Abstract
Purpose
Transverse sinus stenosis is commonly seen in patients with idiopathic intracranial hypertension. It is not clear whether it is the cause or the result of idiopathic intracranial hypertension. Stenting for idiopathic intracranial hypertension has been carried out in several prior series. Our goal was to evaluate the clinical and imaging follow-up results of patients with idiopathic intracranial hypertension that underwent stenting for this condition at our center.
Materials and Methods
We reviewed the clinical, venographic and follow-up imaging data in patients who underwent elective transverse sinus stenting during the period from 2011 to 2017.
Results
In total, 18 patients with idiopathic intracranial hypertension were identified. The mean lumbar cerebrospinal fluid opening pressure recorded was 408 mmH20. Overall, 16 patients met the inclusion criteria and underwent transverse sinus stenting. At venography, the mean pressure gradient across the dominant transverse sinus stenosis was 21 mmHg. The pressure gradient immediately after stenting in all of those measured was negligible. Following stenting, headaches improved in 10 of the 16 cases, with persistent headaches in four patients, one of which had persistent baseline migraines. All cases showed resolution of the papilledema on follow up. Follow-up imaging with computed tomography venography showed that the stents remained widely patent. The follow up in clinic was done for a mean period of 35.5 months. Follow up with computed tomography venography was done for a mean of 10.3 months.
Conclusion
Venous sinus stenting is a safe and effective procedure. It relieves papilledema in all cases and improves headaches in most cases.
Keywords: Headache, idiopathic intracranial hypertension, papilledema, venous sinus stenosis, venous sinus stenting
Introduction
Up to 93% of patients diagnosed with idiopathic intracranial hypertension (IIH) have stenosis within the dural venous sinus system, suggesting a role for sinus stenosis in the poorly understood IIH pathophysiology that could be either a cause or a consequence of the intracranial hypertension.1
IIH is characterized by intracranial hypertension without evidence of a causative factor2 and is a common cause of chronic headache. Although it is considered a benign condition, IIH can lead to disabling headaches and permanent vision loss in 10–20% of affected patients.3
The incidence of this condition ranges between 1–5 cases per 100,000 people in the general population, rising to 19/100,000 in obese women of reproductive age.4 The number of cases is expected to increase dramatically with the worldwide obesity epidemic.
Treatment goals are the stabilization or reduction of intracranial pressure, thereby reducing symptoms and complications. First-line treatment modalities include weight loss, diuretics and repeated lumbar punctures. A lack of response or noncompliance with medical treatment are indications for surgical treatment.5
Surgical cerebrospinal fluid (CSF) diverting procedures have been considered the standard treatment for severe symptomatic IIH. However, in the last 15 years transverse venous sinus stenting has emerged as an effective minimally invasive alternative to surgery with a lower complication rate.6
Methods
Institutional review board approval was obtained. We retrospectively analyzed clinical, radiological and intravascular manometry data of 18 patients with diagnosis of IIH referred to Neurointerventional Radiology for possible treatment with transverse sinus stenting during the period from 2011 to 2017.
For each patient, information on demographics, risk factors and clinical presentation was collected. In total, 18 patients with clinical diagnosis of IIH were identified based on clinical signs and symptoms. Two of the 18 patients were excluded from the study because they did not meet the trans-stenotic pressure gradient criteria.
There were 15 female patients and one male patient. The median age was 35 years. Headache was present in all patients, papilledema in 13, pulsatile tinnitus in four, visual deficits in 11 and diplopia in three. The mean body mass index was 40.65. The mean duration of symptoms before stent placement was 13 months (Table 1).
Table 1.
Patient characteristics.
