Abstract
Background
Cerebral edema is frequent in patients with acute ischemic stroke (AIS) who undergo reperfusion therapy and is associated with high mortality. The impact of collateral pial circulation (CPC) status on the development of edema has not yet been determined.
Methods
We studied consecutive patients with AIS and documented M1–middle cerebral artery (MCA) and/or distal internal carotid artery (ICA) occlusion who underwent reperfusion treatment. Edema was graded on the 24-hour non-contrast computed tomography (NCCT) scan. CPC was evaluated at the acute phase (≤6 hours) by transcranial color-coded Doppler, angiography and/or CT angiography. We performed an ordinal regression model for the effect of CPC on cerebral edema, adjusting for age, baseline National Institutes of Health Stroke Scale, Alberta Stroke Program Early Computed Tomography Score (ASPECTS) on admission, NCCT, parenchymal hemorrhagic transformation at 24 hours and complete recanalization at six hours.
Results
Among the 108 patients included, 49.1% were male and mean age was 74.2 ± 11.6 years. Multivariable analysis showed a significant association between cerebral edema and CPC status (OR 0.22, 95% CI 0.08–0.59, p = 0.003), initial ASPECTS (OR 0.72, 95% CI 0.57–0.92, p = 0.007) and parenchymal hemorrhagic transformation (OR 23.67, 95% CI 4.56–122.8, p < 0.001).
Conclusions
Poor CPC is independently associated with greater cerebral edema 24 hours after AIS in patients who undergo reperfusion treatment.
Keywords: Collateral pial circulation, brain edema, ischemic stroke, stroke outcome
Introduction
Cerebral edema (CE) is frequent in patients with acute ischemic stroke (AIS) treated with reperfusion therapy.1 Approximately 10% of all AIS patients develop malignant middle cerebral artery (MCA) infarction (MMI), with an estimated mortality rate of 80%.2,3 Several predictors of MMI/CE had been evaluated in the acute phase, either using clinical and laboratorial data (coma on admission; nausea, vomiting, or systolic blood pressure; history of hypertension or heart failure; elevated white blood cell count) or through neuroimaging parameters (cranial computed tomography (CT) findings, angiographic findings such as carotid-T occlusion; perfusion parameters; magnetic resonance imaging findings).4–6
The pathophysiology involved in CE is not entirely understood, but ischemia-induced capillary dysfunction can be attributed to de novo synthesis of a specific ensemble of proteins that determine osmotic and hydraulic conductivity in Starling’s equation, resulting in progressive alteration in the permeability of the blood-brain barrier.7,8
Currently, there is no effective medical treatment for CE.1,9 Decompressive craniectomy is a treatment option for selected patients with increased intracranial pressure; when undertaken within 48 hours of stroke onset, it has been shown to reduce mortality and to improve outcome in patients with MMI.3,10–12 Therefore, predicting its occurrence is crucial for therapeutic management at early stages.
Collateral pial circulation (CPC) has been associated with infarction volume, recanalization rates, hemorrhagic transformation, and subsequent clinical outcomes.13–16 However, its potential impact in the development of CE is not definitely established. We aimed to study the influence of brain CPC in the development of CE in patients with AIS who undergo reperfusion therapy.
Participants and methods
Study population
We studied consecutive adult patients with acute ischemic stroke and documented M1–MCA and/or distal internal carotid artery (ICA) occlusion admitted to our institution between January 2015 and December 2016. Only patients treated with reperfusion therapy (intravenous (IV) thrombolysis with or without subsequent mechanical thrombectomy) were included.
Institutional protocol
At arrival, patients underwent a complete evaluation by a vascular neurologist, including assessment of National Institutes of Health Stroke Scale (NIHSS).
In accordance with our institutional protocol, all patients underwent an acute-phase noncontrast CT scan, to exclude hemorrhage and evaluate extent of established infarct. If eligible for endovascular treatment (NIHSS > 10; modified Rankin Scale, mRS, <3; Alberta Stroke Program Early CT Score, ASPECTS, > 6; and time from stroke onset < 6 hours), patients also had an acute-phase cerebral CT angiography (CTA) to confirm proximal occlusion.
