Abstract
Background/Aims:
Ultrasound is used to screen for hepatic steatosis, the most common liver disease in the United States. However, few studies have prospectively evaluated the accuracy of ultrasound to diagnose hepatic steatosis. Therefore, a double blinded prospective study was performed in consecutive patients undergoing liver biopsy to evaluate the accuracy of ultrasound to diagnose hepatic steatosis.
Methods:
Real time ultrasound was performed just prior to the biopsy by a single investigator masked to the clinical diagnosis. The liver biopsy was reviewed by a pathologist masked to the clinical indication or sonographic findings
Results:
Of 73 consecutive patients studied,macrovesicular steatosis of any severity on biopsy was found in 46 (63%) and micro vesicular fat found in 51 (69.9%). The overall impression of the sonographer for the presence of macrovesicular hepatic steatosis of any degree had a sensitivity of 60.9% and a specificity of 100%. The sensitivity increased to 100% and the specificity to 90% when there was ≥ 20% of fat. The zonular distribution of the fat did not alter the diagnostic accuracy of ultrasound. Ultrasound had a poor yield in the diagnosis of microvesicular fat with an overall sensitivity of 43% and a specificity of 73%. The combination of increased echogenicity and portal vein blurring on ultrasound had the greatest sensitivity in the diagnosis of hepatic steatosis.
Conclusion:
Real time ultrasound using a combination of sonographic findings has a high specificity but underestimates the prevalence of hepatic steatosis when there is less than ≥ 20% fat.
Keywords: Hepatic steatosis, Ultrasound, Sensitivity, Specificity
Introduction
Non alcoholic fatty liver disease (NAFLD) is the most common form of liver disease in the United States [1-3]. The diagnosis of NAFLD is established by liver biopsy but ultrasound (US) is being increasingly recognized as a screening tool due to the useful information obtained as well as being non invasive, well tolerated and widely available [4]. Hepatic steatosis appears as a diffuse increase in echogenicity (bright liver) and a number of sonographic alterations in the liver [5,6]. The major limitation of US as a screening tool for hepatic steatosis has been the modest sensitivity of 67% and specificity of 77% that would result in an incorrect diagnosis in up to 33% of patents [7]. In patents with chronic hepatitis C, ultrasound had a sensitivity of 60% and a specificity of 79% making it a relatively inaccurate test [8]. It must be reiterated that the relative utility of ultrasound as a diagnostic tool depends on the clinical setting in which it is being used. Screening asymptomatic patients requires a test with a high sensitivity and specificity so that few patients are undiagnosed and those that are diagnosed are evaluated appropriately [9,10]. However, this requirement for a screening test for NAFLD may not necessarily be clinically appropriate due to the need for a liver biopsy to confirm the diagnosis, the slow rate of progression of disease, and the lack of established treatment protocols [11]. In this clinical situation, one may need a test that has a relatively high specificity to ensure that a large population of false positive subjects is not subjected to a liver biopsy due to the potential for morbidity and mortality. The currently available data on the utility of ultrasound in the diagnosis of hepatic steatosis are either retrospective or done in a well defined population of patients with known hepatic steatosis [4,12-19). Furthermore, hepatic steatosis has been used to refer to macrovesicular steatosis while microvesicular steatosis accompanies a number of hepatic disorders and often occurs with macrovesicular steatosis in NAFLD [20]. The contribution of microvesicular fat to the sonographic abnormalities in patients with hepatic steatosis has not been systematically evaluated. A number of sonographic abnormalities suggest hepatic steatosis but the predictive role of each of these findngs has not been determined in a prospective manner. Their role in identifying fibrosis and inflammation have also not been systematically evaluated prospectively in an unselected population of subjects undergoing liver biopsy. Therefore, the present study prospectively evaluated the diagnostic utility of real time ultrasound performed by a clinical gastroenterologist in diagnosing the presence and severity of hepatic steatosis as well as fibrosis and inflammation in an unselected population of consecutive patients undergoing liver biopsy.
