Abstract
Background
To analyze the clinical outcome of elderly women with early breast cancer who underwent accelerated partial breast irradiation (APBI) based on a post-operative single fraction of multicatheter interstitial high dose–rate brachytherapy (MIB).
Methods
A single institution retrospective cohort study was performed focusing on elderly patients (≥ 65 years old) presenting a low-risk breast carcinoma treated by lumpectomy plus axillary evaluation followed by MIB. A single fraction of 16 Gy was prescribed on the 100% isodose. Clinical outcome at 3 years was reported based on local relapse free survival (3-y LRFS), specific survival (SS) and overall survival (OS). Acute (< 180 days after APBI) and late toxicity were evaluated. Cosmetic results were clinically evaluated by the physician.
Results
Between January 2012 and August 2015, 48 women (51 lesions) were treated. Median age was 77.7 years (range: 65–92) with a median tumor size of 12 mm (range: 3–32). Five patients (pts) presented an axillary lymph node involvement (4 Nmic, 1 N1). Invasive ductal carcinoma was the most frequent histology type (86.3%). With a median follow–up of 40 months (range: 36–42), no local relapse occurred while 1 pt. developed axillary relapse (2.1%). The 3-y LRFS, SS and OS rates were 100%, 100% and 93.1% respectively. Forty-five acute events were remained. The most frequent acute toxicity was grade (G) 1 hyperpigmentation (26.7%), 3 pts. (6.3%) presented G3 acute toxicity (2 breast hematomas, 1 breast abscess). No ≥ G3 late toxicity was observed while 15 late toxicities occurred (G1: 13 events - 86.7%) mainly breast fibrosis). The rate of excellent cosmetic outcome was 76.4%.
Conclusion
We reported promising and encouraging clinical outcome of a post-operative single fraction of MIB ABPI in the elderly. This approach leads to consider a sfAPBI as an attractive alternative to intra-operative radiation therapy while all the patients will be good candidates for APBI in regards to the post-operative pathological report. More mature results (number of patients and follow-up) are needed.
Keywords: Breast cancer, Elderly, Accelerated partial breast irradiation, Brachytherapy
Background
During the last half of century, breast cancer therapeutics progressed steadily and rapidly. In the management of localized breast cancers, total mastectomy has gradually given way to conservative surgical treatments followed by adjuvant radiotherapy. The conservative therapeutic approach is now considered as a standard of care for T1–2 breast cancer [1, 2]. However, the standard adjuvant radiotherapy schedule (25 to 30 fractions) generates much transportation that can be difficult mainly for elderly women. Even using hypofractionated regimen (15 to 16 fractions) [3–5] in place of standard schedule, adjuvant breast irradiation can alter the quality of life and sometimes the adhesion to the treatment. Furthermore, a higher number of transportations generates additional costs for health insurance.
Accelerated partial breast irradiation (APBI) appears as a natural continuity in the process of therapeutic de-escalation. American Society of Radiation Oncology (ASTRO) and Groupe Européen de Curiethérapie of the European Society for Radiotherapy and Oncology (GEC-ESTRO) have considered that, in a well selected population, described as “suitable” (ASTRO) and “low-risk” (ESTRO), adjuvant APBI can be proposed [6, 7]. After two decades of clinical research, APBI is now recognized as an efficient and safe adjuvant treatment for low-risk breast cancer [8, 9].
The aim of this study was to report the early clinical outcome of APBI in the elderly with early breast cancer treated by a post-operative single fraction of MIB APBI (sfAPBI).
Methods
Patient selection
This is a single institution retrospective study including elderly patients presenting with low-risk breast cancer who underwent lumpectomy plus axillary evaluation followed by a single fraction of high-dose rate (HDR) MIB APBI. The patient cohort combined women enrolled in a prospective phase I/II trial (SiFEBI; Clinical.gov #NCT01727011, [10]) and patients previously treated before the SiFEBI trial opening. Briefly, inclusion criteria were as follows: elderly women 65 years and older, histologically proven breast carcinoma with free surgical margins, negative axillary evaluation. Patients were excluded in case of: sarcoma or lymphoma histology, metastatic dissemination. Data were collected from the Antoine Lacassagne Cancer Center institutional database. All the patients treated out of the SiFEBI trial, had the choice between adjuvant WBI and sfAPBI. All APBI indications were validated by the local breast oncologist committee. Patients treated outside of the SiFEBI trial (Clinical.gov #NCT01727011) were carefully selected and fully informed about the new irradiation procedure (advantages and disadvantages) compared to the standard protocol of external beam radiation therapy currently used in our institution. All those patients signed consent form before starting the treatment.
