacute | R-IJV | 45 | 49 | 47 | 49 | 48 | 45 | + | – | + | + | + | + |
| 7 | 39 | Acute | L-IJV, L-SIGMS | 45 | 55 | 63 | 57 | 64 | 46 | + | + | – | – | – | + |
| 8 | 45 | Acute | L-IJV, L-SIGMS | 51 | 55 | 77 | 54 | 78 | 43 | + | + | – | + | – | + |
| 9 | 30 | Acute | SSS, R-IJV, L-IJV, R-SIGMS, L-SIGMS | 68 | 66 | 65 | 64 | 64 | 49 | – | – | – | – | – | + |
| 10 | 36 | Acute | SSS | 77 | 53 | 51 | 52 | 51 | 46 | – | + | + | – | – | + |
| 11 | 25 | Subacute | SSS, R-IJV, R-SIGMS | 67 | 64 | 44 | 66 | 44 | 44 | – | – | + | – | + | + |
| 12 | 91 | Subacute | L-IJV, L-SIGMS | 49 | 53 | 66 | 53 | 67 | 47 | + | + | – | + | – | + |
| 13a | 42 | Subacute | R-IJV, R-SIGMS | 68 | 55 | 52 | 54 | 51 | 50 | + | – | + | – | + | + |
| 14 | 58 | Subacute | SSS, L-IJV, L-SIGMS | 63 | 54 | 61 | 53 | 62 | 54 | – | + | – | – | – | + |
| 15 | 55 | Acute | L-IJV | 49 | 48 | 70 | 49 | 50 | 44 | + | + | – | + | – | + |
| 16 | 47 | Acute | L-IJV, L-SIGMS, SR | 55 | 57 | 67 | 58 | 68 | 64 | + | + | – | + | – | – |
| 17 | 68 | Subacute | SSS, L-IJV, L-SIGMS | 53 | 51 | 63 | 49 | 64 | 48 | – | + | – | + | – | + |
| 18a | 25 | Subacute | L-IJV, L-SIGMS | 52 | 53 | 55 | 54 | 54 | 45 | + | + | – | + | – | + |
| 19 | 27 | Acute | SSS, R-IJV, R-SIGMS | 72 | 65 | 47 | 63 | 48 | 48 | – | – | + | – | + | + |
| 20a | 41 | Subacute | SSS, R-IJV, R-SIGMS | 59 | 57 | 59 | 56 | 58 | 48 | – | – | + | – | + | + |
| 21 | 74 | Acute | SSS, R-IJV, R-SIGMS | 75 | 73 | 48 | 72 | 48 | 49 | – | – | – | – | – | + |
| 22 | 72 | Acute | SSS, SR | 71 | 48 | 49 | 47 | 49 | 70 | – | + | + | + | + | – |
| 23a | 39 | Subacute | SSS, L-IJV, L-SIGMS | 50 | 48 | 51 | 49 | 53 | 46 | – | + | – | + | – | + |
| 24a | 28 | Subacute | R-IJV | 50 | 57 | 50 | 51 | 48 | 44 | + | – | + | – | + | + |
| 25 | 31 | Acute | L-IJV, L-SIGMS | 52 | 53 | 67 | 52 | 68 | 50 | + | + | – | + | – | + |
| 26 | 26 | Acute | SSS, R-IJV | 78 | 79 | 48 | 51 | 49 | 47 | – | – | + | – | + | + |
| 27 | 33 | Subacute | SSS, R-IJV, R-SIGMS | 76 | 70 | 51 | 72 | 51 | 48 | – | – | + | – | + | + |
| 28 | 39 | Subacute | R-IJV | 47 | 66 | 47 | 49 | 48 | 45 | + | – | + | – | + | + |
| 29 | 34 | Acute | SSS, L-IJV, L-SIGMS | 60 | 60 | 53 | 52 | 51 | 56 | – | + | – | + | – | + |
| 30 | 22 | Acute | SSS | 72 | 48 | 54 | 50 | 52 | 55 | – | + | + | + | + | + |
ID: identification; NCCT: non-contrast computed tomography; 3D PC-MRV: three-dimensional phase-contrast magnetic resonance venography; SSS: superior sagittal sinus; R-IJV: right internal jugular vein; L-IJV: left internal jugular vein; R-SIGMS: right sigmoid sinus; L-SIGMS: left sigmoid sinus; SR: sinus rectus.
Venous sinus thrombosis with unremarkable NCCT findings.