Patients and methods
Seventy five consecutive patients undergoing an elective liver biopsy for clinical indications of abnormal liver function or clinical suspicion of liver disease being performed in the Gastroenterology division of Metro Health Medical Center, Cleveland, OH had a real time US by a single investigator (SD) masked to the clinical indication for the liver biopsy as part of confirmation of the site of the biopsy just prior to the procedure. All patients were determined to have normal renal function since one of the sonographic criteria depended on a comparison of the echogenicity of the renal cortex with that of the liver. Real time ultrasound was performed using a Sonosite Micromaxx (Sonosite Inc., Bothell, WA). The technical parameters including gain adjustment, placement of focal zone and the optimum location of the transducer were optimized for each patient. A percutaneous liver biopsy was then performed using an 18G Bard Monopty biopsy gun (Bard Biopsy Systems, Tempe, AZ) with a single pass by the percutaneous route in the right lower intercostal space. Hematoxylin and eosin stained slides were used for assessing the type and degree of steatosis as well as any necroinflammatory changes. Only biopsies deemed to be sufficient specimens were reviewed by a clinical pathologist (AK) who was masked to the clinical indication and the sonographic findings. A quantitative score was assigned based on the estimated pecentage of hepatocytes involved in increments of 5%. The pattern of steatosis was judged as either predominantly macrovesicular if > 75% of involved hepatocytes contained fat droplets larger than the hepatocyte nucleus and displaced the nucleus to one side or predominantly microvesicular if greater than 75% of involved hepatocytes contained fat droplets smaller than the hepatocyte nucleus and without significant nuclear displacement. Biopsies showing at least 25% of each type of fatty change were designated to show a mixed patten. The necroinflammatory changes (portal inflammation, piecemeal necrosis, and lobular inflammation and necrosis) and degree of fibrosis were evaluated as part of histological examination and were related to the sonographic findings. The diagnosis of NASH and steatosis were made using previously described criteria [21]. Significant alcohol intake was defined as > 20 g/day for women and >30 g/day for men. The elective liver biopsy was performed in all patients after at least 6 months of abstinence from alcohol. Serological and biochemical assays were used to diagnose the etiology of liver disease. Adequate liver biopsy was not obtained in 2 patients and they were excluded from the analyses. Severity of hepatic steatosis classified as mild if the area of involvement by fat was 5-35%, moderate when the involvement was 35-65% and severe when the involvement was > 65%.
The US results were interpreted by one of the investigators (SD) with previous experience in performing and interpreting hepatic ultrasound [22-24]. The results were initially categorized into the presence or absence of hepatic steatosis based on the overall impression using the sonographic abnormalities [5]. An attempt was also made to differentiate the degree of steatosis during ultrasound interpretation into no fat, mild fatty liver and severe fatty liver. The liver image was assessed to be normal if the texture was homogenous, exhibited fine level echoes and isoechoic compared to the renal cortex and adequate visualization of the hepatic vessels and diaphragm. The sonographic findings that were specifically evaluated included the hepatorenal contrast, bright hepatic echoes, deep attenuation, vessel blurring and non specific findings of heterogeneous echoes. The diagnosis of hepatorenal echo contrast was based on evidence of sonographic contrast between the liver and right renal cortex in the midaxillary line. The diagnosis of bright liver was based on abnormally intense, high level echoes arising from the hepatic parenchyma, deep attenuation was based on evident attenuation of echo penetration into the deep portion of the liver and impaired visualization of the diaphragm. Vessel blurring was based on an impaired visualization of the borders of the intrahepatic vessels and narrowing of their lumen.
Predefined criteria for determining the severity of hepatic steatosis included the presence of bright echoes or increased hepatorenal contrast indicative of mild steatosis, presence of both bright echhoes and increased hepatorenal contrast as well as vessel blurring indicative of moderate steatosis and severe steatosis was considered to be present when in addition to the criteria for moderate steatosis, there was evidence of posterior beam attenuation and non visualization of the diaphragm. The liver image was assessed to be normal if the texture was homogenous, exhibited fine level echoes and isoechoic compared to the renal cortex and adequate visualization of the hepatic vessels and diaphragm.
Statistical Analyses
Descriptive statistics were computed for all factors. These include means, standard deviations and percentiles for continuous variables and frequiencies for categorical factors. Wilcoxon’s rank sum test was used to compare fat levels between presence and absence of specific ultrasound criteria and p<0.05 accepted as the significance levels. The sensitivity and specificity of each ultrasound criterion in predicting the macrovesicular fat was calculated for the histological severity. In addition, Receiver Operaring Characteristics (ROC) analysis was performed and the areas under the ROC curves (AUC) were estimated with their corresponding 95% confidence intevals (95% CI). Multivariable logistic regression models were used to assess combinations of 2 criteria in prediction of macrovesicular fat levels above 10 and ≥ 20% and the corresponding ROC curves were assessed. Cocharan-Armitage Trend tests were used to assess associations between sonogrpahic findings, fibrosis stage and inflammation grade. Pearson’s Chi-square tests were for presence of NASH [25]. A ρ < 0.05 was considered statistically significant. SAS version 9.2 software (The SAS Institute, Cary, NC) and R version 2.4.1 freeware (The R Foundation of Statistical Computing, Vienna, Austria) were used to perform all analyses.