Treatments
Breast surgery
As previously described, axillary dissection concerned Level I and II axillary lymph node area while sentinel lymph node biopsy alone was also achieved with per-operative exam and conversion to axillary dissection in case of positive biopsy. Then, lumpectomy was performed. Quality of margins was assessed by a per-operative pathological exam. Four to five clips were clamped by the surgeon to mark the tumor bed before closing the tumor bed cavity [11].
Brachytherapy
Brachytherapy was performed according to the GEC-ESTRO Breast Cancer Working Group recommendations for MIB APBI [12]. As previously described, vectors (Sharp Needles™; Elekta AB, Stockholm, Sweden) were placed mainly intra-operatively by the radiation oncologist using 1 to 3 planes in respect with Paris system recommendations [11]. Two days after the implant, a post implant CT (2.5 mm thickness slice) was performed in order to delineate the clinical target volume (CTV) based on clips and surgical cavity (if visible) including a total safety margin of about 2 cm (sum of the resection margin size and “added” safety margins size) [13]. Then, the dose distribution was optimized manually (OncentraBrachy®; Elekta, Sweden) by varying time and stop position of the radioactive source. A single fraction of 16 Gy was prescribed to the 100% isodose. This dose was calculated considering an α/β ratio of 3.4 Gy for breast late toxicity and 4.6 for local control [14]. According to the linear quadratic model, the equivalent dose at 2 Gy (EQD2) for a single fraction of 16 Gy is equal to 53 Gy with a α/β = 4 Gy [15, 16]. Dose constraints were as follow: D90% ≥ 105% of the prescribed dose, D100% ≥ 75%, V100 > 95% of the CTV, V150 ≤ 40%, V200 ≤ 15%; dose non-homogeneity ratio (DNR) ≤ 35% [10]. For organ at risk (skin and thoracic wall), the maximum skin-dose was < 75% of the prescribed dose while the maximum rib dose was < 100% of the prescribed dose.
Systemic therapy
Systemic therapies such as adjuvant chemotherapy and/or hormonal treatments were dispensed according to the protocols used in the Antoine Lacassagne Cancer Center.
Follow up
The radiation oncologist performed iterative monthly post-brachytherapy clinics during 3 months (acute brachytherapy side effects). Then, clinical surveillance was performed alternatively with the surgeon twice a year with a yearly mammogram. Acute (< 180 days after treatment) and late toxicities were evaluated by Common Terminology Criteria for Adverse Event v3 (CTCAE.V3.0) [17]. Cosmetic evaluation was performed according to Harvard criteria [18].
Statistical analysis
Description of the study population and of the different investigated parameters was made using absolute and relative frequencies for the qualitative data and summarized using descriptive statistics such as median, extreme for quantitative data. Survival time was defined between the surgery date and the event date. Local relapse free-survival (LRFS), regional relapse free-survival (RRFS), specific (SS) and overall survivals (OS) were estimated using the Kaplan-Meier method. Patients still alive were censored at the date of last follow-up. Median follow-up with 95% confidence intervals was calculated by reverse Kaplan–Meier method. Data entry and data management were performed on Ennov clinical® system and were analyzed using R 3.2.2 for Windows®.
Results
Patient and tumor characteristics
Between January 2012 and August 2015, a total of 51 lesions from 48 patients (pts) were treated with a sfAPBI. Among these patients, 26 were part of the SiFEBI trial while 22 pts. were treated out of the phase II study. Patient, tumor and treatment features are detailed in Table 1. Patient median age was 77.7 years [range: 65–92]. Most of patients were ECOG Performans Status (PS) 0 (85%). The most frequent location was the upper external quadrant (39.2%). Histological type was mainly invasive ductal carcinoma (86.2%). The median tumor size was 12 mm [range: 3–32] while, 4 pts. presented with a microscopic node involvement (Nmic) and 1 pt. was classified N1. The median surgical margin was 5 mm [range: 1–10]. One lesion was associated with peri-neural invasion. All the tumors but three had positive hormonal receptor status while Her-2 status was over-expressed in 9.8%.
