Summary
Pregnancy infections with Zika virus are associated with a spectrum of fetal brain injuries beyond microcephaly. Non-microcephalic children exposed to Zika virus in utero or early life should undergo neurodevelopmental testing to identify deficits and allow for early intervention. Additionally, long-term monitoring for higher order neurocognitive deficits should be implemented.
Keywords: Congenital Zika syndrome, Zika virus, flavivirus, pregnancy, fetus, neurocognitive testing
The Zika virus (ZIKV) epidemic in the Americas in 2014–2016 became linked to an unexpected surge in congenital microcephaly cases, and was declared a global public health emergency by the World Health Organization [1], ZIKV is a mosquito-transmitted flavivirus that can infect a variety of cells in the fetal brain, including highly vulnerable neural stem and progenitor cells, as well as neurons, astrocytes, and microglia [2], Congenital Zika Syndrome (CZS) describes infants with a severe fetal brain injury including microcephaly, subcortical calcifications, hypertonia, congenital contractures and ocular injuryi. Several studies estimate the occurrence of ZIKV-associated birth defects to be 5–13%, depending on when the infection occurs during pregnancy [3, 4], Interestingly, the isolated diagnosis of microcephaly does not necessarily capture infants with abnormal brain structure by imaging [5, 6], By 1 year of age, some infants exposed to ZIKV in utero with a normal head size at birth have been diagnosed with an array of major neurological defects including postnatal microcephaly (brain growth failure), ocular injury, hemiparesis, seizures/epilepsy and hydrocephalus. The CDC have recommended that healthcare providers “remain alert for any new findings of possible congenital Zika virus infection” in infants without clinical findings of CZSi. We argue that evidence is accumulating to support a stronger recommendation for referral to a developmental specialist even for non-microcephalic infants exposed to ZIKV.
Despite the intense scientific investigation of congenital microcephaly, we are only beginning to understand the greater spectrum of ZIKV-associated injury and neurodevelopmental outcomes for non-microcephalic fetuses. Congenital exposure to ZIKV was recently found to induce a spectrum of subtle and “silent” injuries to the fetal brain that are challenging to detect prenatally, but would result in poor neurodevelopmental outcomes [7], In a pregnant nonhuman primate model, exposure to ZIKV via maternal subcutaneous inoculation led to significant loss of neural stem cell activity and output in both the late fetal cortical subventricular zone and hippocampal subgranular zone; these sites are specialized neurogenic niches that continue to undergo active neurogenesis during late fetal life [8], Injury to hippocampal neural stem and progenitor cells is particularly ominous, because these cells are critical for forming new memories, learning, and maintaining one’s mental health [9], Indeed, abnormalities in hippocampal neurogenesis underlie the progression of severe neurocognitive and psychological behaviors, including spatial pattern recognition, learning and memory, emotional and behavioral regulation, seizures, and later onset schizophrenia, depression, and neurodegenerative disorders such as Alzheimer’s disease [8, 10–12], Consistent with our findings, cell death and loss of neural stem cells in late neurogenic niches was observed in a ZIKV-exposed adult mouse model [13], Further, a recent study in young nonhuman primates correlated a postnatal ZIKV infection with hippocampal growth arrest, deficits in socioemotional processing, and abnormal functional connectivity between the hippocampus and the amygdala [14]. This evidence strongly suggests that neurodevelopmental outcomes should be studied through childhood and adolescence for all infants exposed to ZIKV in utero, regardless of head size at birth, and for children exposed in early life.
Understanding the impact of fetal or early postnatal ZIKV exposure to these specialized and vulnerable hippocampal neural stem and progenitor cells is crucial, because the resulting deficits may not manifest until higher order processing and cognitive behaviors emerge in childhood and adolescence. We recommend frequent assessment and monitoring of ZIKV-exposed children throughout the first 24 months of agei,ii,iii. Because motor, language, and cognitive development are highly interrelated in infancy through early childhood, assessment of multiple domains of development (motor, language/communication, cognition/learning, social-emotional, and adaptive) is needed to fully determine the impact of a fetal brain injury. In addition, magnetoencephalography is a viable measure of neural activity in infants and has the advantages of being quiet, allowing patient movement, and not requiring sedation. Early detection is essential for accessing early interventions, which promote better outcomes in children with neurodevelopmental disabilities. Assessment instruments at later ages should include more specific, nuanced measures of the developmental domains and individualized as indicated by previous evaluation results. Identification of damage to late-onset, higher-order neurocognitive functions requires continued monitoring and evaluation even into adolescence for assessment of mental health, abstract reasoning and language, and independent functioning. From both a quality of life and societal cost perspective, continued monitoring of non-microcephalic infants exposed to ZIKV is a public health imperative and essential for directing care.