All scans were obtained using a 64-slice CT scanner (LightSpeed VCT, GE, CT, USA), with the patient in the supine position. The uniphase CTA was obtained using a helical acquisition with the following parameters: 120 kVp, 300 mAs, collimator of 32 × 0.625 mm and coverage from the aortic arch to the vertex. The acquisition was triggered at the time of maximum arterial contrast opacity at the level of C1/C2, determined by an initial bolus testing (15 ml of contrast). The CT perfusion included eight sections 2 mm thick, beginning approximately at the roof of the orbit. The acquisition was obtained in a period of 60 seconds, after a single bolus of contrast (60 ml). The penumbral information was used to support the decision of endovascular treatment.
Transcranial color-coded Doppler (TCCD) was performed at admission depending on availability and clinical relevance, using a General Electric Logiq 7® with a 3 Mhz sector probe for transcranial examination.
All patients underwent a 24-hour CT scan, as required by our institutional protocol, to evaluate final infarct extension and the presence of hemorrhagic transformation, according to the criteria used in the European Cooperative Acute Stroke Study (ECASS) study (parenchymal hematoma (PH) 2).17
CPC
Using monophasic CTA, the quality of the CPC was evaluated by the extent of retrograde filling of the MCA with respect to the Sylvian fissure, as described by Miteff et al.,18 and comparing to the other side. Scores of 1 (contrast merely in superficial branches) were categorized as poor CPC, while 2 (contrast filling vessels at the Sylvian fissure) and 3 (vessels reconstituted immediately distal to the occlusion) were considered good CPC.
If the patient underwent endovascular treatment, the CPC was also rated by digital subtraction angiography (DSA), according to the grade of reconstitution of the vessels in the occluded territory, using a score previously described and validated.14 Grades 1 and 2 were considered good CPC, in contrast to grades 3, 4 and 5, which were regarded as poor CPC.
If TCCD was performed, the CPC was additionally evaluated by flow diversion in the anterior and posterior cerebral arteries, using the criteria published by Kim et al.19 In this score, the presence of flow diversion (low resistance and high velocity flow, >30% compared to contralateral side) in either the anterior or posterior cerebral arteries was assumed to represent good CPC, and its absence a surrogate of poor CPC.
If a patient underwent more than one vascular examination, the final CPC score was determined hierarchically: DSA > CTA > TCCD. Furthermore, for the purpose of statistical analysis the final CPC score was dichotomized (poor vs good CPC) as specified above.
Treatment
IV fibrinolysis was administered to every patient presenting with an acute stroke of less than 4.5 hours of evolution, no signs of hemorrhage on the initial CT scan and initial ASPECTS >6. The endovascular treatment consisted of mechanical thrombectomy using stent retrievers and was performed for all patients fulfilling the aforementioned criteria.
Recanalization
Recanalization was determined by TCCD (Thrombolysis in Brain Ischemia >3) performed six hours after stroke onset, or final DSA acquisition (Thrombolysis in Cerebral Infarction 2b or 3) when the patient underwent endovascular treatment.
Edema
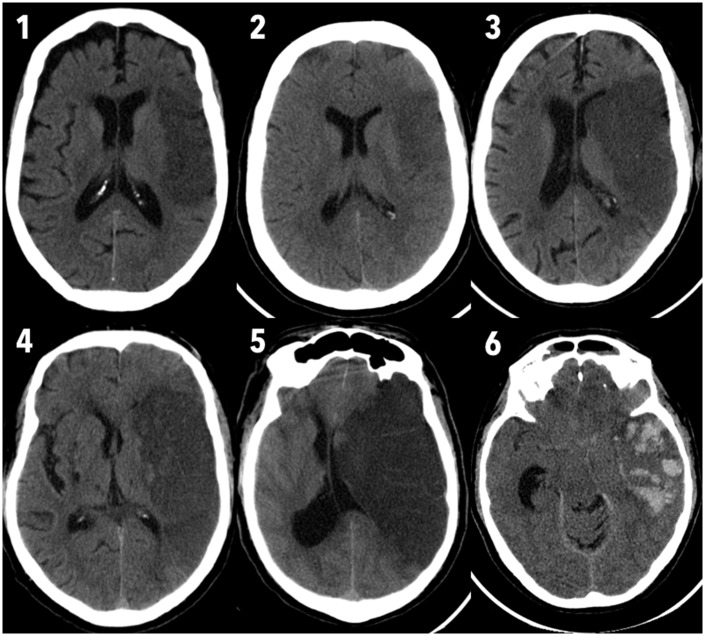
The 24-hour CT was used to grade the extent of CE. This was accomplished by evaluating the effect of the edema on the structures around the infarcted parenchyma, namely the sulci, ventricles, midline shift and basal cisterns. A score previously proposed was used.20 This score ranges from 0 (no edema) to 6 (massive supratentorial edema with basal cisterns effacement), and is illustrated in Figure 1. Notice how comparison to the unaffected side is crucial for the correct rating of edema in the lower grades. Scores ranging from 0 to 2 were considered as minor edema, while scores 3 to 6 were classified as significant/major edema.