Table 1.
Patients, lesions and treatment characteristics
| Patient features | Number of patients | % / (min – max) |
|---|---|---|
| Patients included in SiFEBI trial | ||
| Yes | 26 | 54.2 |
| No | 22 | 45.8 |
| Mean age (years) | 77.7 | (65.2–92.3) |
| ECOG-Performans Status | ||
| 0 | 41 | 85.5 |
| 1 | 7 | 14.5 |
| Tumor side | ||
| Left | 28 | 54.9 |
| Right | 23 | 45.1 |
| Location | ||
| Upper external quadrant | 20 | 39.2 |
| Upper internal quadrant | 5 | 9.8 |
| Lower internal quadrant | 3 | 5.9 |
| Lower external quadrant | 3 | 5.9 |
| Junction of external quadrant | 6 | 11.8 |
| Junction of internal quadrant | 3 | 5.9 |
| Junction of lower quadrant | 3 | 5.9 |
| Junction of upper quadrant | 6 | 11.7 |
| Periareolar | 2 | 3.9 |
| Median tumor size (mm) | 12 | (3–32) |
| Tumor stage | ||
| T1a | 28 | 54.9 |
| T1b | 18 | 35.3 |
| T1c | 5 | 9.8 |
| Axillary lymph node status | ||
| N0 | 46 | 90.1 |
| N1mic | 4 | 7.9 |
| N1 | 1 | 2.0 |
| Histology type | ||
| Invasive ductal carcinoma | 44 | 86.3 |
| Invasive lobular carcinoma | 3 | 5.9 |
| Other | 4 | 7.8 |
| Histological grade | ||
| 1 | 32 | 62.7 |
| 2 | 14 | 27.4 |
| 3 | 5 | 9.8 |
| Hormonal status | ||
| Positive | 48 | 94.1 |
| Negative | 3 | 5.9 |
| Her-2 status | ||
| Over-expressed | 5 | 9.8 |
| Non-over-expressed | 46 | 90.2 |
| Peri-neural invasion | ||
| Yes | 1 | 1.9 |
| No | 50 | 98.1 |
| Median Ki-67 (%) | 10 | (5–60) |
| Median surg. Marg.(mm) | 5 | (1–10) |
| Implant time | ||
| Intra operative | 47 | 92.2 |
| Post-operative | 4 | 7.8 |
| Median time interv. Surg./APBI (d) | 7 | (1–63) |
| Median number of vectors | 11 | (5–15) |
| Median number of planes | 2 | (1–3) |
| Median CTV (cc) | 44 | (11–124) |
| Median V100% (%) | 96 | (86–100) |
| Median V150% (%) | 34 | (23–48) |
| Median V200% (%) | 12 | (8–21) |
| Median DNR | 0.35 | (0.23–0.56) |
Median time interv. Surg./APBI: median time between intervention and sfAPBI; Median surg. Marg.: median surgical margins; DNR: dose non-homogeneity ratio = V100/V150
Treatment characteristics
A median number of 11 vectors [range: 5–15] on 2 planes [range: 1–3] were implanted (depending on the thickness and location of the target volume), mainly intra-operatively (92.2%). The median time between surgery and sfAPBI was 7 days [range: 1–63]. The median CTV was 44 cc [range: 11–124]. The median V100% was 96% [range: 86–100] (Table 1). The median treated volume was 42 cc [range: 10–124].
Oncological outcome
With a median follow–up of 40 months [range: 36–42], no local relapse occurred while 1 pt. developed an axillary relapse (2.1%). Three-year LRFS, RRFS and SS were 100%, 3-year OS was 93.1% [86.4–1] (Fig. 1).
Fig. 1.
