Abstract
NMDA-type ionotropic glutamate receptors (NMDARs) play an important role in the regulation of synapse development and function in the brain. Recently we have shown that NMDARs are critical for GABAergic synapse development in developing hippocampal neurons. However, it remains unclear whether NMDARs are important for establishment of GABAergic synaptic transmission in other types of neurons in the brain. Here we report that in both cortical pyramidal neurons and midbrain dopamine neurons in ventral tegmental area (VTA), genetic deletion of the GluN1 subunit, which is required for assembly of functional NMDARs, leads to a strong reduction of GABAergic synaptic transmission. These data demonstrate that NMDARs play an important role in the development of GABAergic synaptic transmission in two types of neurons with distinct developmental origins, and suggest that NMDARs are commonly involved in development of GABAergic synaptic transmission in different types of neurons in the brain.
Keywords: GABAergic synapse, synapse development, mIPSC, NMDA receptor, dopamine neuron, cortical pyramidal neuron, in utero electroporation
Introduction
In the central nervous system, neurotransmitter-mediated synaptic communication between neurons is essential for proper neural circuit function. The formation and functional maturation of synapses are highly regulated processes and subject to activity-dependent refinement [1, 2]. When synapse development goes awry, devastating neurological and psychiatric diseases may occur [3-6]. Thus, it is important to understand the molecular and cellular mechanisms underlying the regulation of synapse development.
NMDA-type ionotropic glutamate receptors (NMDARs) are a subclass of glutamate-gated ion channels [7]. In mature neurons, NMDARs are highly concentrated at glutamatergic synapses, whereby they contribute to excitatory synaptic transmission and are implicated in several forms of synaptic plasticity [8]. Interestingly, NMDARs are expressed on neuronal surface long before glutamatergic synaptogenesis. For example, functional NMDARs are detectable in embryonic day 14-16 (E14-16) cortical neurons [9-12]. In developing neurons, it has been shown that NMDARs negatively regulate functional maturation of glutamatergic synapses. Indeed, genetic deletion of NMDARs in developing hippocampal, cortical and midbrain dopamine neurons strongly up-regulates AMPA receptor (AMPARs)-mediated synaptic transmission [13-21]. As AMPARs are the primary mediators of fast excitatory synaptic transmission, these data indicate that NMDARs in developing neurons prevent AMPARs from trafficking to synapses, and thus keep glutamatergic synapses in the silent state [13, 15, 16]. In addition, there is evidence showing that NMDARs are not required for glutamatergic synaptogenesis in hippocampal pyramidal neurons [15, 16, 22], but are important in other types of neurons [13, 14, 21].
In addition to regulating glutamatergic synapses, NMDARs have been implicated in inhibitory GABAergic synapse development. Early work has shown that NMDAR activity promotes GABAergic synapse development [23]. Recently we have demonstrated that NMDARs in immature hippocampal neurons are necessary for GABAergic synapse development [24]. Specifically, single-cell genetic deletion of the NMDAR GluN1 subunit in hippocampal progenitor cells at E14 strongly impaired GABAergic synapse formation and reintroduction of NMDARs into the GluN1-deficient neurons rescued the GABAergic synapse deficit [24]. These data demonstrate an important role for NMDARs in the development of GABAergic synaptic transmission in developing hippocampal neurons. However, it remains unclear whether NMDARs are also important for GABAergic synapse development in other neuronal types. The answer to this question will be valuable as it will determine whether NMDAR-dependent GABAergic synapse development is a mechanism widely employed by different types of neurons in the brain. Here we employed a gene inactivation approach to remove NMDARs in developing cortical pyramidal neurons and midbrain dopamine neurons, and examined GABAergic synaptic transmission. We found that GABAergic synaptic transmission is strongly reduced in both types of developing neurons lacking NMDARs, indicating that NMDARs are important for the establishment of GABAergic synaptic transmission in a variety of neuronal types in the brain.
Material and Methods
Mouse genetics
Animal housing and handling was conducted according to the ACUC guidelines at NINDS, NIH. Grin1f/f (#005246) and Ai14 tdTomato (tdTomatoCre) (#007908) mice were purchased from Jackson laboratory. DAT-Cre mice [25] were provided by Dr. Thomas Hnasko at UCSD.