Bold indicates thrombosed sinus segments.
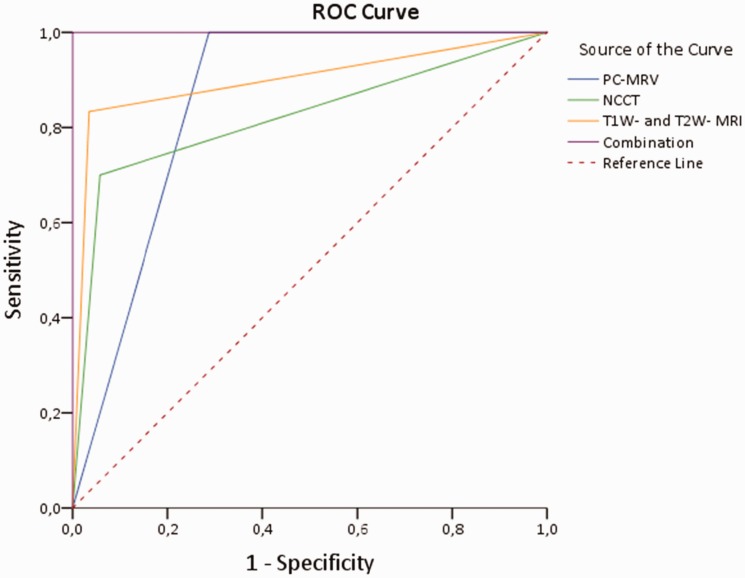
The HU attenuation value was used as a discriminative test and upon generation of an ROC (Figure 1). In our cohort, HU values greater than 60 demonstrate a sensitivity and specificity of 70% (0.58–0.85) and 94% (0.81–0.99), respectively, in the identification of thrombosis (area under curve (AUC), 0.82 (0.72–0.93)) (Figures 1 and 2).
Figure 1.
Receiver operating characteristic (ROC) curve for detection of venous sinus thrombosis was conducted. PCMRV: phase-contrast magnetic resonance venography; NCCT: noncontrast computed tomography.
Figure 2.
Box-plot diagram indicates a comparison between thrombosed and nonthrombosed dural venous sinuses on noncontrast computed tomography (NCCT) with regard to attenuation values. HU: Hounsfield units.
DVST assessment on MRI and 3D PC-MRV
A total of 66 thrombosed venous sinus segments were determined by consensus reading and evaluated. On T1-WI, 17 segments were hypointense, 26 segments were isointense, and 23 segments were hyperintense compared to the gray matter. On T2-WI, 18 segments were hypointense, 33 segments were isointense, and 15 segments were hyperintense compared to the gray matter (Table 2).
Table 2.
Signal intensities of the dural venous sinuses on T1- and T2- weighted MR sequences were demonstrated in patients with acute or subacute DVST.
|
T2WI
|
T1WI
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Age | Phase of thrombosis | Thrombosed segment | SSS | R-IJV | L-IJV | R-SIGMS | L-SIGMS | SR | SSS | R-IJV | L-IJV | R-SIGMS | L-SIGMS | SR |
| 1 | 80 | Subacute | R-IJV | Hypo | Hyper | Hypo | Hypo | Hypo | Hypo | Hypo | Hyper | Hypo | Hypo | Hypo | Hypo |
| 2 | 34 | Subacute | L-IJV, L-SIGMS | Hypo | Hypo | Hyper | Hypo | Hyper | Hypo | Hypo | Hypo | Hyper | Hypo | Hyper | Hypo |
| 3a | 32 | Acute | SSS, R-IJV, R-SIGMS | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo |
| 4 | 67 | Subacute | R-IJV | Hypo | Hyper | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Iso | Hypo | Hypo | Hypo |
| 5 | 31 | Subacute | R-IJV, R-SIGMS | Hypo | Hyper | Hypo | Hyper | Hypo | Hypo | Hypo | Iso | Hypo | Iso | Hypo | Hypo |
| 6 | 63 | Subacute | R-IJV | Hypo | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Hypo | Hypo | Hypo | Hypo |
| 7a | 39 | Acute | L-IJV, L-SIGMS | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo |
| 8 | 45 | Acute | L-IJV, L-SIGMS | Hypo | Hypo | Hypo | Hypo | Iso | Hypo | Hypo | Iso | Iso | Hypo | Iso | Hypo |
| 9 | 30 | Acute | SSS, R-IJV, L-IJV, R-SIGMS, L-SIGMS | Iso | Hypo | Hypo | Iso | Iso | Hypo | Iso | Hypo | Hypo | Iso | Iso | Hypo |
| 10 | 36 | Acute | SSS | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Hypo | Hypo | Hypo | Hypo | Hypo |
| 11 | 25 | Subacute | SSS, R-IJV, R-SIGMS | Iso | Iso | Hypo | Iso | Hypo | Hypo | Hyper | Hyper | Hypo | Hyper | Hypo | Hypo |