Results
The clinical and demographic findings of the patients are shown in Table 1. As shown the most common indications for the biopsy were either fatty liver or hepatitis C. There were 4 patients with cirrhosis of the liver. None of the patients had significant alcohol consumption in the 6 months prior to the biopsy. History of significant alcohol consumption over 6 months prior to the biopsy was observed in 8 patients (7 patients with HCV and 1 cirrhotic patient). On histology, macrovesicular steatosis of any extent was seen in 63% of patients while microvesicular steatosis of any extent was seen in 69% of patients on histology. Sonographic diagnosis of the presence of hepatic steatosis was observed in 28 (38.4%) of patients. The prevalence of the individual criteria for the diagnosis of hepatic steatosis of any severity is shown in Table 2. The sonographic diagnosis of the prevalence of steatosis was observed in 54.6% of patients with a BMI of ≥ 30kg/m2 and 17.7% in those with a BMI < 30 kg/m2.
Table 1.
Clinical and demographic characteristics.
| Number | 73 |
| M:F | 48:25 |
| Mean Age (yrs ± SD) | 48.0±10.7 |
| Body mass index (kg/m2± SD) | 30.6±6.9 |
| Diagnosis | |
| Hepatitis C | 38 (52.1%) |
| Fatty liver | 21 (28.8%) |
| Hepatitis B | 7 (9.6%) |
| Others | 7 (9.6%) |
Table 2.
Prevalence of sonographic findings in obese and non-obese patients.
| Sonographic finding | Number in non obese (n=34) |
Number in obese (n=39) |
Total |
|---|---|---|---|
| Overall sonographic impression | 6 (17.7) | 22 (56.4) | 28 (38.4) |
| Abnormal hepatorenal echoes | 6 (17.7) | 22 (56.4) | 28 (38.4) |
| Bright liver | 6 (17.7) | 22 (56.4) | 28 (38.4) |
| Portal vein blurring | 3 (8.8) | 15 (38.5) | 18 (24.7) |
| Hepatic vein blurring | 6 (17.7) | 22 (56.4) | 28 (38.4) |
| Posterior beam attenuation | 2 (5.9) | 13 (33.3) | 15 (20.6) |
| Poor diaphragm visualization | 4 (11.8) | 10 (25.6) | 14 (19.2) |
| Non specific features | 3 (8.8) | 6 (15.4) | 9 (12.3) |
values presented as N (%)
As shown in Table 3, of the various criteria used to diagnose the presence of hepatic steatosis on ultrasound, the highest sensitivity and specificity for the presence of steatosis confirmed on histology was for macrovesicular steatosis ≥ 20% of total hepatocyte area. With greater area of involvement with fat on histology, the sensitivity increased to 100% with a small reduction in specificity. Of the various sonographic criteria studied, hepatorenal echo contrast and bright liver were able to identify the presence of ≥ 20% area of involvement with fat with a sensitivity of 96.4% and a specificity of 97.8%. Criteria for vascular attenuation that included portal vein blurring and hepatic vein blurring had lower sensitivity and specificity.
Table 3.
Ultrasound findings and macrovesicular fat levels.