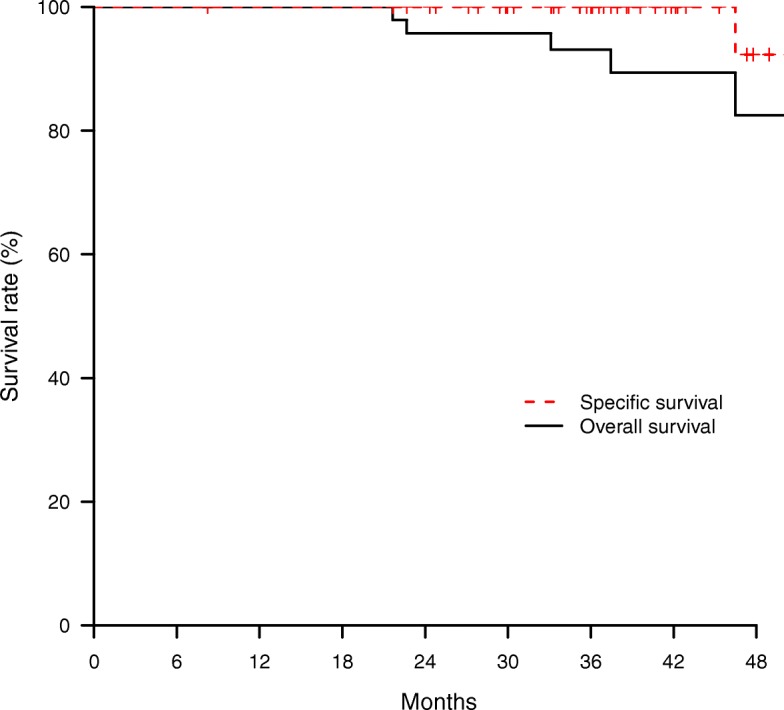
Specific and overall survival
Acute and late toxicity
Forty-five acute events were remained. The most frequent acute toxicity was grade (G) 1 hyperpigmentation (26.7%). Three pts. presented G3 acute toxicity (2 breast hematomas, 1 breast abscess). No ≥G3 late toxicity was observed while 15 late toxicities occurred (G1: 11 events [80%]). G1 breast fibrosis and hypopigmentation of puncture site were the most frequent late side effects. The rate of excellent cosmetic outcome was 76.4%. A breast asymmetry was noticed in 2 pts. (4%) (Table 2).
Table 2.
Acute and late toxicity outcome
| Toxicity | Number of events | % |
|---|---|---|
| Acute | ||
| Grade 1 | ||
| Hyperpigmentation | 12 | 26.7 |
| Epithelitis | 3 | 6.7 |
| Breast hematoma | 2 | 4.4 |
| Other | 13 | 28.9 |
| Total | 30 | 66.7 |
| Grade 2 | ||
| Breast pain | 2 | 4.4 |
| Breast hematoma | 2 | 4.4 |
| Skin hyperpigmentation | 2 | 4.4 |
| Other | 6 | 13.3 |
| Total | 12 | 26.7 |
| Grade 3 | ||
| Breast hematoma | 2 | 4.4 |
| Breast infection | 1 | 2.2 |
| Total | 3 | 6.7 |
| Total number of events | 45 | 100 |
| Late | ||
| Grade 1 | ||
| Breast fibrosis | 4 | 26.7 |
| Puncture site hypopig. | 5 | 33.3 |
| Telangectasia | 2 | 13.3 |
| Epithelitis | 1 | 6.7 |
| Other | 1 | 6.7 |
| Total | 13 | 86.7 |
| Grade 2 | ||
| Breast fibrosis | 2 | 13.3 |
| Total number of events | 15 | 100 |
| Cosmetic outcome | ||
| Excellent | 39 | 76.4 |
| Good | 12 | 25.6 |
Puncture site hypopig.: Puncture site hypopigmentation
Discussion
APBI is now recognized as a validated irradiation option for low-risk breast cancer [12]. The current challenge is to deliver irradiation dose in the shortest treatment duration, in the most appropriate population. From a technical point of view, 2 different APBI approaches can be proposed: Intraoperative irradiation (electron or low-energy X photon [50 kV]) or postoperative irradiation either with brachytherapy (multicatheter interstitial brachytherapy [MIB] / balloon devices) or external beam irradiation (3D or Intensity modulated) [19]. Intraoperative irradiation (IORT) allows the optimal reduction of the treatment duration since the patient is irradiated during the lumpectomy process. However, at least 15% of patients are partially irradiated while the definitive histology is not suitable for this treatment [20]. Consequently, those patients need a post-operative whole breast irradiation (WBI) while the intra-op APBI is considered as a “boost”. Post-operative irradiation does permit treating only validated candidates for APBI due to an appropriate definitive pathological report compatible with APBI criteria while the number of transportation remains significantly higher compared to IORT. Furthermore, MIB-APBI allows best target coverage [21]. In this frame, a post-operative single fraction APBI appeared as an attractive technical option by drastically reducing the number of transportations and in the same time, alleviating the treatment related constraints mainly for elderly patients with frequent comorbidities [22].