| 12 | 91 | Subacute | L-IJV, L-SIGMS | Hypo | Hypo | Iso | Hypo | Iso | Hypo | Hypo | Iso | Hyper | Hypo | Iso | Hypo |
| 13 | 42 | Subacute | R-IJV, R-SIGMS | Hypo | Hyper | Hypo | Iso | Hypo | Hypo | Hyper | Hyper | Hypo | Iso | Hypo | Hyper |
| 14 | 58 | Subacute | SSS, L-IJV, L-SIGMS | Hyper | Hypo | Iso | Iso | Iso | Hypo | Iso | Hypo | Iso | Hypo | Iso | Hypo |
| 15a | 55 | Acute | L-IJV | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo |
| 16 | 47 | Acute | L-IJV, L-SIGMS, SR | Hypo | Hypo | Iso | Hypo | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Iso |
| 17 | 68 | Subacute | SSS, L-IJV, L-SIGMS | Iso | Hypo | Iso | Hypo | Iso | Hypo | Hyper | Hypo | Hyper | Hypo | Hyper | Hypo |
| 18 | 25 | Subacute | L-IJV, L-SIGMS | Hypo | Hypo | Iso | Hypo | Iso | Hypo | Hypo | Hypo | Hyper | Hypo | Hyper | Hypo |
| 19 | 27 | Acute | SSS, R-IJV, R-SIGMS | Iso | Iso | Hypo | Iso | Hypo | Hypo | Iso | Iso | Hypo | Iso | Hypo | Hypo |
| 20 | 41 | Subacute | SSS, R-IJV, R-SIGMS | Hyper | Hyper | Hypo | Hyper | Hypo | Hypo | Hyper | Hyper | Hypo | Hyper | Hypo | Hypo |
| 21 | 74 | Acute | SSS, R-IJV, R-SIGMS | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Iso | Hypo | Iso | Hypo | Hypo |
| 22a | 72 | Acute | SSS, SR | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo |
| 23 | 39 | Subacute | SSS, L-IJV, L-SIGMS | Hyper | Hypo | Hyper | Hypo | Hyper | Hypo | Hyper | Hypo | Hyper | Hypo | Hyper | Hypo |
| 24 | 28 | Subacute | R-IJV | Hypo | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Hyper | Hypo | Hypo | Hypo | Hypo |
| 25 | 31 | Acute | L-IJV, L-SIGMS | Hypo | Hypo | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Hypo | Hypo | Hypo |
| 26 | 26 | Acute | SSS, R-IJV | Iso | Iso | Hypo | Hypo | Hypo | Hypo | Iso | Iso | Hypo | Hypo | Hypo | Hypo |
| 27 | 33 | Subacute | SSS, R-IJV, R-SIGMS | Iso | Iso | Hypo | Iso | Hypo | Hypo | Hyper | Hyper | Hypo | Hyper | Hypo | Hypo |
| 28 | 39 | Subacute | R-IJV | Hypo | Hyper | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Hypo | Hypo | Hypo | Hypo |
| 29a | 34 | Acute | SSS, L-IJV, L-SIGMS | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo | Hypo |
| 30 | 22 | Acute | SSS | Iso | Hypo | Hypo | Hypo | Hypo | Hypo | Iso | Hypo | Hypo | Hypo | Hypo | Hypo |
T1WI: T1-weighted magnetic resonance sequence; T2WI: T2-weighted magnetic resonance sequence; 3D PC-MRV: three-dimensional, phase-contrast magnetic resonance venography; SSS: superior sagittal sinus; R-IJV: right internal jugular vein; L-IJV: left internal jugular vein; R-SIGMS: right sigmoid sinus; L-SIGMS: left sigmoid sinus; SR: sinus rectus.
Hypo, Hyper, and Iso indicate signal values according to the signal intensity of gray matter.
Venous sinus thrombosis with unremarkable T1 and T2 magnetic resonance imaging findings.
Bold indicates thrombosed sinus segments.