| Presence of fat | Hepatorenal echo abnormality | |||||
| % Area fat | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 82.4 | 100 | 0.912 (0.847-0.977) | 82.4 | 100 | 0.912 (0.847-0.977) |
| ≥20 | 96.4 | 97.8 | 0.971 (0.930-1.000) | 96.4 | 97.8 | 0.971 (0.930-1.000) |
| ≥30 | 100 | 84.9 | 0.925 (0.876-0.973) | 100 | 84.9 | 0.925 (0.876-0.973) |
| ≥50 | 100 | 72.6 | 0.863 (0.807-0.919) | 100 | 72.6 | 0.863 (0.807-0.919) |
| Bright liver | PV blurred | |||||
| % Area | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 82.4 | 100 | 0.912 (0.847-0.977) | 50 | 97.4 | 0.737 (0.648-0.826) |
| ≥20 | 96.4 | 97.8 | 0.971 (0.930-1.000) | 60.7 | 97.8 | 0.792 (0.698-0.887) |
| ≥30 | 100 | 84.9 | 0.925 (0.876-0.973) | 70 | 92.5 | 0.812 (0.703-0.921) |
| ≥50 | 100 | 72.6 | 0.863 (0.807-0.919) | 63.6 | 82.3 | 0.729 (0.573-0.886) |
| HV blurred | Posterior attenuation | |||||
| % Area | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 79.4 | 97.4 | 0.884 (0.811-0.958) | 41.2 | 97.4 | 0.693 (0.605-0.781) |
| ≥20 | 92.9 | 95.6 | 0.942 (0.885-0.999) | 46.4 | 95.6 | 0.710 (0.611-0.809) |
| ≥30 | 100 | 84.9 | 0.925 (0.876-0.973) | 55 | 92.5 | 0.737 (0.620-0.855) |
| ≥50 | 100 | 72.6 | 0.863 (0.807-0.919) | 63.6 | 87.1 | 0.754 (0.599-0.909) |
| Poor diaphragm visualization | Nonspecific findings | |||||
| % Area | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 32.4 | 92.3 | 0.623 (0.533-0.714) | 14.7 | 89.7 | 0.522 (0.445-0.600) |
| ≥20 | 39.3 | 93.3 | 0.663 (0.564-0.762) | 17.9 | 91.1 | 0.545 (0.461-0.628) |
| ≥30 | 55 | 94.3 | 0.747 (0.631-0.863) | 20 | 90.6 | 0.553 (0.455-0.651) |
| ≥50 | 72.7 | 90.3 | 0.815 (0.672-0.958) | 90.9 | 12.9 | 0.519 (0.421-0.618) |
AUC, area under ROC curve; C,: confidence interval; PV, portal vein; HV, hepatic vein
As can be seen from the box plots in Figure 1, the overall discrimination by ultrasound for the presence of ≥ 20% fat was high. Similarly, as shown in Table 3, increased echogenicity and the presence of bright liver echoes had a similar discriminatory value. Vascular attenuation (blurring of the portal and hepatic vein) required a higher amount of hepatic fat compared with the increased echogenic shadows that was also reflected in the lower sensitivity and specificity. Posterior beam attenuation and poor visualization of the diaphragm required a higher amount of fat to be identified on ultrasound. Finally, non specific features were a poor indicator of the presence of hepatic fat. Addition of the area of involvement with microvesicular fat to the area of macrovesicular fat did not improve the sensitivity or specificity of the sonographic features.
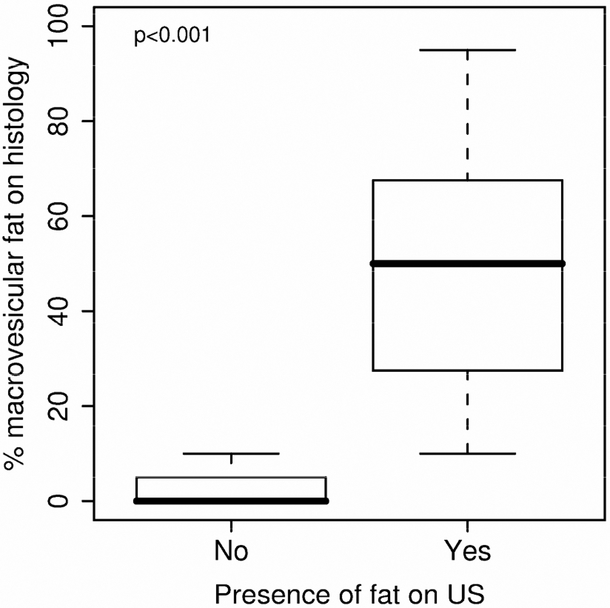
Figure 1.
Histological evidence of total hepatocyte area involved with macrovesicular fat and presence of fat detected on ultrasound based on the overall impression of the sonographer. The box-and-whisker plot is represented by the lower boundary of the box indicating the 25th percentile, the line within the box indicating the median value, the upper boundary of the box indicating the 75th percentile. The whiskers extend to the most extreme data point which is no more than 1.5 times the interquartile range from the box.