Plasmids
Lentiviral vector pFUGW-Cre-mCherry (Cre fused to mCherry, Cre-mCherry) plasmid was used in the current study. In this plasmid, Cre-mCherry expression was driven by a ubiquitin promoter.
In utero electroporation.
In Utero electroporation was performed as described previously [24]. Briefly, a timed-pregnant Grin1f/f mouse at 14.5 day of gestation (E14.5) was anesthetized with isofluorane. The abdominal cavity was opened and 6-8 embryos in the uterine horns were gently exposed. Plasmid DNA (approximately 1 to 2 μl) were manually injected into the lateral ventricles of each embryo at the concentration of 2 μg/μl mixed with 0.05% fast green (Sigma 68724). The injection glass pipettes were beveled with the BV-10 micropipette Beveller (Sutter) before injection. After each injection, voltage steps via tweezertrodes (5 mm round, platinum electrodes and BTX electroporator, BTX, ECM830) positioned on either side of the head were applied across the uterus to target hippocampal or cortical neural progenitors. Voltage was 45 V for 5 pulses at 1 Hz, each pulse lasting 50 ms. The embryos were moistened with warmed PBS and returned to the abdominal cavity. Buprenex (0.1 mg/kg) was put into to the abdominal cavity before the wound was sutured. Ketoprofen (5 mg/kg) was administered daily for three days after surgery.
Immunohistochemistry.
Mice were perfused via cardiac infusion with 4% paraformaldehyde in cold PBS. To obtain frozen sections, brain tissues were removed and submerged in 30% sucrose for 24 h and sectioned at 40 μm thickness with a cryostat (Leica CM1850). Three to five brain slices containing the ventral tegmental area (VTA) or the Cre-mCherry targeted area of primary motor cortex in in utero electroporation were permeabilized in 0.3% Triton X-100 for 15 minutes. Slices were incubated in primary antibodies (with 3% normal goat serum and 0.3% Triton X-100) overnight at 4 °C after blocking in 10% normal goat serum for 1 h at room temperature. After extensively washed in PBS, slices were incubated with Alexa 488, Alexa 594 or Alex 633-conjugated secondary antibodies (1:500). Slices were mounted with DAPI Fluoromount-G (SouthernBitotech 0100-01) and imaged by LSM510 confocal microscope with 20X objective for overview images of primary motor cortex and VTA, and 40X objective for detailed images of dopaminergic neuron in VTA. Antibodies specific to tyrosine hydroxylase (TH) (Millipore AB152, 1:500) and NeuN (Millipore MAB377, 1:1000) were used to stain dopaminergic neurons and all neurons in VTA, respectively.
Electrophysiology.
For whole-cell recording in acute slices, coronal cortical slices (300 μm) or horizontal midbrain slices (for recording in VTA dopamine neurons, 200 μm) were cut from dissected brain on a DTK Microslicer vibratome (Ted Pella) in chilled high sucrose cutting solution, containing (in mM): KCl 2.5, CaCl2 0.5, MgCl2 7, NaH2PO4 1.25, NaHCO3 25, glucose 7, sucrose 210 and ascorbic acid 1.3. Freshly cut slices were placed in an incubating chamber containing carbogenated artificial cerebrospinal fluid (ACSF), containing (in mM) NaCl 119, KCl 2.5, NaHCO3 26, Na2PO4 1, glucose 11, CaCl2 2.5, MgCl2 1.3, and recovered at 32°C for ~30-60 min. Slices were then maintained in ACSF at room temperature prior to recording. After 0.5-1 h incubation at room temperature, slices were transferred to a submersion chamber on an upright Olympus microscope, perfused in ACSF. For recording NMDAR-mediated EPSCs at +40 mV, NBQX (10 μM) and picrotoxin (100 μM) were added to ACSF. For recording mIPSCs at −70 mV, NBQX (10 μM) and TTX (0.5 μM) were added to ACSF. All the pharmacological reagents were purchased from Abcam.