Figure 1.
Score used for edema grading in the 24-hour unenhanced computed tomography scan.
Outcome
The mRS score was used to evaluate the functional outcome at 90 days. A score less than 3 was considered a good outcome.
Statistical analysis
All images were reviewed by two trained observers (OG and MB), regarding CPC status, hemorrhagic transformation and CE at 24 hours. Inter-rater agreement level was determined by Cohen’s Kappa coefficient.
Univariate correlations between the CE at 24 hours and the various vascular risk factors and appropriate clinical and imaging parameters were determined with Spearman correlation and Mann–Whitney U test, for quantitative and nominal variables, respectively. All variables showing a correlation with the grade of edema at 24 hours with p < 0.20 were included in the multivariable analysis. Ordinal regression models were obtained to identify variables independently associated with the grade of CE at 24 hours. CE was used as an ordinal variable for the entire analysis, and only later dichotomized to allow for easier group characterization.
The level of significance used was 5%. All statistical analyses were performed using SPSS (version 22.0; IBM, NY, USA).
Results
A total of 108 patients with acute ischemic stroke and MCA and/or ICA occlusion were included. The baseline characteristics of all patients are shown in Table 1. Fifty-three (49.1%) individuals were male. The mean age was 74.2 years (SD 11.6) and the median baseline NIHSS was 17 (interquartile range (IQR) 11). On CTA, 21 patients presented with a thrombus in the distal ICA, while the remainder had only an M1–MCA occlusion. All patients in our study population underwent IV fibrinolysis. Mechanical trombectomy was also performed in 29 cases (26.9%): Patients were excluded because of previous mRS > 2 (n = 9), admission ASPECTS < 7 (n = 18), age >85 years (n = 14), spontaneous/IV fibrinolysis-related recanalization (n = 3) and time window (n = 5); for 30 additional patients, this treatment was not yet approved at the time of the study (see Supplementary Figure). The rate of significant hemorrhagic transformation (PH2) was 5.6% (six patients).
Table 1.
Clinical characteristics of the studied patients.
| Minor edema (n = 80) | Significant edema (n = 28) | p value | |
|---|---|---|---|
| Age, mean (SD) | 75.0 (11.5) | 71.9 (11.7) | 0.469 |
| Male gender, n (%) | 38 (47.5) | 15 (53.6) | 0.582 |
| Vascular risk factors | |||
| Arterial hypertension | 66 (85.7) | 23 (85.2) | 0.946 |
| Diabetes mellitus | 17 (22.1) | 6 (22.2) | 0.988 |
| Dyslipidemia | 54 (70.1) | 13 (48.1) | 0.061 |
| Smoking | 5 (5.3) | 1 (3.6) | 0.664 |
| Atrial fibrillation | 42 (54.5) | 13 (46.4) | 0.461 |
| Coronary disease | 8 (11.8) | 2 (7.4) | 0.533 |
| Obesity | 14 (20.6) | 6 (22.2) | 0.631 |
| Peripheral artery disease | 3 (4.5) | 2 (7.4) | 0.567 |
| Baseline NIHSS, mean (SD) | 16.9 (7.2) | 17.7 (5.6) | 0.138 |
| ASPECTS at admission, median (IQR) | 9 (1.75) | 7 (3) | <0.001 |
| Thrombus location, n (%) | 0.354 | ||
| Distal ICA | 2 (2.5) | 2 (7.1) | |
| M1–MCA | 68 (85.0) | 19 (67.9) | |
| ICA + M1–MCA | 10 (12.5) | 7 (25.0) | |
| IV fibrinolysis | 80 (100.0) | 28 (100.0) | |
| Mechanical thrombectomy | 21 (26.3) | 8 (28.6) | 0.297 |
| Recanalization at six hours | 59 (73.8) | 10 (35.7) | <0.001 |
| Hemorrhagic transformation (PH2) | 1 (1.3) | 5 (17.9) | 0.002 |
| Good CPC score, n (%) | 58 (72.5) | 11 (39.3) | <0.001 |
The p values shown are the results of comparison of both groups (minor and significant edema) for the corresponding variable.