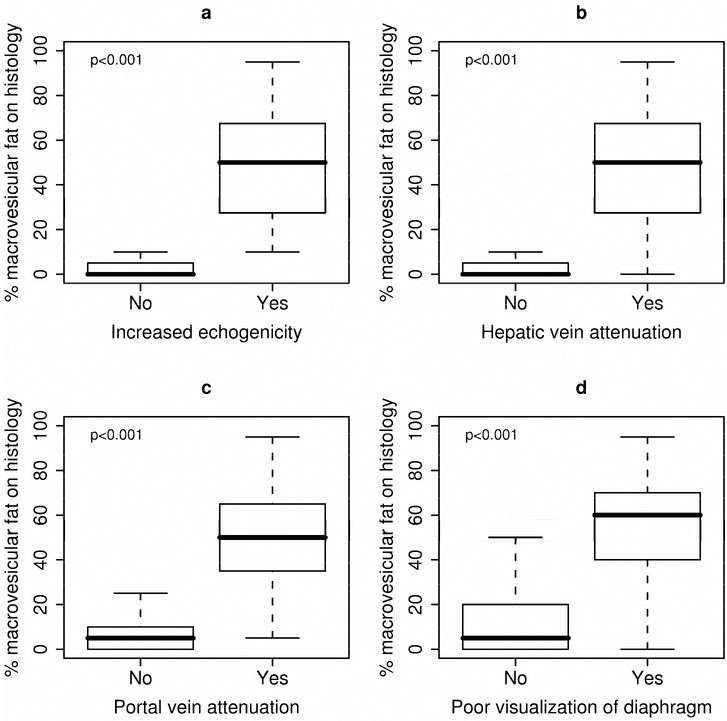
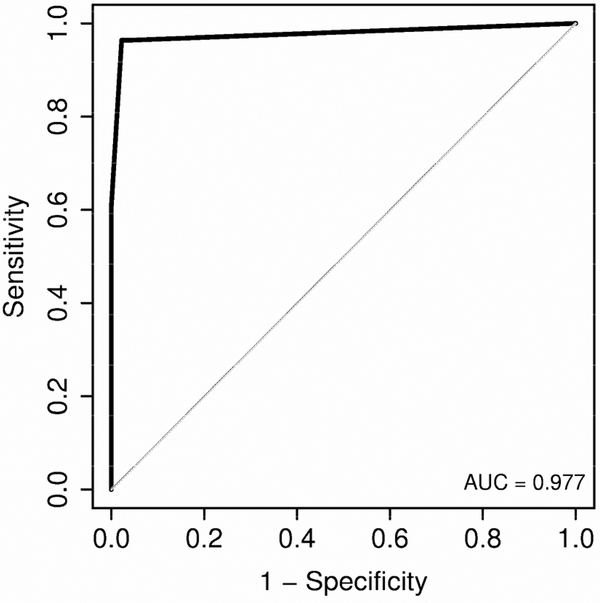
Macrovesicular fat levels were significantly higher in patients whose ultrasound was suggestive of fat presence (Median (25th, 75th percentiles): 50 (27, 67.5) vs. 0 (0, 5); ρ < 0.001) (Figure 1). Furthermore, each specific ultrasound criteion was significantly associated with increased macrovesicular fat levels (Figure 2). A logistic regression analysis showed that the best combination for identification of ≥ 20% hepatic steatosis was increased hepatic echogenicity and portal vein blurring with the highest area under the curve (0.977; 95% CI - 0.94-1.00). Other combinations of sonographic abnormalities did not yield a similar diagnostic accuracy. A receiver operated curve that was constructed (Figure 3) and showed that a combination of increased hepatic echogenicity with portal vein blurring had the best sensitivity and specificity. Furthermore, for various combinations, the lowest amount of hepatic steatosis that could be detected by ultrasound with an area under the curve of 0.95 was ≥ 20%.
Figure 2.
Macrovesicular fat levels by ultrasound criteria: (A) increased echogenicity, (B) hepatic vein blurring, (C) portal vein blurring and (D) poor visualization of diaphragm. Macrovesicular fat levels by ultrasound criteria: (A) increased echogenicity, (B) hepatic vein blurring (C) portal vein blurring and (d) poor visualization of diaphragm. PV- portal vein; HV hepatic vein
Figure 3.
Receiver Operating Characteristics assessment of increased echogenicity and portal vein blurring to predict Macrovesicular fat levels ≥ 20%.