Analysis of thrombosed venous sinus intensity in 14 patients examined during the first five days (acute) following the onset of symptoms showed 17 isointense and 16 hypointense signals on T1-WI, and 18 hypointense and 15 isointense signals on T2-WI. Between days 5 and 15 (subacute), 16 patients had MRI showing 23 hyperintense and 10 isointense clots on T1-WI. Fifteen hyperintense and 18 isointense clots were seen on T2-WI (Table 2). None of the patients were examined more than two weeks (between two weeks and three months) after the onset of symptoms. Therefore, the calculated sensitivity of T1- and T2-weighted MRI sequences was 83% (0.70–0.94), with a specificity of 96% (0.84–0.99) (AUC, 0.90 (0.82–0.98)) (Figure 1).
3D PC-MRV was conducted for every patient always after the standard MRI. DSA showed abnormalities in the 80 venous sinus segments of these 30 patients, consisting of a defect (22 times) or a lack of visualization (58 times) of these sinuses (Figure 3). All 30 patients with DVST were accurately confirmed by DSA according to the nonvisualization of dural sinuses related to acute or subacute thrombosis (Table 1). Therefore, the calculated sensitivity of 3D PC-MRV was 100% (0.86–1.00), with a specificity of 71% (0.59–0.82) (AUC, 0.86 (0.79–0.92)) (Figure 1). Comparison and combination with NCCT, 3D PC-MRV and T1- and T2-weighted MRI sequences allowed diagnosis of thrombosis in all these cases (100% (0.86–1.00) specificity and 100% (0.86–1.00) sensitivity in combination) (AUC, 1.00) (Figure 1).
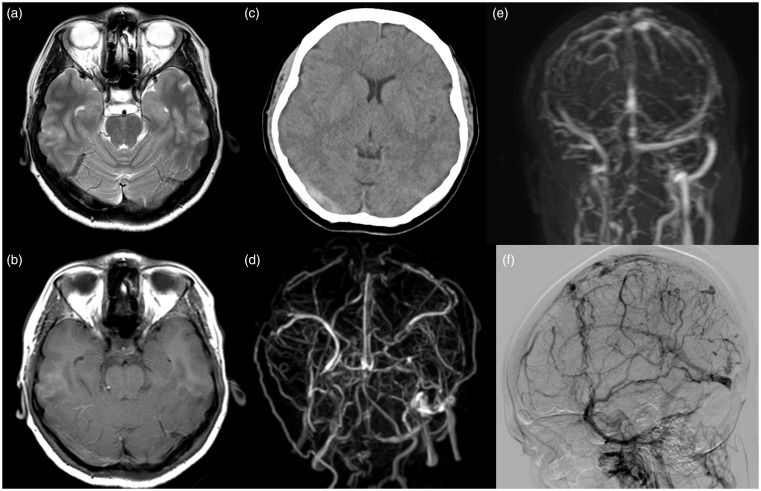
Figure 3.
An acute dural venous sinus thrombosis (DVST) was demonstrated in a postpartum patient admitted to the emergency department with the symptom of headache. On imaging, both transverse sinuses, sigmoid sinuses and superior sagittal sinus (SSS) were found to be thrombosed according to the signal characteristic on T2-weighted imaging (WI) magnetic resonance imaging (MRI) showing a hypointense thrombus in the right transverse sinus (a); T1-WI with iso- to hyperintense signal appearance (b); noncontrast computed tomography with hyperdense right transverse sinus (c); loss of flow in SSS, both transverse and sigmoid sinuses on three-dimensional phase-contrast magnetic resonance venography (MRV) (d); confirmation of venous thrombosis with four-dimensional contrast-enhanced MRV (e) and digital subtraction angiography (f).
In all 30 cases having DVST, clinical presentation with radiological findings was considered sufficient to establish the diagnosis of venous sinus thrombosis and to start anticoagulative treatment.
Discussion
DVST mostly occurs in young adults, unlike arterial stroke, and may cause significant morbidity and mortality. Early diagnosis is required to initiate appropriate treatment.16
NCCT has been utilized primarily as a first-step diagnostic method to assess patients with nonspecific neurological symptoms in the emergency department because of its availability and cost-effectiveness. In DVST, hyperattenuation of the dural venous sinuses is a unique finding on NCCT that mostly indicates an acute phase of venous thrombosis at a time when treatment is most effective and can have a substantial impact on clinical outcome.17 The calculation of attenuation in the venous sinus might be a more accurate method of comparing with visual evaluation of a thrombus because an apparent increase in attenuation can be misleading and may not be visually discovered.18 Increased attenuation in the sinus may be the only finding suggestive of sinus thrombosis on NCCT images, and patients with this sign should be further assessed with MRI, MRV, DSA or both, in the proper clinical scenario.