ASPECTS: Alberta Stroke Program Early Computed Tomography Score; CPC: collateral pial circulation; ICA: internal carotid artery; IQR: interquartile range; IV: intravenous; MCA: middle cerebral artery; NIHSS: National Institutes of Health Stroke Scale; PH: parenchymal hematoma; p-values in bold are significant.
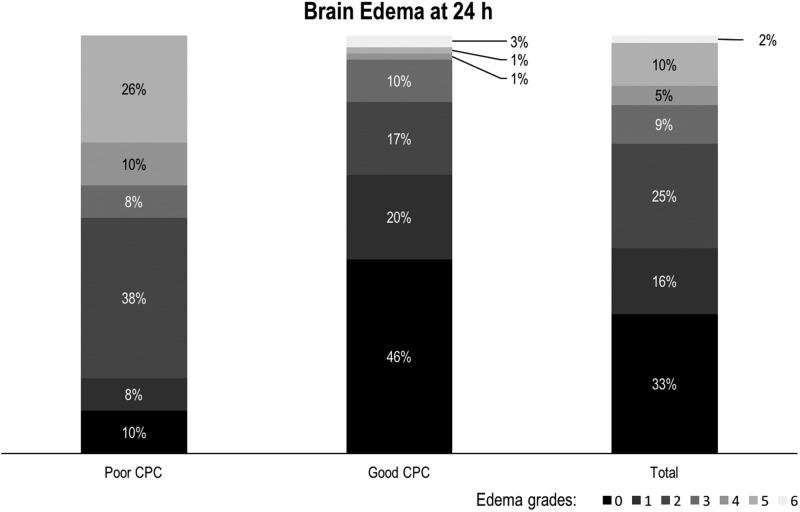
The CPC scoring was performed with TCCD in 55 cases, CTA in 34 and DSA in 19. Seventeen patients had both CTA and DSA. The CPC status was graded as good in 69 patients (63.9%). Two cases (1.9%) showed maximum edema (grade 6) at 24 hours and developed malignant MCA infarction, requiring decompressive craniectomy (Figure 2). We found significant (score greater than 2) CE at 24 hours in a total of 28 patients (25.9%), while 53 (49%) had only minor edema (grades 0 or 1).
Figure 2.
Brain edema scores at 24 hours.
Univariate analysis (see Table 1) showed a significant correlation between poor CPC and greater edema at 24 hours (p < 0.001). Patients with poor CPC displayed a greater proportion of significant CE (43.6% vs 15.9%).
Patients with hemorrhagic conversion (PH2) showed a greater median CE score (2 IQR 2 vs 4 IQR 2.5; p = 0.002). Lower ASPECTS was associated with greater edema (Spearman’s correlation, p < 0.001), while complete recanalization up to six hours correlated to lower edema score at 24 hours (Mann–Whitney, p < 0.001).
In our study, there was a good level of interobserver agreement in the evaluation of CPC status (Cohen’s Kappa coefficient: 0.87; 95% confidence interval (CI) 0.78–0.90; p < 0.001) and cerebral edema at 24 hours (Cohen’s Kappa coefficient: 0.83; 95% CI 0.75–0.86; p < 0.001), and excellent level of agreement for hemorrhagic transformation (Cohen’s Kappa coefficient: 0.94; 95% CI 0.88–0.97; p < 0.001).
