Abstract
Aim
To evaluate voxelwise computed diffusion-weighted imaging (vcDWI) for the detection of cytotoxic oedema in brain imaging and to quantify the benefit of lesion contrast in comparison to standard b = 1000 s/mm2 by the example of acute ischaemic stroke.
Materials and methods
A retrospective evaluation of 66 patients (63 ± 15.9 years) suspected for acute ischaemic stroke who received diffusion-weighted magnetic resonance imaging and fluid-attenuated inversion recovery sequence. A neuroradiologist evaluated all examinations for acute ischaemic stroke based on diffusion-weighted imaging, the apparent diffusion coefficient and fluid-attenuated inversion recovery (reference standard) and 6 weeks later the vcDWI in a randomised manner. Time of analysis was noted. Signal intensities were acquired in lesions, in healthy tissue as well as in the cerebrospinal fluid. Contrast ratios and coefficients of variation were computed.
Results
A total of 218 lesions was found in 46/66 patients. vcDWI identified all patients and lesions correctly. The median evaluation time was 36 seconds (4–126 s) for the vcDWI and 44 seconds (9–186 s; P < 0.001) for the diffusion-weighted imaging/apparent diffusion coefficient reading. The contrast ratio in vcDWI (mean value 2.57, range 1.73–4.11) was higher than in b = 1000 s/mm2 (2.33, 0.83–3.85, P = 0.03) and the apparent diffusion coefficient map (1.83, 1.00–3.00, P < 0.001), respectively. Coefficients of variation in lesions and tissue did not differ significantly between vcDWI and b = 1000 s/mm2 (P = 0.81/P = 0.26). The signal intensity of cerebrospinal fluid was lower in vcDWI than in b = 1000 mm2/s (0.08 and 34.8, P < 0.001).
Conclusion
It could be shown that vcDWI has the potential to accelerate the detection of diffusion-restricted lesions in neuroimaging by improving the contrast ratios and reducing the T2 shine-through effect in comparison to standard diffusion-weighted imaging in brain imaging.
Keywords: DWI, vcDWI, acute ischaemic stroke, neuroimaging
Introduction
Diffusion-weighted magnetic resonance imaging (MRI) sensitively detects areas of impaired diffusivity which can be used for the diagnosis of a broad spectrum of neurological diseases such as, for example, ischaemia or infectious diseases.1 As diffusion-weighted imaging (DWI) is technically based on a standard T2-weighted spin-echo sequence the signal intensity does not only depend on the diffusion restriction of the investigated tissue but also on its T2 relaxation time (T2 shine-through effect).2 To differentiate a ‘true’ diffusion restriction caused by, for example, cytotoxic oedema from a T2 shine-through effect in gliosis, the additional evalution of the corresponding apparent diffusion coefficient (ADC) map is mandatory. In individual cases, however, a combined assessment of diffusion-weighted images and ADC maps can be time-consuming, e.g. for small or linear cortical lesions.3 Gatidis et al.4 recently proposed an approach to reduce the T2 shine-through effect and improve the visibility of diffusion-restricted lesions in whole-body DWI: voxelwise computed diffusion-weighted imaging (vcDWI). For brain imaging, the image characteristics of vcDWI have not yet been evaluated. One of the most frequent applications of DWI in neuroimaging is the diagnosis of acute ischaemic stroke (AIS), in which a rapid and secure diagnosis as well as the exact definition of localisation and the extent of acute ischaemic lesions are mandatory for the further management.5
The aim of this pilot study was to evaluate the image characteristics of vcDWI and the reliability in detecting diffusion-restricted lesions in comparison to standard DWI in neuroimaging by the example of AIS.
Materials and methods
Technique
vcDWI is a post-processing technique computing the presented signal intensity (SI) of each voxel (x) separately, based on two acquired b values, b0 and b1, and the respective ADC value with the following equation (1):4
| (1) |
with SI0 being the signal intensity of the respective voxel measured at b0. Therefore, voxels with a low ADC are presented with a signal intensity of low b values (resulting in increased signal intensities in vcDWI) and vice versa (Figure 1).