In our study, after a median follow-up of 40 months, no local relapse was observed while SS rate was 100%. Two studies already reported oncological outcome after a MIB sfAPBI. In the SiFEBI prospective phase II trial, 26 elderly patients were treated with a 16 Gy sfAPBI. After a median follow-up of 37 months, there was no local relapse [10]. Recently, Latorre et al. reported the results of 20 pts. treated with a MIB sfAPBI of 18 Gy. After a median follow-up of 20 months, there was no local relapse [23]. Other teams investigated very hypofractionnated APBI, based on different regimens (1 to 7 fractions in 1 to 2 consecutive days) and HDR irradiation techniques (Per-operative and balloon devices) [24–27]. The results of those studies (oncological outcome and toxicities) are summarized in Table 3.
Table 3.
Very hypo-fractionnated APBI discribed in the litterature
| Authors | Year | # pts | MFU (months) | APBI techniques | Total dose (Gy) | D/f (Gy) | AG3 to (%) | LG3 tox (%) | LF (%) | RF (%) | DM (%) | Ex/good cosmetic result (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sacchini | 2008 | 18/34 | 31 | HDRIORT | 20/18 | 20/18 | 7.7 | – | 0 | – | – | c |
| Khan | 2013 | 30 | 11 | Balloona | 28 | 7 (BID) | 0 | 0 | – | – | – | – |
| Wilkinson | 2012/17 | 45 | 74 | Balloonb | 28 | 7 (BID) | 13.3 | 2 | 0 | 0 | 0 | 91 |
| Showalter | 2016 | 28 | 6 | HDRIORT | 12.5 | 12.5 | 0 | – | – | – | – | 93 |
| Hannoun-Levi | 2017 | 26 | 37 | HDRMIB | 16 | 16 | 7.6 | 0 | 0 | 0 | – | 88 |
| Latorre | 2018 | 20 | 24 | HDRMIB | 18 | 18 | 0 | 0 | 0 | 0 | 5 | 80 |
| Present study | 2018 | 48 | 40 | HDRMIB | 16 | 16 | 6.3 | 0 | 0 | 0 | 0 | 100 |
aContura™
bMammosite™
cCosmetic results were better with 18 Gy compared to 20 Gy
# Pts: number of patients; MFU: median follow-up; APBI: accelerated partial breast irradiation; HDRIORT: high-dose rate brachytherapy performed intraoperatively; MIB: multicatheter interstitial high-dose rate brachytherapy; D/f: dose per fraction; AG3 tox: acute ≥ Grade 3 toxicity; LG3 tox: late ≥ Grade 3 toxicity; LF: local failure; RF: regional failure; DM: distant metastasis; Ex/good cosmetic: percentages of excellent and good cosmetic results
Regarding MIB sfAPBI side effects, we did not report G ≥ 3 late toxicity confirming the safety of this approach already reported by others (Table 3). However, Sacchini et al. had to decrease the per-operative delivered dose from 20 Gy to 18 Gy due to the unexpectedly high-rate of acute toxicity.
Specifically for elderly patients presenting with a low-risk positive hormonal status breast cancer, the omission of adjuvant radiation therapy was suggested in order to alleviate the treatment. In those phase III randomized trials (Surgery + hormonal therapy with or without adjuvant WBI), there was no significant difference in terms of overall survival between irradiated and non-irradiated patients [28, 29]. However, there was a significant over-risk of local recurrence for patient without adjuvant breast irradiation. In elderly patients, the impact on functional status must be taken in account in treatment decision. The GERICO-O3 phase II trial aimed to evaluate the impact on functional status of MIB APBI in a cohort of 46 elderly women (median age: 74 years). Activity Daily Living (ADL) and Instrumental Activity Daily Living (IADL) scales were evaluated before and after ABPI. The scores remained unchanged at 6 and 12 months after APBI confirming no deleterious impact of MIB APBI [30].