| Case no. | Clinical presentation | BMI | Lumbar CSF opening P. (cmH2O) | Duration of symptoms (months) | Lesion location | % stenosis |
|---|---|---|---|---|---|---|
| 1 | Headache, papilledema, visual obscuration | 60.9 | 35 | 72 | B/L R dominant | 70% |
| 2 | Headache, papilledema, visual obscuration, pulsatile tinnitus | NA | 44 | 18 | B/L R dominant | 70% |
| 3 | Headache, papilledema, visual obscuration, diplopia, pulsatile tinnitus | 49 | 34 | 6 | B/L R dominant | 70% |
| 4 | Headache, papilledema, visual obscuration | 35.6 | 27 | 2 | B/L L dominant | 70% |
| 5 | Headache, papilledema, visual obscuration | NA | 45 | 19 | B/L codominant | 80% |
| 6 | Headache, visual obscuration, pulsatile tinnitus | 22.8 | ND due to Chiari | 24 | B/L codominant | 80% |
| 7 | Headache, papilledema | 43.4 | 36 | 1 | B/L R dominant | |
| 8 | Headache, visual obscuration, papilledema, diplopia | 30.34 | 50 | 24 | Dominant R TS stenosis | 80% |
| 9 | Headache | 37.3 | 35 | 2 | Dominant R TS stenosis | 80% |
| 10 | Headache, visual obscuration, papilledema, pulsatile tinnitus | 41.1 | 55 | 6 | B/L codominant | 70% |
| 11 | Headache, visual obscuration, papilledema | 31.41 | 31 | 3 | B/L L dominant | 70% |
| 12 | Headache, papilledema | 37.5 | 51 | 12 | L dominant | 70% |
| 13 | Headache, visual obscuration | 42.49 | 31 | 12 | B/L R dominant | 90% |
| 14 | Headache, papilledema, diplopia | 32 | 52 | 0.5 | B/L R dominant | 80% |
| 15 | Headache, papilledema | 55.23 | 44 | 1 | B/L R dominant | 70% |
| 16 | Headache, papilledema | 50.11 | 39 | 12 | B/L R dominant | 70% |
BMI: body mass index; B/L: bilateral; L: left; NA: not available; ND: not done; R: right; TS: transverse sinus.
All patients underwent lumbar puncture with CSF opening pressure measurements. The mean CSF opening pressure was 408 mmH20. At venography, the mean pressure gradient across the dominant transverse sinus stenosis was 21 mmHg (range of 10–45 mmHg).
The indications for endovascular treatment were symptomatic IIH patients unresponsive to conservative treatment with evidence of transverse sinus stenosis and a pressure gradient greater than 8 mmHg documented on a catheter venogram.
Post-deployment venography and computed tomography (CT) venography were obtained in all cases.
All 16 patients underwent transfemoral cerebral diagnostic angiogram, venogram and manometry under sedation to avoid the general anesthetic effects on intracranial pressure.
The technique was similar for diagnostic venogram and interventional stenting cases. Femoral arterial and venous access were obtained under ultrasound guidance with a 6 French Neuron Max catheter (Penumbra) in the right common femoral vein and a 4 French 10 cm sheath in the common left femoral artery.
Super-selective catheterization of the dural sinuses was performed with a neuro Renegade microcatheter over a 0.014” Synchro standard microwire. Then an injection through the microcatheter was performed (with venography assessment of the superior sagittal sinus and both transverse and sigmoid sinuses) demonstrating the site and percentage of stenosis. Manometry was subsequently performed at the superior sagittal sinus, transverse sinus, sigmoid sinus and internal jugular vein. Pressures were measured proximal and distal to the stenosis using a transducer attached to the catheter (in cases of bilateral stenosis, measurements were performed bilaterally).
The intra-arterial sheath was then removed and hemostasis was achieved with 5 French Mynx grip (Cordis) closure device and 5 minutes of manual compression. Finally, the Neuron Max catheter was withdrawn and hemostasis achieved with manual compression for 10 minutes.
For stenting cases dual antiplatelet therapy was started 5 days before the procedure in all patients. All patients underwent a trans-femoral cerebral angiogram and venogram under general anesthesia using the technique described above.
A neuro Renegade microcatheter over a 0.014” Synchro standard microwire was advanced through the transverse sinus stenosis and was placed within the anterior portion of the superior sagittal sinus. The microcatheter was advanced and the wire was withdrawn and replaced for an exchange 0.014 Ironman microwire to achieve optimal support. The microcatheter was removed and a previously selected Acculink stent was advanced; if the stenosis was bilateral the dominant transverse sinus was chosen for stent placement. The length of the stent used was determined by measurement of the caliber of the sinuses, with most cases requiring 8 x 40 mm stents.
The Acculink stent was deployed under continuous fluoroscopy. Finally, a control injection was performed in every case to confirm apposition of the stent to the vessel wall. The microcatheter was re-advanced across the stent and manometry was performed to document resolution of the pressure gradient.