Neurons were visualized by infrared differential interference contrast microscopy. mCherry positive neurons were identified by epifluorescence microscopy. The intracellular solution for NMDA EPSC recording contained (in mM) CsMeSO4 135, NaCl 8, HEPEs 10, Na3GTP 0.3, MgATP4, EGTA 0.3, QX-314 5, and spermine 0.1. The intracellular solution for GABA mIPSC recording contained (in mM) CsMeSO4 70, CsCl 70, NaCl 8, EGTA 0.3, HEPES 20, MgATP 4 and Na3GTP 0.3. Osmolality was adjusted to 285-290 mOsm and pH was buffered at 7.25-7.35. Neurons were recorded with 3- to 5- MΩ borosilicate glass pipettes. For recording evoked responses in VTA dopamine neurons, the tip of the monopolar glass electrode was placed 50-100 μm from the recorded neurons. For recording evoked responses in layer 2/3 neurons in primary motor cortical region, the monopolar glass electrodes were placed ~50 μm lateral to layer 2/3 pyramidal neurons. All evoked responses in acute slices were paired recordings involved simultaneous whole-cell recordings from one fluorescence-positive neuron and a neighboring fluorescence-negative neuron. The stimulus was adjusted to evoke a measurable, monosynaptic EPSC in both cells. Most mIPSC recordings in acute brain slices were also paired recordings involved simultaneous whole-cell recordings from one fluorescence-positive neuron and one neighboring fluorescence-negative neuron. Series resistance was monitored and not compensated, and cells in which series resistance varied by 25% during a recording session were discarded. Synaptic responses were collected with a Multiclamp 700B-amplifier (Axon Instruments, Foster City, CA), filtered at 2 kHz, digitized at 10 Hz. All recordings were performed at room temperature.
Statistics
Statistical analysis was performed using GraphPad Prism 6. All data were presented as Mean ± SEM (standard error of mean). Paired whole-cell recordings were compared with paired t-test. Other comparisons between two groups were made using two-tailed, unpaired Student's t-test (Mann-Whitney test). Cumulative distributions were compared by the Kolmogorov-Smirnov test. Statistical significance was defined as p < 0.05, 0.01 or 0.001 (indicated as *, ** or ***, respectively). p values ≥0.05 were considered not significant.
Results
We have previously shown that NMDARs are important for the development of GABAergic synaptic transmission in developing hippocampal neurons[24]. However, it remained unclear whether NMDAR-dependent development of GABAergic synaptic transmission is a hippocampal neuron-specific phenomenon, or, NMDARs are also important for establishing GABAergic synaptic transmission in other types of neurons in the brain. To study this question, we examined GABAergic synaptic transmission in cortical neurons or midbrain dopamine neurons in which the GluN1 subunit of the NMDAR was genetically deleted.
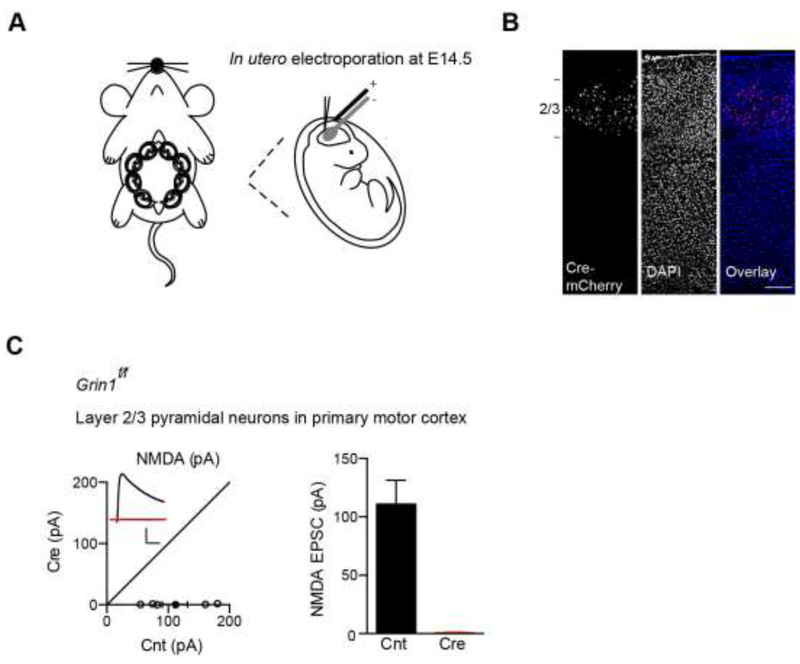
We first performed in utero electroporation to express Cre fused to mCherry (Cre-mCherry) in layer 2-3 primary motor cortical progenitor neurons of E14.5 Grin1f/f mice (Fig. 1A). About three weeks after in utero electroporation, we performed a histological assay and found that Cre-mCherry were specifically expressed in layer 2-3 pyramidal neurons in the primary motor cortex (Fig. 1B). We then prepared acute coronal cortical slices and performed simultaneous dual whole-cell recordings to measure NMDAR-mediated excitatory postsynaptic currents (EPSCs) at +40 mV between a transfected Cre-mCherry-positive layer 2/3 pyramidal neurons and a nearby control pyramidal cell in the primary motor cortex in the presence of NBQX and picrotoxin to block AMPAR- and GABAAR-mediated synaptic transmission, respectively. We found that NMDAR-mediated EPSCs were lost in Cre-positive neurons, demonstrating that functional NMDARs are genetically eliminated in these cells (Fig. 1C).