Conclusion
We reported promising and encouraging clinical outcome of a post-operative single fraction of MIB ABPI in the elderly. This approach leads to consider a sfAPBI as an attractive alternative to intra-operative radiation therapy while all the patients will be good candidates for APBI in regards to the post-operative pathological report. SfAPBI allows to drastically reducing the number of transportations and could benefit to the patients by decreasing fatigue and to the society by lowering cost related to transportations. More mature results (number of patients and follow-up) are needed to confirm the results.
Acknowledgments
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ADL
Activity Daily Living
- APBI
Accelerated partial breast irradiation
- ASTRO
American Society of Radiation Oncology
- DNR
Dose non-homogeneity ratio
- GEC-ESTRO
Groupe Européen de Curiethérapie of the European Society for Radiotherapy and Oncology
- HDR
High-dose rate
- IADL
Instrumental Activity Daily Living
- IORT
Intraoperative radiation therapy
- LRFS
Local relapse-free survival
- MIB
Multicatheter interstitial brachytherapy
- OS
Overall survival
- PS
Performans Status
- RRFS
Regional relapse-free survival
- sfAPBI
Single fraction APBI
- SS
Specific survival
- WBI
Whole breast irradiation
Authors’ contributions
RK: acquisition of data and analysis, manuscript writing and final approval, MEC: data analysis and final approval, JG: statistical analysis and final approval, MG: data analysis and final approval, LM: acquisition of data and analysis and final approval, DLCK: data analysis and final approval, JMHL: Study concept, design, acquisition of data and analysis, manuscript writing and final approval.
Ethics approval and consent to participate
This study was approved by the local ethic committee of the Antoine Lacassagne Cancer Center.
Consent for publication
Institutional consent form was obtained.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rémy Kinj, Email: remy.kinj@nice.unicancer.fr.
Marie-Eve Chand, Email: Marie-Eve.CHAND@nice.unicancer.fr.
Jocelyn Gal, Email: jocelyn.gal@nice.unicancer.fr.
Mathieu Gautier, Email: mathieu.gautier@nice.unicancer.fr.
Lucile Montagné, Email: lucile.montagne@nice.unicancer.fr.
Daniel Lam Cham Kee, Email: Daniel.LAMCHAMKEE@nice.unicancer.fr.
Jean Michel Hannoun-Lévi, Phone: (+33) 4 92 03 16 23, Email: jean-michel.hannoun-levi@nice.unicancer.fr.
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing Total for the treatment of invasive breast Cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trialists TS. The UK Standardisation of breast radiotherapy (START) trial a of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.START Trialists’ Group TST. Bentzen SM, Agrawal RK, et al. The UK standardisation of breast radiotherapy (START) trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whelan T. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast Cancer. CancerSpectrum Knowl Environ. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 6.Correa C, Harris EE, Leonardi MC, et al. Accelerated partial breast irradiation: executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;7:73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Polgár C, Van Limbergen E, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strnad V, Ott OJ, Hildebrandt G, et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast : a randomised, phase 3, non-inferiority trial. Lancet. 2016;387:229–238. doi: 10.1016/S0140-6736(15)00471-7. [DOI] [PubMed] [Google Scholar]
- 10.Hannoun-Lévi JM, Cham Kee DL, Gal J, et al. Accelerated partial breast irradiation for suitable elderly women using a single fraction of multicatheter interstitial high-dose-rate brachytherapy: early results of the single-fraction elderly breast irradiation (SiFEBI) phase I/II trial. Brachytherapy. 2018;17:407–414. doi: 10.1016/j.brachy.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Genebes C, Chand M-E, Gal J, et al. Accelerated partial breast irradiation in the elderly: 5-year results of high-dose rate multi-catheter brachytherapy. Radiat Oncol. 2014;9:115. doi: 10.1186/1748-717X-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strnad V, Major T, Polgar C, et al. ESTRO-ACROP guideline: Interstitial multi-catheter breast brachytherapy as Accelerated Partial Breast Irradiation alone or as boost - GEC-ESTRO Breast Cancer Working Group practical recommendations. Radiother Oncol. 2018; in press [DOI] [PubMed]