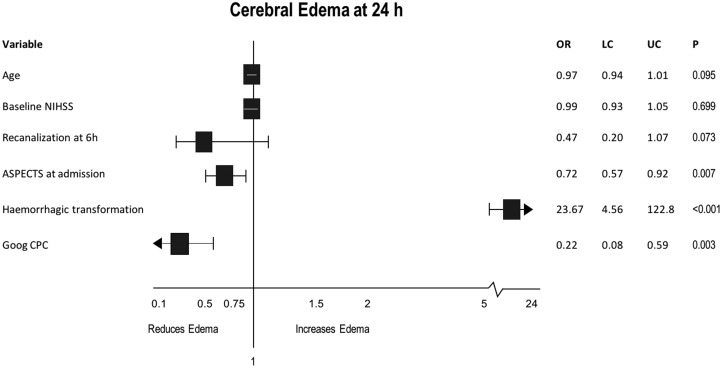
Multivariable analysis (Figure 3) adjusted by age, NIHSS at admission and recanalization at six hours showed that poor CPC status (odds ratio (OR) 0.22, 95% CI 0.08–0.59, p = 0.003), hemorrhagic transformation (OR 23.67, 95% CI 4.56–122.8, p < 0.001) and ASPECTS at admission (OR 0.72, 95% CI 0.57–0.92, p = 0.007) were independent predictors of CE at 24 hours.
Figure 3.
Multivariable analysis for predictors of cerebral edema at 24 hours.
Discussion
In our study, CPC detected at the baseline TCCD, CTA and/or DSA was independently associated with the grade of radiological CE at 24 hours. We showed that this association remained even after adjusting for the occurrence of hemorrhagic conversion and initial lesion extension.
The development of edema is part of the pathophysiological cascade of ischemic injury to any tissue.21 An understanding of the pathophysiology of ischemic brain edema is a prerequisite to applying effective treatments.8,21 While cytotoxic edema is an early change reflecting irreversible cellular injury that requires absence of blood flow and is not accompanied by tissue swelling, vasogenic edema and hemorrhagic conversion, however, require active blood flow and therefore can occur only after postischemic reperfusion.7,8 Thus, tissue swelling develops from the periphery to the center of the ischemic lesion, and requires reperfusion.
The effects of CE can be profound, leading to increased intracranial pressure, brain herniation, and consequent clinical deterioration and poor outcome.1,21 As there is no effective medical treatment, preventing severe edema and implementing early treatment may be a more effective approach.1,9 Therefore, efforts have been made to understand the factors that influence the development of cerebral edema after ischemic stroke, and to establish predictors of malignant MCA infarction.4–6 Several risk factors for the development of edema have been identified either using clinical and laboratorial data or through neuroimaging parameters.4–6 CPC assessment could improve the accuracy of previously proposed predictors and could emerge as an early predictor of brain edema.
Our results indicate that poor CPC is independently associated with greater CE at 24 hours in ischemic stroke patients who undergo reperfusion treatment, corroborating data published previously.22 Moreover, CPC influenced the final infarct volume (ASPECTS at 24 hours), which is known to be in direct relation with the amount of CE.22,23 Our results are supported by previous studies that showed that CPC influences the extent of penumbral tissue and improves clinical and imaging outcomes independently of recanalization,13–16,24,25 and that poor CPC is related to increased risk of MMI.22 However, our study further demonstrates an association of CPC status and the degree of radiological CE, establishing the former as an independent factor in the pathophysiology of postischemic edema.
While complete recanalization at six hours was related to CE in the univariate analysis, it did not constitute an independent predictor of CE. This can be explained by the action of intervenient factors, namely the infarct volume and hemorrhagic transformation, which are dependent on the reestablishment of blood flow.26 Flores et al.22 also reported no significant association between recanalization and MMI evolution, concluding that an evaluation after 24 hours by TCCD may have lost relevance on ischemic tissue outcome. In our study recanalization was assessed earlier (six hours of stroke evolution) but we had a lower percentage of endovascular reperfusion treatment (26.9% vs 64.6%), which is reported to be associated with lower risk of MMI, suggesting a protective effect of early recanalization.22 It has been suggested that there is an increased risk of brain edema following spontaneous or thrombolytic recanalization of the MCA, which may be explained by the active blood flow and the formation of vasogenic edema.7,21,27,28 In contrast, massive ischemic brain edema occurs more often when there is no collateral flow or after unsuccessful thrombolysis.2,21,29,30 Strbian et al. reported post-thrombolysis edema to be independently associated with worse three-month outcome.1
The role of veins in the clinical outcome after stroke is increasingly recognized.31 These vessels can induce a vicious cycle of outflow obstruction and growing intracranial pressure, determined by a combination of venous compression by CE, smooth muscle and endothelium phenotype changes driven by ischemia, dural sinus hypoplasia and use of dihydropyridines.32 We did not study the status of venous outflow, but in the context of ischemic stroke it is believed that arterial recanalization without adequate venous outflow would ultimately increase venous pressure and contribute to CE.31 It is plausible that adequate CPC might reduce venous stasis and outflow obstruction by maintaining minimal perfusion to the microcirculation.