MRI appears very sensitive in the detection of thrombosis (83% of sensitivity in this study, more than 80%) for all the main series in the literature.19 Thrombosis induces time-dependent modifications of the signal in the sinus. On MRI, fast-flowing blood appears as a decreased signal intensity or flow void within the vessel lumen. When thrombosis first occurs in a vessel, the expected signal void of flowing blood is replaced by an isointense signal on T1-WI and decreased signal intensity on T2-WI, indicating acute thrombosis. These signal intensities change to increased signal intensity by degrees on both T1- and T2 weighted images, representing the transformation of deoxyhemoglobin to methemoglobin. Ultimately, resolution of the thrombus occurs in the chronic phase with the reappearance of attenuated signal intensity within the venous sinus, representing flow void both on T1-WI and T2-WI sequences.20
MRV is also a noninvasive diagnostic method capable of demonstrating dural venous sinuses. There are two main methods by which MRV may be gathered, PC and time-of-flight. The flowing blood seems increased in signal intensity while the stationary tissue is suppressed in both techniques. For both PC and time-of-flight techniques, the diagnostic value of MRV alone is limited because the differentiation between thrombosis and hypoplasia may not be possible, mainly a characteristic diagnostic dilemma for lateral sinuses.21
Even with the combination of MRI and MRV, the diagnosis can still be troublesome. An absence of flow on MRV with a corresponding abnormal signal in a sinus is desired for complete confidence in diagnosing DVST, but expert radiologic consideration is needed to avoid diagnostic and technical pitfalls.22 It may be essential to combine NCCT, T1- and T2-weighted MRI sequences and MRV at least for the initial diagnosis. The combination may be necessary to minimize confusion with venous sinus aplasia/hypoplasia and flow-related artifacts with thrombus, and not to mistake the T2-weighted hypointense signal of deoxyhemoglobin, intracellular methemoglobin with flow void and hyperdense appearance of venous sinuses on NCCT.
Our findings recommend a combination to evaluate acute or subacute DVST in the emergency department, especially in patients with a limitation to the contrast agent application: If an absence of flow on MRV is present, the suspicion of a DVST should be high, and an NCCT or T1- and T2- weighted MRI sequences should be performed and checked to confirm the diagnosis. The presence of a hyperattenuating dural venous sinus on NCCT (with or without any hemorrhage or edema) makes an acute DVST very likely, or the time-dependent modifications of the signal in the sinus related to the subacute venous thrombosis on T1- and T2- weighted MR sequences suggest the diagnosis of subacute DVST.
Some limitations of our study need to be considered. Consensus reading of two observers was applied to evaluate the NCCT with ROI measurement, T1- and T2- weighted MR sequences with qualitative signal estimation and 3D PC-MRV, so interobserver variability and consistency were not calculated. The relatively small size of this single-center study was another limitation. In addition, DVST is not a common disease and the size of our population is comparable to previous studies. Our study should serve as the basis for multicenter studies. The data were also gathered retrospectively and therefore were not always complete for all examinations, which could lead to indeterminate estimations of sensitivity.
Our patients have included only acute and subacute cases of DVST, so the effect of thrombus heterogeneity secondary to chronic or partial venous thrombosis was not referred. This study was also retrospective in nature and the presence of indirect signs such as edema or hemorrhage in some cases with DVST might bias the reader toward the diagnosis of DVST. Clinical aging of venous thrombus can be inaccurate because of variable symptoms. Fortunately, most of our patients had a relatively defined onset of a single symptom (headache), increasing the accuracy of our temporal data. The signal intensity on T1- and T2- weighted MRI evaluation was subjective, though the signal intensity was classified by consensus reading of experienced radiologists.
Nevertheless, our protocol comprises 3D PC-MRV, NCCT, and conventional T1- with T2-weighted MRI sequences, as this technique does not require the intravenous application of contrast agent, which constitutes a major advantage, especially in patients with impaired renal function. Perhaps, further prospective studies need to be performed to determine the proposed algorithm for evaluation of acute or subacute DVST.