Hemostasis was obtained in the same way as described above. All of the patients were transferred to intensive care for recovery over 1 day before discharge with dual daily aspirin 325 mg and Plavix 75 mg antiplatelet therapy for 6 weeks and 81 mg aspirin thereafter.
Post-treatment clinical and imaging follow up with CT venography was performed in all cases. Follow up in clinic was done for a mean period of 35.5 months (range of 4 months to 65 months). Follow up with CT venography was done for a mean of 10.3 months (range of 2 months to 36 months). Diagnosis to stenting time was done with a mean of 4.6 months (range of 2 months to 9 months).
Results
Immediate post-deployment venography showed restoration of venous flow and venous sinus diameter with residual stenosis percentage ranging between 0–10%. The pressure gradient immediately after stenting in all of those measured was negligible (Table 2). There were no technical problems encountered with the device and no complications were observed (Figure 1).
Table 2.
Results.
| Case no. | Venous pressure gradient pre-procedure | Venous pressure gradient post-procedure (mmHg) | Stent location | Clinical progress |
|---|---|---|---|---|
| 1 | 10 | 0 | R distal TS | Improved headache severity, no papilledema |
| 2 | 25 | 1 | R distal TS | Persistent headache, papilledema resolved |
| 3 | 14 | 0 | R distal TS | Persistent headache, papilledema resolved |
| 4 | 12 | 0 | L distal TS | Improved headache, papilledema and visual obscuration resolved |
| 5 | 19 | 0 | R distal TS | Improved headache severity (from 9/10 to 7/10) |
| 6 | 23 | 2 | R Mid TS | Improved headache but recurred |
| 7 | 18 | 1 | R distal TS | Improved headache and papilledema |
| 8 | 18 | 0 | R distal TS | Improved headache, resolved papilledema |
| 9 | 16 | 1 | R distal TS | Complete headache relief |
| 10 | 13 | 0 | R distal TS | Headache recurrence and increased severity increased medical treatment dose needed; resolved papilledema |
| 11 | 37 | 1 | L distal TS | Improved headache and resolved papilledema |
| 12 | 16.8 | 1 | R distal TS | Persistent headache, resolved papilledema |
| 13 | 10 | 3 | R distal TS | Improved headache, persistent intermittent visual obscuration |
| 14 | 28 | ND | L distal TS | Persistent headache, resolved papilledema |
| 15 | 24 | 3 | R distal TS | Improved headache and resolved papilledema |
| 16 | 19 | 1 | R distal TS | Improved headache and resolved papilledema |
R: right; L: left; TS: transverse sinus; ND: not done.
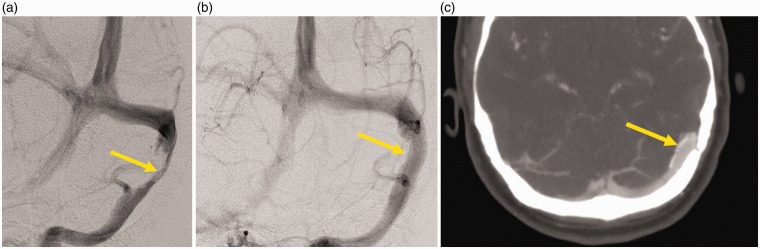
Figure 1.
Patient number 4. (a) Pre-stenting venographic injection showing a 70% transverse sinus stenosis (arrow) (b), (c) immediate post-stent placement venographic injection and CT venography showing wide patency of the stented venous sinus and contrast filling (arrows).
The dominant transverse sinus was stented in all cases, on the right side in 75% of the cases (12/16). None of the cases required re-stenting.
Following stenting, headache improved in 10 of the 16 cases treated. Persistent headache was observed in four patients, one of whom had persistent baseline migraine. One patient had complete relief of headache, whereas one required increased medication for increased headache. The patients with persistent headache had normal opening pressure on a lumbar puncture (LP) and pressure gradient on follow-up venography was absent. All cases showed no papilledema on follow up. Only one patient had persistent visual obscurations and all the patients who had diplopia pre-stenting had a complete recovery. Follow-up imaging with CT venography showed the stents remained widely patent. No in-stent stenosis or stenosis adjacent to the stent was demonstrated.