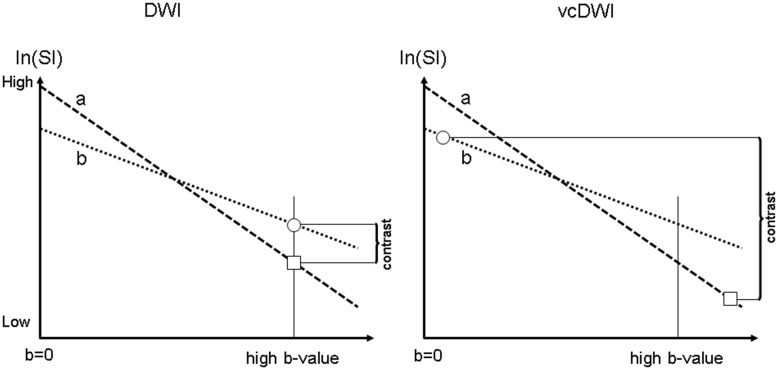
Figure 1.
Scheme of a simplified linear signal decay model of conventional diffusion-weighted imaging (DWI) (left hand side) and the technique of voxelwise computed diffusion-weighted imaging (vcDWI) in the computation of the presented signal intensity (right hand side) of two tissue types with high (a) and low (b) apparent diffusion coefficient (ADC) values. In high b value images in DWI (e.g. b = 1000 s/mm2), tissue (b) provides higher signal intensity (SI) than tissue (a). vcDWI computes the SI of each voxel based on the respective ADC value. Therefore, tissue (b) is presented with the SI of a lower b value while tissue (a) is presented with the SI of a higher b value. This reduces the T2 shine-through of tissue (a) and increases the contrast ratio (CR) between tissue (a) and tissue (b).4
Patient cohort and image analysis
We retrospectively analysed 66 patients (63 ± 15.87 years, 32 women) consecutively examined from January 2016 until July 2017. Inclusion criteria were a clinical-neurological suspected stroke and a MRI at admission (time window <12 hours) that excluded acute intracranial haemorrhage. MRI examinations were performed either in a 3 T (n = 35) or a 1.5 T (n = 31) magnetic resonance scanner (Siemens Magnetom Skyra/Aera, Siemens Healthineers, Erlangen, Germany) with a DWI protocol containing at least the two b values of b = 0 and b = 1000 s/mm2 and a time inversion recovery sequence (fluid-attenuated inversion recovery; FLAIR). Further parameters were: acquisition matrix 128 × 128 (1.5 T) or 224 × 224 (3 T), slice thickness 4 mm, number of averages 1. The delay from symptom onset until MRI was less than 72 hours. The local ethics committee waived informed consent for the retrospective pseudomised scientific evaluation. vcDWI was computed for all examinations based on the b = 0 mm2/s and the b = 1000 s/mm2 using MATLAB (MathWorks Inc., Natick, MA, USA).
A radiologist with 6 years of experience in neuroradiological MRI (GB) evaluated all examinations based on the DWI side by side with the ADC map and the FLAIR sequence for AIS (reference standard). Six weeks later, the same neuroradiologist evaluated the vcDWI of all patients. The time from the start of the analysis until the complete documentation was noted. In vcDWI, lesions were rated positive for AIS if the signal was increased as compared to the surrounding tissue by region of interest (ROI) analysis. The evaluations were performed in a randomised manner. Maximum lesion size in the axial plane and the lesion’s location were recorded.
Based on the first reading, freehand two-dimensional ROIs were drawn: (a) in AIS lesions of 1 cm or greater in diameter (maximum one lesion per patient); (b) in healthy brain tissue of the same kind (e.g. white matter) in the same slice position as the AIS lesion; and (c) in the cerebrospinal fluid (CSF) of the right ventricle. The mean signal intensity and the standard deviation (SD) within each ROI were acquired. The ROIs were drawn in the b = 1000 s/mm2 image and copied to the vcDWI image and the ADC map using the software Imagine (Imagine 2.0; Matlab Central File Exchange, MathWorks Inc., Natick, MA, USA). Care was taken to avoid lesion borders.
CRs of vcDWI and b = 1000 s/mm2 images were defined as follows: mean SI (lesion)/mean SI (tissue). For the ADC map, the CRADC was defined as mean SI (tissue)/mean SI (lesion).
The coefficient of variation (COV) in lesions and in tissue was defined as: SD/(mean SI) within a ROI.