Figure 1. Genetic deletion of NMDARs in layer 2-3 pyramidal neurons in motor cortex.
(A) Schematic of in utero electroporation of Cre-mCherry performed at E14.5 GRIN1f/f embryos.
(B)Expression of Cre-mCherry in layer 2/3 pyramidal neurons in Grin1f/f primary motor cortex.
(C)Dual recordings showed that Cre expression led to loss of NMDA EPSCs in layer 2/3 pyramidal neurons in cortical slices made from P13-P17 mice (Control (Cnt), 110.9 ± 20.5 pA, n = 6; Cre, 0.80 ± 0.32 pA, n = 6; p < 0.01; paired t-test). Scale bar: 50 pA and 50 ms.
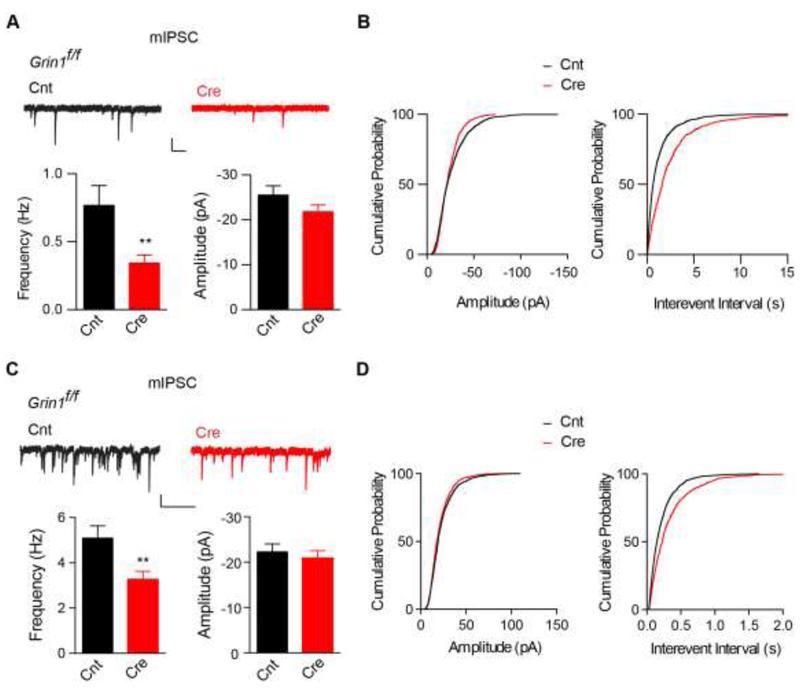
We next measured miniature inhibitory postsynaptic currents (mIPSCs) at the −70 mV to examine GABAergic synaptic transmission in Cre-mCherry positive layer 2-3 neurons in the primary motor cortex in the presence of NBQX and AP-5 to block glutamatergic synaptic transmission. In developing neurons from P9-10 cortical slices, GABAergic synaptic transmission was strongly reduced in Cre-mCherry positive, layer 2-3 pyramidal neurons as there was a nearly 60% reduction of mIPSC frequency (Fig. 2A-B). In addition, although there was a trend for lower mIPSC amplitude in Cre-mCherry positive neurons, it was not significant (Fig. 2A-B). Similarly, in layer 2-3 neurons at P17-18 mice, genetic deletion of NMDARs also caused a substantial reduction of mIPSC frequency, but not amplitude (Fig. 2C-D), suggesting that genetic deletion of NMDARs during development has a lasting effect on GABAergic synaptic transmission in these neurons. Taken together, these data indicate that loss of NMDARs in cortical pyramidal neurons strongly impairs the establishment of GABAergic synaptic transmission.