Discussion
In the literature, the relationship of dural venous sinus stenosis to IIH is speculative. However, a positive feedback cycle has been described as a cause of the dysregulated CSF dynamics in IIH: an increased ICP (caused by an unknown factor) can produce stenosis in the venous sinus, which leads to a decreased CSF resorption, further increase in intracranial pressure (ICP) and even greater sinus stenosis.7,8
In an interesting laboratory and mathematical analysis, Stevens et al. suggest that transient increases in ICP can cause compression of the transverse sinus, altering hemodynamics between the sagittal sinus and the transverse sinus (Starling-like resistor effect) and be the stimulus for the development of IIH and transverse sinus stenosis.9 These transient and intermittent episodes of increased pressure may be seen especially in obese patients in whom sleep apnea produces hypercapnia, which in turn produces increased cerebral blood flow.
In our series, the transverse sinus stenosis was bilateral in 75% of cases, with a higher grade of stenosis affecting the dominant right sinus.7 This demonstrates that IIH is symptomatic if the stenosis affects a dominant transverse sinus or both transverse sinuses similar to other causes of ICP, which may also be symptomatic if they affect the dominant or both transverse sinuses. For example, the venographic filling defects produced by trabeculae or large arachnoid granulations may be associated with symptoms of intracranial hypertension.10
Stent placement was done in the dominant sinus in all cases, resulting in an immediate reduction of intracranial pressure and the resolution of the pressure gradient. This supports the premise that sinus stenting removes the positive feedback in the vicious cycle of IIH independent of its role as the cause or as the consequence of the intracranial hypertension.11,12 Additionally, the stent supports the walls of the sinus, minimizing the chance of compression of the sinus and the Starling-like resistor effect. Furthermore, these results suggest the dominant sinus is needed for the maintenance of normal CSF dynamics. This is evident in our results and in those reported in the literature, where unilateral stenting has been effective in patients with bilateral stenosis.11
In our patients, there was 100% resolution of papilledema, diplopia and tinnitus. Only one patient had persistent visual loss. We speculate this is related to irreversible damage to the optic nerve. Headaches disappeared in 7% and improved in 57% of patients. Persistent headache was present in 28% and 7% of patients had a worsening of symptoms. During the clinical follow up, the characteristics of the headaches changed in all cases and the patients with persistent headaches had primary headache syndromes such as migraine and tension headache. We speculate that once the ICP is normal, other causes of headaches become more noticeable by the patient.
There were no complications in our series. An incidence of adverse events ranging between 2.9% and 4.4% has been described. This includes ipsilateral headache as a result of dural stretching secondary to the stent placement, subdural hematoma and transient hearing loss.7,13
In general, our outcomes were similar to those reported in several case series and meta-analyses. The first case report was written in 2002 by Higgins,14 and since then several case series reports have been published. In 2013, Puffer et al. performed a literature review of patients with IIH treated with stenting. A total of 143 cases were identified (85% women), with headache the most common symptom (90%), followed by papilledema (89%) and visual changes (62%). Venous stenting had high clinical effectiveness, with improvement of headaches in 88%, resolution of papilledema in 97% and resolution of pulsatile tinnitus in 93% of cases.
More recently, Dinkin et al. performed a meta-analysis to evaluate the efficacy and safety of sinus stenting. A total of 25 studies were found with 282 patients undergoing sinus stenting. The results showed a reduction in trans-stenotic gradient and significant clinical improvement in most of the patients. Headache improved in 50.4% and resolved in 32.3% of cases, with 17% of patients showing no improvement. Papilledema resolution rate was 72% and improvement 24%. There was a 97% complete resolution of visual symptoms.7
The outcomes of endovascular technique have been compared to those of the available two surgical modalities: optic nerve sheath fenestration (ONSF) and CSF diverting procedures. Satti et al.5 performed a large meta-analysis comparing 136 stented patients with 712 who underwent ONSF and 435 who underwent CSF diversion procedures.