Statistical analysis
Statistical analyses were performed with SPSS (SPSS Statistics 19, IBM, Armonk, NY, USA). The results from the reading were compared using a receiver operating characteristic (ROC) with area under the curve (AUC) analysis. CR and COV of vcDWI, DWI and ADC were statistically compared using analysis of variance with Bonferroni post hoc correction and the Wilcoxon test for comparison of non-parametric values. With the same test, the CR and the COV in lesions and tissues of vcDWI, ADC and b = 1000 were compared between the different scanners (1.5 T and 3 T). Moreover, the differences in the percentage of CR between vcDWI and DWI as well as between vcDWI and ADC, respectively, were computed as follows: (CR (vcDWI) – CR (ADC or DWI))/CR (ADC or DWI)*100 and statistically compared between the two scanners. A P value of less than 0.05 was considered as significant.
Results
Overall, 218 AIS lesions were found in 46/66 patients (n = 15 with the 3 T scanner, n = 31 with the 1.5 T scanner) with the reference standard (median, three lesions per affected patient; range, one to 23 lesions). Overall, 141 lesions (64.7%) were located in the subcortical white matter, 36 (16.5%) were located cortically and in 41 cases (18.8%), the cortex as well as the underlying white matter were affected. Forty-nine lesions were located in the frontal lobe (mean size 12.2 ± 12.5 mm), 42 in the occipital lobe (12.3 ± 17.5 mm), 34 cerebellar (18.8 ± 17.9 mm), 30 parietal (14.1 ± 13.3 mm), 29 in the basal ganglia (14.0 ± 13.4 mm), 23 in the temporal lobe (21.2 ± 27.4 mm), seven in the pons (9.7 ± 7.3 mm) and two mesencephal (each 5.0 mm). In two cases, the whole MCA territory was affected. All patients and lesions were identified correctly with vcDWI. Lesion sizes did not differ significantly between vcDWI and the ADC map (P = 0.6). No false positive or false negative lesions were found. The corresponding AUC was 1.0. Examples are given in Figure 2. The median evaluation time was 36 seconds for the vcDWI analysis (range 4–126 s) and 44 seconds for the DWI/ADC reading (range 9–186 s; P < 0.001). In 46 patients, lesions with a diameter of 1 cm or greater could be identified. The lesion CRs were significantly higher than in the b = 1000 s/mm2 images (P = 0.03) and the ADC map (P < 0.001), see Figure 3. COV in lesions and tissue did not differ significantly between vcDWI and the b = 1000 s/mm2 image (P = 0.81 and P = 0.26, respectively). CSF SI was significantly lower in vcDWI than in the b = 1000 s/mm2 image (mean values 0.08 and 34.8, P < 0.001).
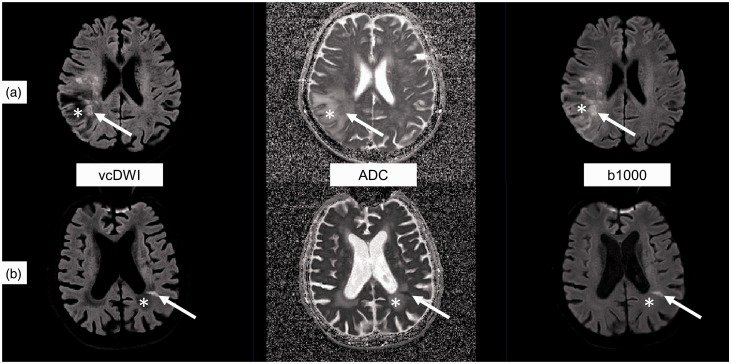
Figure 2.
Two examples of patients (A and B) with acute ischaemic stroke (white arrow) in areas of gliosis (*) in the right or left parietal lobe, respectively, in voxelwise computed diffusion-weighted imaging (vcDWI), the apparent diffusion coefficient map (ADC) and the b = 1000 s/mm2 image (b1000). The T2 shine-through effect of gliosis is suppressed by vcDWI which leads to an improved contrast ratio as compared to the ADC map or to the b = 1000 s/mm2 image.