In conclusion, this study demonstrates that the combination of NCCT, T1- and T2-weighted MRI and 3D PC-MRV may help to allow diagnosis of acute or subacute DVST and may obviate the need for contrast usage in patients who have impaired renal function.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ferro J. Prognosis of cerebral vein and dural sinus thrombosis: Results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 2004; 35: 664–670. [DOI] [PubMed] [Google Scholar]
- 2.Bousser M, Ferro J. Cerebral venous thrombosis: An update. Lancet Neurol 2007; 6: 162–170. [DOI] [PubMed] [Google Scholar]
- 3.Van Gijn J. Cerebral venous thrombosis: Pathogenesis, presentation and prognosis. J R Soc Med 2000; 93: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashkoush AI, Ma H, Agarwal N, et al. Cerebral venous sinus thrombosis in pregnancy and puerperium: A pooled, systematic review. J Clin Neurosci 2017; 39: 9–15. [DOI] [PubMed] [Google Scholar]
- 5.Chiewvit P, Piyapittayanan S, Poungvarin N. Cerebral venous thrombosis: Diagnosis dilemma. Neurol Int 2011; 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuhr F, Mehraein S. Cerebral venous and sinus thrombosis: Patients with a fatal outcome during intravenous dose-adjusted heparin treatment. Neurocrit Care 2004; 1: 355–362. [DOI] [PubMed] [Google Scholar]
- 7.Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med 2005; 352: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 8.Roland T, Jacobs J, Rappaport A, et al. Unenhanced brain CT is useful to decide on further imaging in suspected venous sinus thrombosis. Clin Radiol 2010; 65: 34–39. [DOI] [PubMed] [Google Scholar]
- 9.Teasdale E. Cerebral venous thrombosis: Making the most of imaging. J R Soc Med 2000; 93: 234–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buyck P, De Keyzer F, Vanneste D, et al. CT density measurement and H:H ratio are useful in diagnosing acute cerebral venous sinus thrombosis. AJNR Am J Neuroradiol 2013; 34: 1568–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferro JM, Bousser MG, Canhão P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—endorsed by the European Academy of Neurology. Eur J Neurol 2017; 24: 1203–1213. [DOI] [PubMed] [Google Scholar]
- 12.Connor S, Jarosz J. Magnetic resonance imaging of cerebral venous sinus thrombosis. Clin Radiol 2002; 57: 449–461. [DOI] [PubMed] [Google Scholar]
- 13.Patel D, Machnowska M, Symons S, et al. Diagnostic performance of routine brain MRI sequences for dural venous sinus thrombosis. AJNR Am J Neuroradiol 2016; 37: 2026–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonneville F. Imaging of cerebral venous thrombosis. Diagn Interv Imaging 2014; 95: 1145–1150. [DOI] [PubMed] [Google Scholar]
- 15.Liauw L, van Buchem M, Spilt A, et al. MR angiography of the intracranial venous system. Radiology 2000; 214: 678–682. [DOI] [PubMed] [Google Scholar]
- 16.Bergman EM, Henriksson KM, Åsberg S, et al. National registry-based case-control study: Comorbidity and stroke in young adults. Acta Neurol Scand 2015; 131: 394–399. [DOI] [PubMed] [Google Scholar]
- 17.Linn J, Pfefferkorn T, Ivanicova K, et al. Noncontrast CT in deep cerebral venous thrombosis and sinus thrombosis: Comparison of its diagnostic value for both entities. AJNR Am J Neuroradiol 2009; 30: 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black D, Rad A, Gray L, et al. Cerebral venous sinus density on noncontrast CT correlates with hematocrit. AJNR Am J Neuroradiol 2011; 32: 1354–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferro JM, Canhão P. Cerebral venous sinus thrombosis: Update on diagnosis and management. Curr Cardiol Rep 2014; 16: 523. [DOI] [PubMed] [Google Scholar]
- 20.Krieger DA, Dehkharghani S. Magnetic resonance imaging in ischemic stroke and cerebral venous thrombosis. Top Magn Reson Imaging 2015; 24: 331–352. [DOI] [PubMed] [Google Scholar]
- 21.Fera F, Bono F, Messina D, et al. Comparison of different MR venography techniques for detecting transverse sinus stenosis in idiopathic intracranial hypertension. J Neurol 2005; 252: 1021–1025. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo L, Crasto SG, Rudà R, et al. Cerebral venous thrombosis: Role of CT, MRI and MRA in the emergency setting. Radiol Med 2010; 115: 313–325. [DOI] [PubMed] [Google Scholar]