Of the 21 patients with NAFLD, there were 8 patients with definite NASH and 13 with hepatic steatosis alone. None of the sonographic findings could distinguish between steatosis and NASH (p > 0.1). Similarly, there was no significant association between sonographic findings and either stage of fibrosis or the grade of inflammation (p > 0.1). Sonographic findings are not useful for identifying patients with severe fibrosis (stages 3-4) or moderate inflammation (grades 2-3); areas under the curve ranged between 0.503 and 0.605 and there was no evidence to suggest these were significantly higher than 0.5 (chance assignment).
Discussion
With the increasing recognition of NAFLD as being the most common cause of chronic liver disease, ultrasound is likely to become the screening modality of choice before a confirmatory liver biopsy is performed. Previous studies on the diagnostic accuracy of ultrasound for identification of hepatic steatosis have been either retrospective or performed in patients with documented fatty liver [26,27]. We report the diagnostic accuracy of each of the sonographic criteria used to document hepatic steatosis in a prospective double blind manner in which neither the sonographer nor the pathologist was aware of each other’s findings. The present findings demonstrate convincingly that ultrasound is an accurate method to identify hepatic steatosis when the total area of steatosis exceeded 20%.
As shown in Table 2 and Figures 1-3, ultrasound had a high accuracy in the diagnosis of fatty liver when the total area of hepatic steatosis exceeded 20%. When the total area of fat was less than this, the sensitivity of US was lower. This was related primarily to the low sensitivity and consequent high false negative rate. These results are similar to previous reports [8,18]. Our observations suggest that even at a relatively low percentage area of fat on histology (≥ 20%), a combination of sonographic abnormalities of abnormal echogenicity or bright liver with portal vein blurring had a high sensitivity and specificity. Even though the prevalence of steatosis based on the sonographer’s overall impression was only 28% despite the mean BMI being 30.6 kg/m2, when patients were stratified into obese and non-obsese subjects, the prevalence of steatosis on ultrasound was 56.4% in obese subjects. Furthermore, lower sensitivity of some of the sonographic findings used to diagnose steatosis (poor diaphragm visualization, posterior beam attenuation) contributed to the lowered the sensitivity of the sonographer’s overall impression. In the present study, ultrasound was unable to discriminate between NASH and hepatic steatosis that was similar to previous reports [28]. This may be related to the diagnostic criteria for NASH that requires histological evidence of ballooning changes [21] as well as the low diagnostic accuracy of ultrasound to diagnose fibrosis or inflammation. These data are consistent with previous reports that ultrasound is a sensitive method to diagnose hepatic steatosis but not for fibrosis or inflammation [29,30]. The major strengths of this study include its prospective nature, simultaneous timing of US and liver biopsy, blinded method of evaluation of sonographic diagnosis of hepatic steatosis and the use of an unselected population of patients undergoing liver biopsy. This allowed us to determine the true sensitivity and specificity due to the inclusion of patients who did not have fat on histology. The use of predetermined sonographic criteria demonstrated that bright hepatic echoes and increased hepatorenal echogenicity had similar sensitivity and specificity while the vascular blurring of either the portal vein or hepatic vein had similar sensitivity and specificity for hepatic steatosis occupying areas of 20% to 65%. It therefore is clear that using a combination of increased echogenicity and vascular blurring would be the best method to determine the presence of hepatic steatosis as demonstrated by the high area under the curve in Figure 3. Another observation was that even though the sensitivity and specificity of hepatic vein blurring was higher than that of portal vein blurring, the combination of increased hepatic echogenicity and portal vein blurring was a more accurate predictor of hepatic steatosis. This was related to the high concordance between increased echogenicity and hepatic vein blurring (97.3% agreement) so that adding hepatic vein blurring would add any additional information to this. Hence, the combination of portal vein blurring was a better sonographic finding in combination with increased hepatic echogenicity.
Our study also demonstrated that using these criteria did not have a high degree of accuracy in quantifying the amount of fat. Similarly, the presence of microvesicular fat did not alter the sonographic findings. Previous studies on the role of imaging in the diagnosis of microvesicular fat have had limited success and this was similar to our own observations [20]. Similarly, the zonular location of the fat did not determine the sonographic findings and this was also similar to previous observations. This is unlike the more recently used magnetic resonance spectroscopy that has a much higher sensitivity with normal liver demonstrating about 9% fat [31]. This high sensitivity will result in a much higher number of patients being evaluated for hepatic steatosis than may even be clinically relevant. The use of CT and MRI also suffer from the much higher cost, non availability in a ‘field setting’ for screening and the potential exposure to radiation with CT. Given these reasons, our double blind evaluation of predetermined sonographic criteria for hepatic steatosis shows the clinical benefit of US as a potential screening method for hepatic steatosis.