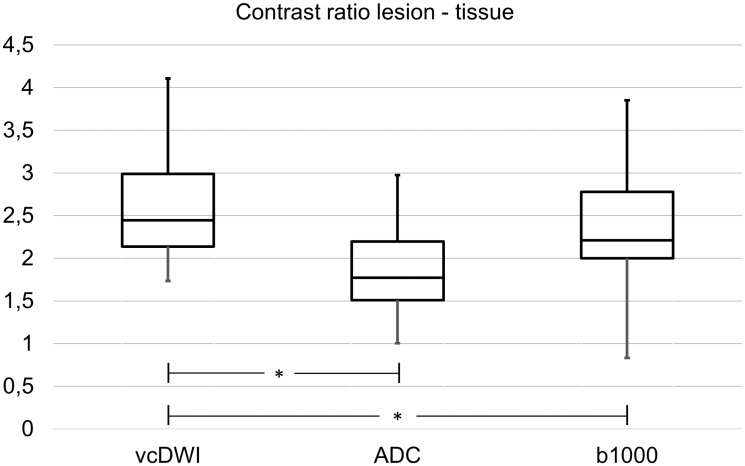
Figure 3.
Box-plot diagram of the contrast ratios (CRs) of ischaemic lesions in voxelwise computed diffusion-weighted imaging (vcDWI), the apparent diffusion coefficient maps (ADC) and the b = 1000 s/mm2 images (b1000) including median, the first and third quartile and minimum and maximum values. The lesion CR in vcDWI was significantly higher (P < 0.05, marked with *) than the lesion CRs in b = 1000 s/mm2 images and in the ADC map.
Compared to the 1.5 T scanner, higher CRs were found in vcDWI at 3 T (2.8 vs. 2.4, P = 0.02) while CRs in ADC and DWI did not differ significantly (P > 0.05). However, the relative difference of CR in percentage between vcDWI and ADC as well as between vcDWI and DWI did not differ significantly between 3 T and 1.5 T (mean values 62.4% vs. 39.7% and 20.3% vs. 10.8%, P > 0.05). COV was significantly higher in 3 T in tissues and lesions as compared to 1.5 T in vcDWI, ADC and DWI, respectively.
Discussion
In this study, we could show that vcDWI reliably detects lesions of restricted diffusivity and can provide an increased lesion CR in brain imaging as compared to standard b = 1000 s/mm2 images.
The vcDWI approach has recently been introduced by Gatidis et al.4 for body imaging. Nevertheless, there is still the need to evaluate its potential in brain imaging. With the aim of improving lesion detection in oncological body imaging, Blackledge et al. introduced an approach to compute high b value images (cDWI) based on lower acquired b values images.6 However, for brain imaging, there are only few studies evaluating this technique.7 The computations of cDWI, vcDWI and the ADC map, respectively, are based on a mono-exponential decay which assumes that the distribution of diffusion-driven displacements obeys Gaussian law. However, it could be shown that this simplified model does not reflect the true diffusion properties of brain tissue and the content of diffusion-weighted images is affected by several parameters, as summarised by Le Bihan.8 Nevertheless, despite this limitation, the mono-exponential model has been shown to be useful and is commonly applied in daily routine. In comparison to cDWI, vcDWI further increases the lesion contrast by computing the presented b value of each voxel separately in dependence on its ADC value. This might especially be of value in lesions of restricted diffusivity located in regions with a high T2 signal, as shown in Figure 2.
Our standard of reference for the detection of AIS lesions was set by a reading of the b = 1000 s/mm2 image, the ADC map and the FLAIR image. The reading time of solely vcDWI was less time consuming and all AIS lesions were identified correctly. vcDWI includes the information of DWI and the ADC map in one image and, of course, one can expect that the vcDWI reading is faster than the reading of DWI, ADC map and FLAIR images side by side. Nevertheless, as our analysis did not reveal any information loss, this information is still important: vcDWI reading is faster and not worse as compared to a standard DWI/ADC reading. CR of vcDWI was significantly higher as compared to the b = 1000 s/mm2 images or the ADC map, respectively. No false positive lesions were found. Morever, the lesion size could reliably be assessed in vcDWI. The b = 1000 s/mm2 image is commonly applied and was therefore used for the computation of vcDWI in this study.9 vcDWI is aimed to improve the visibility of lesions of restricted diffusivity and reduce the T2 shine-through effect. To demonstrate the reduction of the T2 shine-through effect, we acquired the SI in the CSF of the right ventricle which was significantly lower in vcDWI as compared to the b = 1000 s/mm2 images. This can improve the visibility of AIS lesions, e.g. when located in regions of gliosis as demonstrated in Figure 2. The COV (as an indicator of image noise) did not show a significant difference between the vcDWI and the b = 1000 s/mm2 image. We performed an analysis of AIS for this first introduction of vcDWI in brain imaging as this is one of the major areas of application for the detection of diffusion-restricted lesions in neuroradiology. Nevertheless, also in further areas of application such as in patients with infectious diseases or post-ictal changes, vcDWI might be beneficial. In those cases, vcDWI could improve the conspicuity of diffusion-restricted cortical lesions, as ADC hypointense signals in the cortex can be difficult to detect in individual cases.10
It should be noted that (a) vcDWI is a post-processing tool which does not influence the acquisition time (but the reading time), and (b) in contrast to the ADC map, does not provide quantitative values. Therefore, although the reading time could be accelerated by vcDWI in specific clinical questions (such as AIS) as the additional evaluation of the ADC map might be omitted, vcDWI can not replace the ADC map in general.