- 13.Strnad V, Hannoun-Levi J-M, Guinot J-L, et al. Recommendations from GEC ESTRO breast Cancer working group (I): target definition and target delineation for accelerated or boost partial breast irradiation using multicatheter interstitial brachytherapy after breast conserving closed cavity surgery. Radiother Oncol. 2015;115:342–348. doi: 10.1016/j.radonc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Haviland JS, Owen JR, Dewar JA, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 15.Dutreix J, Cosset JM, Girinsky T. Biological equivalency of high single doses used in intraoperative irradiation. Bull Cancer Radiother. 1990;77:125–134. [PubMed] [Google Scholar]
- 16.Wheldon TE, Deehan C, Wheldon EG, Barrett A. The linear-quadratic transformation of dose-volume histograms in fractionated radiotherapy. Radiother Oncol. 1998;46:285–295. doi: 10.1016/S0167-8140(97)00162-X. [DOI] [PubMed] [Google Scholar]
- 17.Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 18.Harris JR, Levene MB, Svensson G, Hellman S. Analysis of cosmetic results following primary radiation therapy for stages I and II carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979;5:257–261. doi: 10.1016/0360-3016(79)90729-6. [DOI] [PubMed] [Google Scholar]
- 19.Chand-Fouché M, Hannoun-Lévi JM. State of the art and perspectives of accelerated partial breast irradiation in 2014. Cancer Radiother. 2014;18:693–700. doi: 10.1016/j.canrad.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 21.Cozzi S, Laplana M, Najjari D, et al. Advantages of intraoperative implant for interstitial brachytherapy for accelerated partial breast irradiation either frail patients with early-stage disease or in locally recurrent breast cancer. J Contemp Brachytherapy. 2018;10:97–104. doi: 10.5114/jcb.2018.75594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannoun-Levi JM, Courdi A, Marsiglia H, Namer M, Gerard JP. Breast cancer in elderly women: is partial breast irradiation a good alternative? Breast Cancer Res Treat. 2003;81:243–251. doi: 10.1023/A:1026166518203. [DOI] [PubMed] [Google Scholar]
- 23.Latorre JA, Galdós P, Buznego LA, et al. Accelerated partial breast irradiation in a single 18 Gy fraction with high-dose-rate brachytherapy: preliminary results. J Contemp Brachytherapy. 2018;10:58–63. doi: 10.5114/jcb.2018.73994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacchini V, Beal K, Goldberg J, Montgomery L, Port E, McCormick B. Study of quadrant high-dose intraoperative radiation therapy for early-stage breast cancer. Br J Surg. 2008;95:1105–1110. doi: 10.1002/bjs.6208. [DOI] [PubMed] [Google Scholar]
- 25.Khan AJ, Vicini FA, Brown S, et al. Dosimetric feasibility and acute toxicity in a prospective trial of ultrashort-course accelerated partial breast irradiation (APBI) using a multi-lumen balloon brachytherapy device. Ann Surg Oncol. 2013;20:1295–1301. doi: 10.1245/s10434-012-2671-1. [DOI] [PubMed] [Google Scholar]
- 26.Ben WJ, Martinez AA, Chen PY, et al. Four-year results using balloon-based brachytherapy to deliver accelerated partial breast irradiation with a 2-day dose fractionation schedule. Brachytherapy. 2012;11:97–104. doi: 10.1016/j.brachy.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson JB, Chen PY, Wallace MF, et al. Six-year results from a phase I/II trial for Hypofractionated accelerated partial breast irradiation using a 2-day dose schedule. Am J Clin Oncol. 2017;1 10.1097/COC.0000000000000402. [DOI] [PubMed]
- 28.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunkler IH, Williams LJ, Jack WJL, Cameron DA, Dixon JM. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 30.Hannoun-Levi J-M, Gourgou-Bourgade S, Belkacemi Y, et al. GERICO-03 phase II trial of accelerated and partial breast irradiation in elderly women: feasibility, reproducibility, and impact on functional status. Brachytherapy. 2013;12:285–292. doi: 10.1016/j.brachy.2012.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