The challenge of implementing developmental assessments would be formidable to include all children in the Americas exposed to ZIKV either in utero or in early life. The cumulative number of pregnancies exposed to ZIKV in the Americas between 2014 and 2017 is likely in the hundreds of thousands; more precise estimates are not possible to obtain. Currently, the reporting of cumulative cases of CZS and “suspected” ZIKV infections is strikingly inconsistent across countries with known local transmission during the recent ZIKV epidemic in the Americas (Fig. 1). For example, Trinidad and Tobago, a small island nation with a population of ~1.4 million people, has nearly the same number of ZIKV-associated birth defects as the entire country of Mexico (~130 million people). If we extrapolate from data obtained in the U.S. and French Territories, we could multiply known cases with a birth defect by a factor of 12–20 to determine the total number of pregnancies exposed to ZIKV [3, 4], Although the cumulative number of cases in the Americas with birth defects attributed to ZIKA is only ~4,000, this is likely a significant underestimate and would translate to at least ~80,000 exposed pregnancies. Even this estimate would not include children exposed to ZIKV in early life. In many ways, the ZIKV epidemic served to highlight what we already knew - the public health surveillance of pregnancy and neonatal outcomes in the Americas is completely inadequate. The U.S. Pregnancy Zika Registry provides a model for moving forward to prepare for detection of adverse maternal-fetal outcomes with the next viral epidemici.
Figure 1.
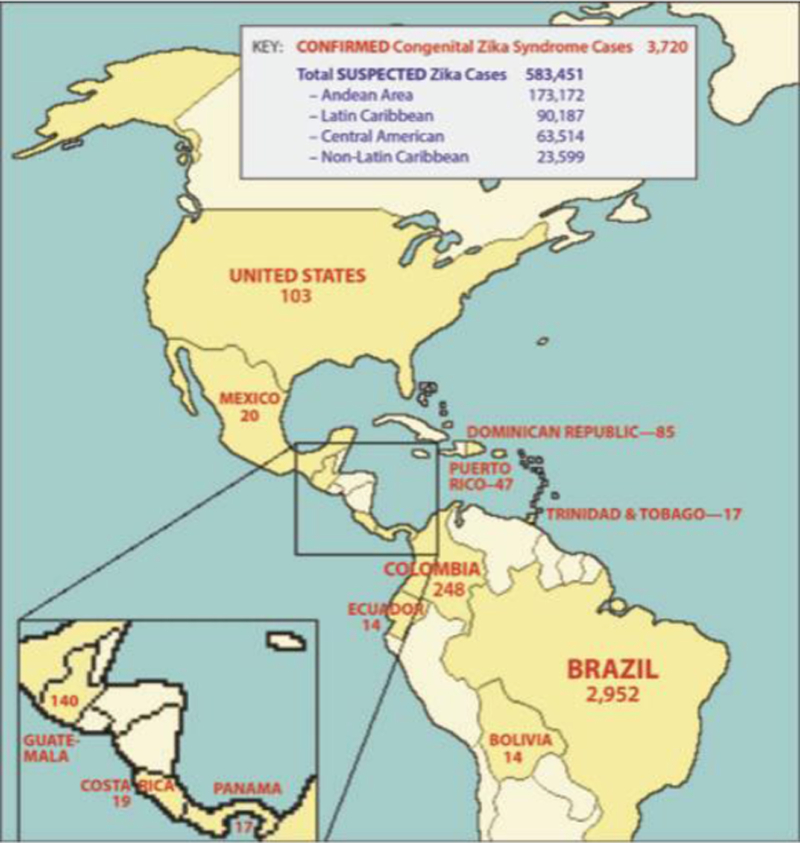
Cumulative Number of Confirmed Congenital Zika Syndrome Cases and Suspected ZIKV Infections from 2015–2107. A world map shows the cumulative number of reported Congenital Zika Syndrome cases (red, countries in dark yellow) and the number of suspected ZIKV infections (includes non-pregnant individuals, blue) as reported to the Pan American Health Organization/World Health Organization by each country between 2015–2017ii.Striking and unexpected differences in numbers across regions highlight disparities in ZIKV surveillance and reporting, public health, vector control and ecological constraints on mosquito-borne virus transmission. Countries in pale yellow have not reported any ZIKV-associated birth defects, but are in regions with likely or confirmed local transmission.