Figure 2. Loss of NMDARs in layer 2-3 pyramidal neurons in motor cortex decreases GABAergic synaptic transmission.
(A-B) Cre expression caused a strong reduction of mIPSC frequency in layer 2/3 pyramidal neurons from primary motor cortex from P9-10 Grin1f/f mice (Frequency: Cnt (0.77 ± 0.14 Hz, n = 10), Cre (0.34 ± 0.06 Hz, n = 11), p <0.01, t-test; Amplitude: Cnt (−25.5 ± 2.1 pA, n = 10), Cre (−21.8 ± 1.6 pA, n = 11), p = 0.67, t-test). Cumulative distributions were compared by the Kolmogorov-Smirnov test (p ≥ 0.05 for amplitude and p < 0.001 for interevent interval). Scale bar: 20 pA and 1s.
(C-D) Cre expression caused a significant reduction of mIPSC frequency in layer 2/3 pyramidal neurons from primary motor cortex from P17-18 Grin1f/f mice (Frequency: Cnt (5.1 ± 0.5 Hz, n = 9), Cre (3.3 ± 0.3 Hz, n = 8), p < 0.01, t-test; Amplitude: Cnt (−22.4 ±1.6 pA, n = 9), Cre (−21.0 ± 1.5 pA, n = 8), p = 0.74, t-test). Cumulative distributions were compared by the Kolmogorov-Smirnov test (p ≥ 0.05 for amplitude and p < 0.001 for interevent interval). Scale bar: 20 pA and 1s.
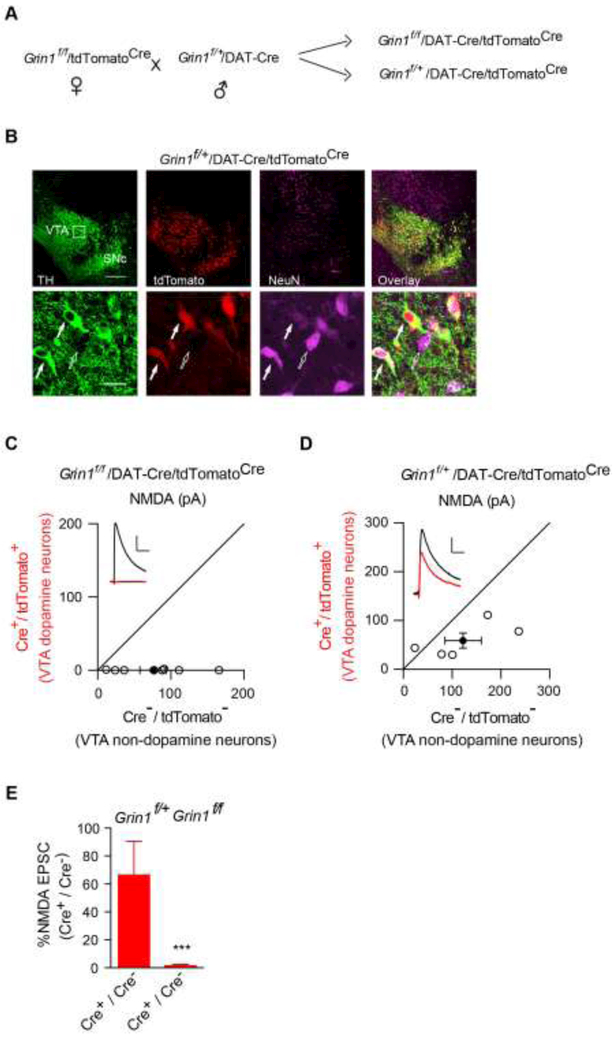
It is well-known that pyramidal neurons in hippocampus and cortex share many common morphological and functional properties[26]. We thus sought to test whether NMDARs are important for the establishment of GABAergic synaptic transmission in a distinct type of neurons, such as midbrain dopamine neurons. To this end, we crossed a well-characterized knockin mouse line in which the expression of Cre is under the control of the DAT (dopamine transporter) promoter[25] that expresses Cre specifically in midbrain dopamine neurons at around E15 with the conditional GluN1 knockout (Grin1f/f) mice. In addition, we crossed a Cre-dependent tdTomato reporter mouse line (Ai14, tdTomatoCre) with Grin1f/f. We then crossed Grin1f/+/DAT-Cre with Grin1f/f/tdTomatoCre to generate Grin1f/f/DAT-Cre/tdTomatoCre mice or Grin1f/+/DAT-Cre/tdTomatoCre mice that served as the control (Fig. 3A). In this three-gene modified mouse line, Cre expression led to genetic deletion of the conditional GluN1 alleles and simultaneously the expression of tdTomato, a red fluorescent protein, in dopamine neurons, to facilitate electrophysiological recordings (Fig. 3B). We further confirmed the loss of NMDARs in dopamine neurons with a dual whole-cell recording approach in horizontal brain slices containing the ventral tegmental area (VTA) as described before[27]. In these experiments, we performed paired whole-cell recordings of NMDAR-mediated evoked EPSCs at +40 mV from a tdTomato positive neuron (dopamine neuron) and a neighboring tdTomato negative neuron (non-dopamine neuron) in Grin1f/f/DAT-Cre/tdTomatoCre mice or Grin1f/+/DAT-Cre/tdTomatoCre mice. The stimulation electrode was placed at the rostral part of the VTA area to evoke a measurable, monosynaptic EPSC in both cells. We found that NMDAR-mediated EPSCs were eliminated in dopamine neurons in Grin1f/f/DAT-Cre/tdTomatoCre mice, but not in Grin1f/+/DAT-Cre/tdTomatoCre mice (Fig. 3C-E), demonstrating the loss of NMDARs in dopamine neurons from Grin1f/f/DAT-Cre/tdTomatoCre mice.
Figure 3. Genetic deletion of NMDARs in VTA dopamine neurons.
(A)Breeding scheme to generate Grin1f/f/DAT-Cre/tdTomatoCre mice.
(B)Immunohistochemical assays in P13 Grin1f/+/DAT-Cre/tdTomatoCre mice confirmed Cre expression selectively in dopaminergic neurons as indicated by complete colocalization of tdTomato, the product of Cre-mediated recombination and tyrosine hydroxylase (TH, green), a cellular marker for dopamine neurons. Note that there were both tdTomato-positive dopamine neurons (solid arrow) and tdTomato-negative nondopamine neurons (open arrow) in the VTA region. NeuN is a neuronal marker (purple). SNc, Substantia nigra pars compacta.
(C-D) Scatter plots and sample traces of NMDA EPSCs in tdTomato-expressing VTA dopamine neurons and nearby tdTomato-negative non-dopamine control neurons from the same slices showed the loss of NMDA EPSCs in dopamine neurons from Grin1f/f/DAT-Cre/tdTomatoCre (C), but not in Grin1f/+/DAT-Cre/tdTomatoCre mice (D).
(E) Bar graph showed normalized NMDA EPSC ratio in dopamine neurons vs nondopamine neurons from Cre+/Cre-in Grin1f/+/DAT-Cre/tdTomatoCremice (66.36 ± 24.02 %, n = 6) and in Grin1f/f/DAT-Cre/tdTomatoCre mice (1.35 ± 1.01 %, n = 9), p < 0.001, t-test. Deletion of the GluN1 subunit abolished NMDA EPSCs in VTA DA neurons from Grin1f/f/DAT-Cre/tdTomatoCre mice. Scale bar: 50 pA and 50 ms.