The retrospective study design is a limitation of our study as it only enables a first demonstration of possible advantages but does not allow for a general recommendation of the usage of vcDWI in daily routine. Moreover, we evaluated the data from two scanners with different field strengths (1.5 and 3.0 T). However, the relative improvement of CR in the percentage of vcDWI as compared to the b = 1000 image or the ADC map, respectively, did not differ significantly between the scanners which indicates that vcDWI can be beneficial at both field strengths. The higher COV (as an indicator of image noise) in tissues and lesions in vcDWI, DWI and the ADC map at 3 T as compared to 1.5 T can be explained by the higher magnetic field strength11 on the one hand and the higher acquisition matrix on the other hand.12
Conclusion
In conclusion, in this pilot study it could be shown that vcDWI has the potential to improve the CR of diffusion-restricted lesions and reduce the T2 shine-through effect in comparison to standard DWI in brain imaging. Further studies with larger patient cohorts are needed to evaluate a potential diagnostic benefit in clinical routine.
Funding
This research received no specific grant from any finding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Renard D, Nerrant E, Lechiche C. DWI and FLAIR imaging in herpes simplex encephalitis: a comparative and topographical analysis. J Neurol 2015; 262: 2101–2105. [DOI] [PubMed] [Google Scholar]
- 2.Roberts TP, Rowley HA. Diffusion weighted magnetic resonance imaging in stroke. Eur J Radiol 2003; 45: 185–194. [DOI] [PubMed] [Google Scholar]
- 3.Inoue M, Mlynash M, Christensen S, et al. Early diffusion-weighted imaging reversal after endovascular reperfusion is typically transient in patients imaged 3 to 6 hours after onset. Stroke 2014; 45: 1024–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatidis S, Schmidt H, Martirosian P, et al. Apparent diffusion coefficient-dependent voxelwise computed diffusion-weighted imaging: an approach for improving SNR and reducing T2 shine-through effects. J Magn Reson Imaging 2016; 43: 824–832. [DOI] [PubMed] [Google Scholar]
- 5.Sanelli PC, Sykes JB, Ford AL, et al. Imaging and treatment of patients with acute stroke: an evidence-based review. AJNR Am J Neuroradiol 2014; 35: 1045–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackledge MD, Leach MO, Collins DJ, et al. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 2011; 261: 573–581. [DOI] [PubMed] [Google Scholar]
- 7.Ogura A, Koyama D, Hayashi N, et al. Optimal b values for generation of computed high b-value DW images. AJR Am J Roentgenol 2016; 206: 713–718. [DOI] [PubMed] [Google Scholar]
- 8.Le Bihan D. Apparent diffusion coefficient and beyond: what diffusion MR imaging can tell us about tissue structure. Radiology 2013; 268: 318–322. [DOI] [PubMed] [Google Scholar]
- 9.Meyer JR, Gutierrez A, Mock B, et al. High-b-value diffusion-weighted MR imaging of suspected brain infarction. AJNR Am J Neuroradiol 2000; 21: 1821–1829. [PMC free article] [PubMed] [Google Scholar]
- 10.Vitali P, Maccagnano E, Caverzasi E, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology 2011; 76: 1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohmann R, Speck O, Scheffler K. Signal-to-noise ratio and MR tissue parameters in human brain imaging at 3, 7, and 9.4 tesla using current receive coil arrays. Magn Reson Med 2016; 75: 801–809. [DOI] [PubMed] [Google Scholar]
- 12.Constable RT, Henkelman RM. Contrast, resolution, and detectability in MR imaging. J Comput Assist Tomogr 1991; 15: 297–303. [DOI] [PubMed] [Google Scholar]