The hypothesis that children exposed to ZIKV might be at greater risk for poor neurodevelopmental outcomes has strong precedent across history for a number of other teratogenic viruses exist including: cytomegalovirus, herpes simplex viruses, parvovirus B19, human immunodeficiency virus, and Chikungunya virus. We argue that prenatal and early postnatal ZIKV exposure is a risk factor for neurodevelopmental delay, in the absence of microcephaly or another ZIKV-associated birth defect; therefore, these children should be eligible for early intervention services in the United States under the Individuals with Disabilities Education Act (IDEA). Early neurodevelopmental evaluation using standardized assessments should be performed even for the non-microcephalic ZIKV-exposed children at regular intervals, at least matching the U.S. well-child visit schedule, but more frequently if developmental delays are identified (Table 1). We also advocate for a long-term differentiated, individualized evaluation through adolescence in order to detect later developing cognitive processes (i.e. executive functioning) and mental health. The next step to act on this information to improve early child development is no small task. Rigorous implementation science research is needed to evaluate and scale early interventions in low- and middle-income countries that are culturally appropriate for each population.
Table 1.
Common Assessments Appropriate for ZIKV Developmental Monitoring
| Age Range | Common Assessment Tools* | Developmental Areas |
|---|---|---|
| Birth-5:0 | Peabody Developmental Motor Scales, Second Edition (PDMS-2) |
Fine and Gross Motor |
| Birth-7:11 | Preschool Language Scales, Fifth Edition (PLS-5) | Receptive and Expressive Language |
| Birth-90:0 | Vineland Adaptive Behavior Scales, Third Edition (VABS-3) Adaptive Behavior Rating Scales, Third Edition (ABAS-3) |
Adaptive |
| Birth-14:11 | Sensory Profile, Second Edition (SP-2) | Sensory Processing |
| 0:1–3:6 | Bayley Scales of Infant and Toddler Development, Third Edition (BSID-III) | Cognition, Language, Motor, Adaptive, Social-Emotional |
| 1:6–90:0 2:0–21:11 |
Achenbach Child Behavior Checklist (CBCL) Behavior Assessment Scales for Children, Third Edition (BASC-3) |
Social-Emotional/Behavior |
| 2:0–99:0 | Beery-Buktenika Developmental Test of Visual Motor Integration, Sixth Edition (VMI-6) |
Visual Motor Integration, Visual Perception, Motor Coordination |
| 2:6–7:7 2:6–17:11 5:0–95:0 6:0–16:11 |
Wechsler Preschool and Primary Scales of Intelligence, Fourth Edition (WPPSI-IV) Differential Ability Scales, Second Edition (DAS-2) Batería III Woodcock-Munoz: Pruebas de habilidades cognitivas (Batería III-COG) Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V) |
Cognitive |
| 2:6–5:11 2:6–7:11 5:0–95:0 |
Developmental Indicators for Assessment of Learning, Fourth Edition (DIAL-4) Bracken Basic Concepts Scale, Third Edition: Receptive and Expressive (BBCS-3:R & E) Bateria III Woodcock-Munoz: Pruebas de aprovechmient (Bateria III-APROV) |
Pre-Academic/Academic Learning |
| 3:0–16:11 8:0–89:11 |
Neuropsychological Assessment for Children, Second Edition (NEPSY-2) Delis-Kaplan Executive Functioning Scales (D-KEFS) |
Executive Functioning |
| 5:0–16:11 16–89:11 |
Children’s Memory Scale (CMS) Wechsler Memory Scales (WMS) |
Memory |
| 5:0–80:0 | Quick Neurological Screening Test, Third Edition Revised (QNST-3R) | Neurological Soft Signs of Possible Neurological and/or Learning Problems |
| 13:0–18:0 | Millon Adolescent Clinical Inventory (MACI) Minnesota Multiphasic Personality Inventory, Second Edition (MMPI-2) |
Personality, Mental Health |
Available in multiple languages; may also have multicultural norms. Age range is denoted as Years Months of age – Years Months of age.