Our data could have potential practical implications in clinical surveillance, as CPC assessment could allow early identification of candidates for neurointensive care unit admission, tighter vigilance and additional imaging controls, as well as consideration of early preventive medical treatment and early decompressive craniectomy, all of which can influence the prognosis of these patients.3,9–12,33 However, the clinical outcomes related to the various degrees of radiological CE in our study were not measured and further studies should focus on establishing this relation. Previous work22 has shown significant association between poor CPC and development of MMI, but the impact of minor degrees of edema remains unclear. Minor edema is probably an essential part of the healing process, without relevant clinical repercussions.
We analyzed a homogeneous population that nonetheless translates easily to real practice. Moreover, recanalization rate was assessed early, at six hours after the onset of stroke; this provides confidence that the edema subsequently observed is not caused by growth of the infarct area.
There are some limitations to our study, however, that need to be taken under consideration. Firstly, edema assessment at 24 hours is not an accurate depiction of maximum edema after stroke; we tried to constrain this limitation by grading the edema associated with stroke using a scale20 instead of a simple dichotomic outcome (i.e. presence of malignant edema). Secondly, we used heterogeneous methods to determine CPC status and early recanalization; both are limitations that demand caution when trying to extrapolate our results to other populations. Categorizing CPC status as either “good” or “poor” is also most likely an oversimplification. Further studies should try to use only one method of CPC evaluation, in our opinion preferentially DSA based, and additional grades of CPC status. Thirdly, we used a monophasic acquisition of CTA, which is dependent on timing of the arterial phase; multiphasic CTA would provide a more accurate estimate of CPC status, but inevitably involves higher radiation exposure.
In conclusion, our study shows that poor CPC is independently associated with greater radiological CE 24 hours after ischemic stroke treated with reperfusion therapy, and could potentially be incorporated as an imaging predictor of clinically relevant edema.
Supplementary Material
Acknowledgments
Author contributions include the following: Orlando Galego: data collection, analysis and interpretation, literature search and manuscript writing; Joana Jesus-Ribeiro: data collection, analysis and interpretation, literature search and manuscript writing; Mariana Baptista: data collection, analysis and interpretation; João Sargento-Freitas: study design, data analysis and manuscript review; Ana Inês Martins: data collection; Fernando Silva: literature search and data review; Gustavo Cordeiro Santos: literature search and data review; Luís Cunha: literature search and manuscript review; César Nunes: data collection; and Egídio Machado: study design, data interpretation and manuscript review.
The study protocol was submitted to and approved by the institution’s medical and research ethics committee. All patients provided informed consent for participation on this study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Strbian D, Meretoja A, Putaala J, et al. Cerebral edema in acute ischemic stroke patients treated with intravenous thrombolysis. Int J Stroke 2013; 8: 529–534. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Schwab S, Horn M, et al. ‘Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol 1996; 53: 309–315. [DOI] [PubMed] [Google Scholar]
- 3.Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke 2007; 38: 3084–3094. [DOI] [PubMed] [Google Scholar]
- 4.Thomalla GJ, Kucinski T, Schoder V, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke 2003; 34: 1892–1899. [DOI] [PubMed] [Google Scholar]
- 5.Kasner SE, Demchuk AM, Berrouschot J, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke 2001; 32: 2117–2123. [DOI] [PubMed] [Google Scholar]
- 6.Oppenheim C, Samson Y, Manai R, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke 2000; 31: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 7.Simard JM, Kent TA, Chen M, et al. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol 2007; 6: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahle KT, Simard JM, Staley KJ, et al. Molecular mechanisms of ischemic cerebral edema: Role of electroneutral ion transport. Physiology (Bethesda) 2009; 24: 257–265. [DOI] [PubMed] [Google Scholar]