In the ONSF group, there was vision improvement in 59%, 44% with headache improvement and 80% with papilledema improvement. With re-intervention, major and minor complication rates of 1.5% and 16.4% respectively were noted. Within the CSF diversion group, there was improvement of vision in 54% of cases, headache improvement in 80% and papilledema improvement in 70%. With re-intervention, major and minor complication rates of 7.6% and 32.9% respectively were observed. In the stented group of patients, improvement of vision occurred in 78%, improvement in headaches in 83% and papilledema in 97%. With re-intervention, the incidence of major and minor complications was 2.9% and 4.4% respectively.
These results confirm the high technical and clinical efficacy of sinus stenting and its lower complication rate in comparison with surgical modalities.13 These results only apply to a specific population with refractory IIH with evidence of hemodynamically significant dural venous sinus stenosis.
In all of our patients, venographic follow up showed neither in-stent stenosis nor stenosis adjacent to the stent that has been previously described on other clinical series.
Limitations of our study include the relatively small number of cases treated, single center experience and the lack of long-term follow up in some cases.
Conclusion
Our clinical experience has shown that dural venous sinus stenting is a safe and effective procedure with immediate normalization of the intracranial pressure and resolution of papilledema in all cases. The procedure also improves headaches in most cases. Further randomized studies with larger populations are warranted.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Puffer RC, Mustafa W, Lanzino G. Venous sinus stenting for idiopathic intracranial hypertension: A review of the literature. J Neurointerv Surg 2013; 5(5): 483–6. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Perez M, Martinez-Moreno R, Kurre W, et al. Endovascular treatment of idiopathic intracranial hypertension: Retrospective analysis of immediate and long-term results in 51 patients. Neuroradiology 2017; 59(3): 277–87. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed RM, Wilkinson M, Parker GD, et al. Transverse sinus stenting for idiopathic intracranial hypertension: A review of 52 patients and of model predictions. AJNR Am J Neuroradiol 2011; 32(8): 1408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galgano MA, Deshaies EM. An update on the management of pseudotumor cerebri. Clin Neurol Neurosurg 2013; 115(3): 252–9. [DOI] [PubMed] [Google Scholar]
- 5.Satti SR, Leishangthem L, Chaudry MI. Meta-analysis of CSF diversion procedures and dural venous sinus stenting in the setting of medically refractory idiopathic intracranial hypertension. AJNR Am J Neuroradiol 2015; 36(10): 1899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lenck S, Vallee F, Labeyrie MA, et al. Stenting of the lateral sinus in idiopathic intracranial hypertension according to the type of stenosis. Neurosurg 2017; 80(3): 393–400. [DOI] [PubMed] [Google Scholar]
- 7.Dinkin MJ, Patsalides A. Venous sinus stenting for idiopathic intracranial hypertension: Where are we now? Neurologic Clin 2017; 35(1): 59–81. [DOI] [PubMed] [Google Scholar]
- 8.Levitt MR, Albuquerque FC, Ducruet AF, et al. Venous sinus stenting for idiopathic intracranial hypertension is not associated with cortical venous occlusion. J Neuroint Surg 2016; 8(6): 594–5. [DOI] [PubMed] [Google Scholar]
- 9.Stevens SA, Thakore NJ, Lakin WD, et al. A modeling study of idiopathic intracranial hypertension: Etiology and diagnosis. Neurol Res 2007; 29(8): 777–786. [DOI] [PubMed] [Google Scholar]
- 10.Arjona A, Delgado F, Fernandez-Romero E. Intracranial hypertension secondary to giant arachnoid granulations. J Neurol Neurosurg Psych 2003; 74(4): 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu K, Yu T, Yuan Y, et al. Current status of the application of intracranial venous sinus stenting. Int J Med Sci 2015; 12(10): 780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buell TJ, Raper DMS, Pomeraniec IJ, et al. Transient resolution of venous sinus stenosis after high-volume lumbar puncture in a patient with idiopathic intracranial hypertension. J Neurosurg 2017; 25: 1–4. [DOI] [PubMed] [Google Scholar]
- 13.Albuquerque FC, Gross BA, Levitt MR. Time to re-assess the treatment of idiopathic intracranial hypertension. J Neuroint Surg 2016; 8(6): 549–50. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JN, Owler BK, Cousins C, et al. Venous sinus stenting for refractory benign intracranial hypertension. Lancet 2002; 359(9302): 228–30. [DOI] [PubMed] [Google Scholar]