In conclusion, hepatic ultrasound has a high diagnostic accuracy in patients with hepatic steatosis and the use of predefined criteria have a high sensitivity and specificity especially when the total area of hepatocytes with steatosis exceeds 20%. At lower levels of fat content, the specificity of ultrasound remans high but the sensitivity is significantly reduced, resulting in high false negative rates. Our studies show that ultrasound is not an accurate measure of the degree of fibrosis or inflammation, but can be used as a screening modality for hepatic steatosis in an unselected population because of the high accuracy at significant fat content levels. These observations also demonstrate that serial ultrasound can be used to non invasively monitor the therapeutic efficacy of interventions in the management of these patients.
Table 4.
Ultrasound findings and microvesicular fat levels.
| Presence of fat | Hepatorenal echo abnormality | |||||
| % Area fat | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 59.3 | 73.9 | 0.666 (0.552, 0.780) | 59.3 | 73.9 | 0.666 (0.52,0.780) |
| ≥20 | 66.7 | 65.6 | 0.661 (0.488, 0.835) | 66.7 | 65.6 | 0.661 (0.488, 0.835) |
| Bright liver | PV blurred | |||||
| % Area | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 59.3 | 73.9 | 0.666 (0.552-0.780) | 29.6 | 78.3 | 0.601(0.498-0.646) |
| ≥20 | 66.7 | 65.6 | 0.661 (0.488-0.835) | 33.3 | 76.6 | 0.549 (0.378-0.72) |
| HV blurred | Posterior attenuation | |||||
| % Area | Sensitivity | Specificity | AUC (95% CI) | Sensitivity | Specificity | AUC (95% CI) |
| ≥5 | 59.3 | 73.9 | 0.666 (0.552-0.780) | 33.3 | 87 | 0.601 (0.498-0.705) |
| ≥20 | 77.8 | 67.2 | 0.725 (0.570-0.880) | 55.6 | 84.4 | 0.700 (0.522-0.878) |
| Poor diaphragm visualization | Non specific findings | |||||
| ≥5 | 25.9 | 84.8 | 0.554 (0.454-0.653) | 25.9 | 95.7 | 0.608 (0.519-0.697) |
| ≥20 | 22.2 | 81.3 | 0.517 (0.365-0.669) | 66.7 | 95.3. | 0.810 (0.644-0.975) |
AUC, area under ROC curve; CI, Confidence interval.
Abbreviation:
- NAFLD
non alcoholic fatty liver disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content,and all legal disclaimers that apply to the journal pertain.
References
- 1.Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younossi ZM, et al. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. Am J Gastroenterol 2008;103:2263–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarantino G Non-alcoholic fatty liver disease, obesity and other illnesses. Clin Invest Med 2008;31 :E290–E295. [DOI] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatities. Gastroenterology 2008; 134:1682–1698. [DOI] [PubMed] [Google Scholar]
- 4.Perez NE, Siddiqui FA, Mutchnick MG, Dhar R, Tobi M, Ullah N, et al. Ultrasound diagnosis of fatty liver in patients with chronic liver disease: a retrospective observational study. J Clin Gastroenterol 2007;41:624–629. [DOI] [PubMed] [Google Scholar]
- 5.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. AJR Am J Roentgenol 2007; 189:W320–W323. [DOI] [PubMed] [Google Scholar]
- 6.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102:2708–2715. [DOI] [PubMed] [Google Scholar]
- 7.Graif M, Yanuka M, Baraz M, Blank A, Moshkovitz M, Kessler A, et al. Quantitative estimation of attenuation in ultrasound video images: correlation with histology in diffuse liver disease. Invest Radiol 2000;35:319–324. [DOI] [PubMed] [Google Scholar]
- 8.Hepburn MJ, Vos JA, Fillman EP, Lawitz EJ. The accuracy of the report of hepatic steatosis on ultrasonography in patients infected with hepatitis C in a clinical setting: a retrospective observational study. BMC Gastroenterol 2005;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert R, Logan S, Moyer VA, Elliott EJ. Assessing diagnostic and screening tests: Part 2. How to use the research literature on diagnosis. West J Med 2001;175:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert R, Logan S, Moyer VA, Elliott EJ. Assessing diagnostic and screening tests: Part 1. Concepts. West J Med 2001;174:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology 2009;49:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnetic resonance imaging of parenchymal liver disease: a comparison with ultrasound, radionuclide scintigraphy and X-ray computed tomography. The Clinical NMR Group. Clin Radiol 1987;38:495–502. [DOI] [PubMed] [Google Scholar]