- 9.Demchuk AM, Krieger DW. Mass effect with cerebral infarction. Curr Treat Options Neurol 1999; 1: 189–199. [DOI] [PubMed] [Google Scholar]
- 10.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomised controlled trials. Lancet Neurol 2007; 6: 215–222. [DOI] [PubMed] [Google Scholar]
- 11.Schwab S, Steiner T, Aschoff A, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke 1998; 29: 1888–1893. [DOI] [PubMed] [Google Scholar]
- 12.Maciel CB, Sheth KN. Malignant MCA stroke: An update on surgical decompression and future directions. Curr Atheroscler Rep 2015; 17: 40. [DOI] [PubMed] [Google Scholar]
- 13.Liebeskind DS, Tomsick TA, Foster LD, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke 2014; 45: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christoforidis GA, Karakasis C, Mohammad Y, et al. Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: The role of pial collateral formation. AJNR Am J Neuroradiol 2009; 30: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bang OY, Saver JL, Kim SJ, et al. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke 2011; 42: 2235–2239. [DOI] [PubMed] [Google Scholar]
- 16.Mohammad YM, Christoforidis GA, Bourekas EC, et al. Qureshi grading scheme predicts subsequent volume of brain infarction following intra-arterial thrombolysis in patients with acute anterior circulation ischemic stroke. J Neuroimaging 2008; 18: 262–267. [DOI] [PubMed] [Google Scholar]
- 17.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: Relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999; 30: 2280–2284. [DOI] [PubMed] [Google Scholar]
- 18.Miteff F, Levi CR, Bateman GA, et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain 2009; 132: 2231–2238. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Sin DS, Park HY, et al. Relationship between flow diversion on transcranial Doppler sonography and leptomeningeal collateral circulation in patients with middle cerebral artery occlusive disorder. J Neuroimaging 2009; 19: 23–26. [DOI] [PubMed] [Google Scholar]
- 20.Wardlaw JM, Sellar R. A simple practical classification of cerebral infarcts on CT and its interobserver reliability. AJNR Am J Neuroradiol 1994; 15: 1933–1939. [PMC free article] [PubMed] [Google Scholar]
- 21.Ayata C, Ropper AH. Ischaemic brain oedema. J Clin Neurosci 2002; 9: 113–124. [DOI] [PubMed] [Google Scholar]
- 22.Flores A, Rubiera M, Ribó M, et al. Poor collateral circulation assessed by multiphase computed tomographic angiography predicts malignant middle cerebral artery evolution after reperfusion therapies. Stroke 2015; 46: 3149–3153. [DOI] [PubMed] [Google Scholar]
- 23.Ribó M, Flores A, Rubiera M, et al. Extending the time window for endovascular procedures according to collateral pial circulation. Stroke 2011; 42: 3465–3469. [DOI] [PubMed] [Google Scholar]
- 24.Cho TH, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015; 46: 1582–1589. [DOI] [PubMed] [Google Scholar]
- 25.Campbell BC, Christensen S, Tress BM, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 2013; 33: 1168–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina A, Montaner J, Abilleira S, et al. Timing of spontaneous recanalization and risk of hemorrhagic transformation in acute cardioembolic stroke. Stroke 2001; 32: 1079–1084. [DOI] [PubMed] [Google Scholar]
- 27.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–1025. [PubMed] [Google Scholar]
- 28.Koudstaal PJ, Stibbe J, Vermeulen M. Fatal ischaemic brain oedema after early thrombolysis with tissue plasminogen activator in acute stroke. BMJ 1988; 297: 1571–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Kummer R, Holle R, Rosin L, et al. Does arterial recanalization improve outcome in carotid territory stroke? Stroke 1995; 26: 581–587. [DOI] [PubMed] [Google Scholar]
- 30.Rudolf J, Grond M, Stenzel C, et al. Incidence of space-occupying brain edema following systemic thrombolysis of acute supratentorial ischemia. Cerebrovasc Dis 1998; 8: 166–171. [DOI] [PubMed] [Google Scholar]
- 31.Zhang JH, Obenaus A, Liebeskind DS, et al. Recanalization, reperfusion, and recirculation in stroke. J Cereb Blood Flow Metab 2017; 37: 3818–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Chen Y, Xu L, et al. Venous system in acute brain injury: Mechanisms of pathophysiological change and function. Exp Neurol 2015; 272: 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang MH, Lin HY, Fu J, et al. Decompressive hemicraniectomy in patients with malignant middle cerebral artery infarction: A systematic review and meta-analysis. Surgeon 2015; 13: 230–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.