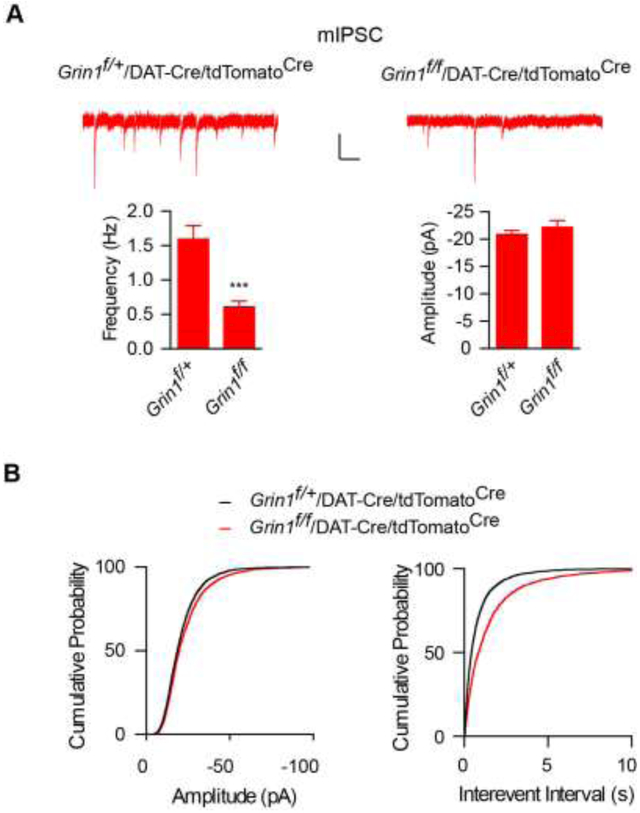
We next measured mIPSCs in dopamine neurons to examine GABAergic synaptic transmission. We found that mIPSC frequency was strongly reduced in dopamine neurons in Grin1f/f/DAT-Cre/tdTomatoCre mice (Fig. 4A-B). However, there was no significant difference of mIPSC amplitudes in dopamine neurons from Grin1f/f/DAT-Cre/tdTomatoCre and Grin1f/+/DAT-Cre/tdTomatoCre mice (Fig. 4A-B). These data indicate that NMDARs are also important for proper establishment of inhibitory synaptic transmission in midbrain dopamine neurons.
Figure 4. GABAergic synapse development in cortical neurons and in VTA dopamine neurons requires NMDARs.
(A-B) mIPSC frequency was strongly reduced in Cre-positive VTA dopamine neurons from Grin1f/f/DAT-Cre/tdTomatoCremice (Frequency: Grin1f/+ (1.59 ± 0.20 Hz, n = 21), Grin1f/f (0.61 ± 0.08 Hz, n = 22), p < 0.001, t-test; Amplitude: Grin1f/+ (−20.84 ± 0.79 pA, n = 21), Grin1f/f (−21.62 ± 1.26 pA, n = 22), p = 0.61, t-test). Cumulative distributions were compared by the Kolmogorov-Smirnov test (p ≥ 0.05 for amplitude and p < 0.001 for interevent interval). Scale bar: 20 pA and 1s.
Discussion
Neural circuit function relies on rapid communication through both excitatory and inhibitory chemical synapses, and synaptic dysfunctions can lead to devastating neurological and psychiatric disorders [3-6, 28-30]. Thus, it is important to understand the regulatory mechanisms underlying the formation and maturation of synapses. Hippocampal neurons have been widely used to study the molecular and cellular mechanisms for synapse development in the central nervous system. However, as enormous diversity of neuronal types and function exist in the brain, it is important to examine whether the mechanisms discovered in hippocampal neurons also apply in other neuronal types. We have previously demonstrated that NMDARs play a critical role in controlling the establishment of GABAergic synaptic transmission in developing hippocampal neurons[24]. Here we examined the role of NMDARs in development of GABAergic synaptic transmission in two distinct types of neurons.
Our data show that single-cell genetic deletion of the GluN1 subunit leads to a strong reduction of GABAergic synaptic transmission in cortical layer 2-3 pyramidal neurons. Similarly, in midbrain dopamine neurons loss of NMDARs causes a significant decrease of inhibitory synaptic transmission. Taken together with our previous findings of an important role of NMDARs in GABAergic synapse development in hippocampal neurons[24], these data collectively demonstrate that neurons with distinct developmental origins commonly rely on NMDAR-dependent mechanisms for establishing GABAergic synaptic transmission during development. It is also important to note that it is a partial loss of GABAergic synaptic transmission in neurons lacking NMDARs. This suggests that there are other mechanisms existing in neurons important for GABAergic synapse development. Currently, the molecular mechanisms for either NMDAR-dependent or independent processes for GABAergic synapse development remain largely unclear. Recent studies have shown the importance of several postsynaptic cell surface proteins in GABAergic synapse development [31-42]. Thus, it will be critical to investigate the functional interactions between NMDARs and above-mentioned molecules in GABAergic synaptogenesis in the future.