- 13.Bartolotta TV, Taibbi A, Galia M, Runza G, Matranga D, Midiri M, et al. Characterization of hypoechoic focal hepatic lesions in patients with fatty liver: diagnostic performance and confidence of contrast-enhanced ultrasound. Eur Radiol 2007; 17:650–661. [DOI] [PubMed] [Google Scholar]
- 14.Davies RJ, Saverymuttu SH, Fallowfield M, Joseph AE. Paradoxical lack of ultrasound attenuation with gross fatty change in the liver. Clin Radiol 1991; 43:393–396 [DOI] [PubMed] [Google Scholar]
- 15.de Korte PJ, van der Loos TL, van den Tweel JG, Cremers PT, Veldhuijzen van Zanten GO, Lustermans FA. Interpretation of a ‘bright’ liver in ultrasound examination. Neth J Med 1986;29:5–7. [PubMed] [Google Scholar]
- 16.de Moura AA, Cotrim HP, Barbosa DB, de Athayde LG, Santos AS, Bitencourt AG, et al. Fatty liver disease in severe obese patients: diagnostic value of abdominal ultrasound. World J Gastroenterol 2008;14:1415–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsberg L, Floren CH, Hederstrom E, Prytz H. Ultrasound examination in diffuse liver disease. Clinical significance of enlarged lymph nodes in the hepato-duodenal ligament. Acta Radiol 1987;28:281–284. [PubMed] [Google Scholar]
- 18.Foster KJ, Dewbury KC, Griffith AH, Wright R. The accuracy of ultrasound in the detection of fatty infiltration of the liver. Br J Radiol 1980;53:440–442. [DOI] [PubMed] [Google Scholar]
- 19.Harrison SA. Abnormal liver tests and fatty liver on ultrasound clin Gastoentology Hepatol 2008;6:26–29. [DOI] [PubMed] [Google Scholar]
- 20.Fishbein M, Castro F, Cheruku S, Jain S, Webb B, Gleason T, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol 2005;39:619–625 [DOI] [PubMed] [Google Scholar]
- 21.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis 2001;21:3–16. [DOI] [PubMed] [Google Scholar]
- 22.Sharma MP, Duphare HV, Nijhawan S, Dasarathy S. Gallstone disease in north India: clinical and ultrasound profile in a referral hospital. J Clin Gastroenterol 1990;12:547–549. [DOI] [PubMed] [Google Scholar]
- 23.Sharma MP, Dasarathy S. Gallbladder abnormalities in acute viral hepatitis: a rospective ultrasound evaluation. J Clin Gastroenterol 1991;13:697–700. [DOI] [PubMed] [Google Scholar]
- 24.Sharma MP, Dasarathy S, Misra SC, Saksena S, Sundaram KR. Sonographic signs in portal hypertendon: a multivariate analysis. Trap Gastroenterol 1996;17:23–29. [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more receiver operating characteristic curves: a nonparametric approach. 44 ed. 1988:837–845. [PubMed] [Google Scholar]
- 26.Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis 2007;11:37–54. [DOI] [PubMed] [Google Scholar]
- 27.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD). Am J Gastroenterol 2007; 102:2716–2717. [DOI] [PubMed] [Google Scholar]
- 28.Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utili radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 29.Mathiesen UL, Franzen LE, Aselius H, Resjo M, Jacobsson L, Foberg U, et al. Increased liver echogenicity at ultrasound examination reflects degree of steatosis but not of fibrosis in asymptomatic patients with mild/moderate abnormalities of liver transaminases. Dig Liver Dis 2002;34:516–522. [DOI] [PubMed] [Google Scholar]
- 30.Mendler MH, Bouillet P, Le SA, Lavoine E, Labrousse F, Sautereau D, et al. Dual-energy CT in the diagnosis and quantification of fatty liver: limited clinical value in comparison to ultrasound scan and single-energy CT, with special reference to iron overload. J Hepatol 1998;28:785–794. [DOI] [PubMed] [Google Scholar]
- 31.Fishbein MH, Gardner KG, Potter CJ, Schmalbrock P, Smith MA. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging 1997;15:287–293. [DOI] [PubMed] [Google Scholar]