Reports of recent ZIKV outbreaks in West Africa suggest that this virus will remain a contemporary problem for pregnancies in many parts of the world. In addition to vaccine efforts, we must also focus investigation on the thousands of children exposed to ZIKV that are now growing up now as infants and toddlers. While the concept of microcephaly as a marker for fetal brain injury was useful in the beginning to direct scientific efforts, we are now aware that the deleterious effects of ZIKV may be subtle and challenging to detect during pregnancy. Recent evidence that fetal or early postnatal ZIKV exposure can injure neural stem cell and progenitor cell populations in the hippocampus is particularly concerning for the possible development of neurocognitive deficits, memory disorders or psychopathologies in childhood. Early detection and intervention in these cases is critical to promote the best neurodevelopmental outcome. We need long-term studies of the development and mental health of children exposed to ZIKV, regardless of head size at birth, similar to ongoing studies in Colombia and Brazili. Unfortunately, ZIKV is not the only virus of concern; a recent study revealed that West Nile virus, a neuroinvasive virus related to ZIKV, can also infect the placenta and, presumably, the fetus [15] As we recognize an expanding list of viruses that may damage the fetal brain, we need toolkits to rapidly implement a global public health response and to address the long-term public health challenges of fetal brain injury. Most importantly, we must take care of the children.
Acknowledgments
We would like to acknowledge Jan Hamanishi for technical assistance with preparation of the figure. This work was supported by the University of Washington Department of Obstetrics & Gynecology, Seattle Children’s Research Institute and the National Institutes of Health, Grant # R01 AM 00989 and R01AI33976 (L.R and K. A. W) and R21OD023838 (B.R.N). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Resources
Contributor Information
Kristina M. ADAMS WALDORF, Seattle, WA; Department of Obstetrics & Gynecology, Center for Innate Immunity and Immune Disease, and Department of Global Health University of Washington; Sahlgrenska Academy, Gothenburg University, Sweden..
Erin M. OLSON, Seattle WA; Department of Epidemiology, School of Public Health and Department of Educational Psychology School Psychology Program, University of Washington..
Branden R. NELSON, Seattle WA; Center for Integrative Brain Research, Seattle Children’s Research Institute..
Marie-Térèse E. LITTLE, Seattle, WA; 4th Dimension Biomedical Research Communications, Victoria, Canada..
Lakshmi RAJAGOPAL, Seattle, WA; Center for Innate Immunity and Immune Disease, Department of Pediatrics, University of Washington; Center for Global Infectious Disease Research, Seattle Children’s Research Institute..
References
- 1.Gulland A (2016) Zika virus is a global public health emergency, declares WHO. BMJ 352, i657. [DOI] [PubMed] [Google Scholar]
- 2.Qian X et al. (2016) Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165 (5), 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shapiro-Mendoza CK et al. (2017) Pregnancy Outcomes After Maternal Zika Virus Infection During Pregnancy - U.S. Territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep 66 (23), 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoen B et al. (2018) Pregnancy Outcomes after ZIKV Infection in French Territories in the Americas. N Engl J Med 378 (11), 985–994. [DOI] [PubMed] [Google Scholar]
- 5.Franca GVA et al. (2016) Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 388 (10047), 891–897. [DOI] [PubMed] [Google Scholar]
- 6.Honein MA et al. (2017) Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. JAMA 317 (1), 59–68. [DOI] [PubMed] [Google Scholar]
- 7.Adams Waldorf KM et al. (2018) Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat Med 24 (3), 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spalding KL et al. (2013) Dynamics of hippocampal neurogenesis in adult humans. Cell 153 (6), 1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goncalves JT et al. (2016) Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167 (4), 897–914. [DOI] [PubMed] [Google Scholar]
- 10.Mu Y and Gage FH (2011) Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener 6, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan X et al. (2007) Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130 (6), 1146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs BL et al. (2000) Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry 5 (3), 262–9. [DOI] [PubMed] [Google Scholar]
- 13.Li H et al. (2016) Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell 19 (5), 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavigner M et al. (2018) Postnatal Zika virus infection is associated with persistent abnormalities in brain structure, function, and behavior in infant macaques. Sci Transl Med 10 (435), DOI: 10.1126/scitranslmed.aao6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platt DJ et al. (2018) Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci Transl Med 10 (426), DOI: 10.1126/scitranslmed.aao7090. [DOI] [PMC free article] [PubMed] [Google Scholar]