Previous work has shown that NMDARs negatively regulate AMPAR-mediated excitatory synaptic transmission in pyramidal neurons and dopamine neurons. In both hippocampal CA1 and cortical pyramidal neurons, genetic deletion of NMDARs strongly up-regulate AMPA EPSCs [13-17]. Similarly, loss of NMDARs in midbrain dopamine neurons significantly increases AMPAR-mediated synaptic transmission [18, 19]. Furthermore, it has been shown that NMDARs negatively regulates AMPA EPSCs in hippocampal neurogliaform interneurons and adult-born granule cells [20, 21]. Taken together, these data indicate that NMDARs suppress functional maturation of AMPAR-mediated fast excitatory synaptic transmission and maintain the excitatory synapses in a silent state during development [15]. Thus, NMDARs in developing neurons promote GABAergic synapse development as demonstrated here and before [24], and at the same time suppress the maturation of glutamatergic synaptic transmission [13-21]. One potential advantage for such dual regulation of synapse development by NMDARs may be to prevent AMPAR-mediated over-excitation of neurons before proper development and maturation of GABAergic inhibitory transmission. Therefore, it is tempting to speculate that the NMDAR is a critical signaling molecule for controlling the establishment of excitation/inhibition balance during development.
Our findings that GABAergic synaptic transmission is strongly reduced in different types of neurons lacking NMDARs may have important implications. Genetic deletion of NMDARs in different brain regions has been widely used to probe the function of NMDAR-dependent synaptic transmission and/or plasticity in animal behavior [18, 19, 27, 43-50]. The reduction of inhibitory synaptic transmission in several types of neurons lacking of NMDARs as shown by us indicates that the behavioral outcomes induced by NMDAR knockout could be generated by a combined effect of loss of NMDARs, increased AMPAR-mediated synaptic transmission and/or reduced synaptic inhibition. Thus, innovative approaches to precisely manipulate NMDAR function, instead of simply genetically deleting the receptors, may be the key to determine the role of NMDAR activity in neural circuit function and behavior.
Highlight.
GluN1 knockout in developing cortical neurons suppresses GABAergic transmission
Loss of NMDARs in developing dopamine neurons reduces synaptic inhibition
NMDARs are commonly involved in development of GABAergic synaptic transmission
Acknowledgement
This work was supported by NINDS/NIH intramural research program to WL. The authors appreciate Xia Mao for mouse colony management.
Abbreviations:
- NMDA
N-methyl-D-aspartate
- GABA
γ-Aminobutyric acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- VTA
ventral tegmental area
- TH
tyrosine hydroxylase
- DAT
dopamine transporter
- mIPSC
miniature inhibitory postsynaptic currents
- EPSC
excitatory postsynaptic currents
- PBS
phosphate-buffered saline
- NBQX
2,3-dihydroxy-6-nitro-7-sulfamoyl-benzoquinoxaline-2,3-dione.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest None
References
- [1].McAllister AK, Dynamic aspects of CNS synapse formation, Annu. Rev. Neurosci, 30 (2007) 425–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shen K, Scheiffele P, Genetics and cell biology of building specific synaptic connectivity, Annu. Rev. Neurosci, 33 (2010) 473–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zoghbi HY, Bear MF, Synaptic dysfunction in neurodevelopmental disorders associated with autism and intellectual disabilities, Cold Spring Harb. Perspect. Biol., 4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baig DN, Yanagawa T, Tabuchi K, Distortion of the normal function of synaptic cell adhesion molecules by genetic variants as a risk for autism spectrum disorders, Brain Res. Bull, DOI 10.1016/j.brainresbull.2016.10.006(2016). [DOI] [PubMed] [Google Scholar]
- [5].Lisman J, Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia?, Curr. Opin. Neurobiol, 22 (2012) 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dudek FE, Epileptogenesis: a new twist on the balance of excitation and inhibition, Epilepsy Curr, 9 (2009) 174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L, Structure, function, and pharmacology of NMDA receptor channels, Physiol. Res, 63 Suppl 1 (2014) S191–203. [DOI] [PubMed] [Google Scholar]
- [8].Malenka RC, Bear MF, LTP and LTD: an embarrassment of riches, Neuron, 44 (2004) 5–21. [DOI] [PubMed] [Google Scholar]
- [9].Blanton MG, Kriegstein AR, Properties of amino acid neurotransmitter receptors of embryonic cortical neurons when activated by exogenous and endogenous agonists, J. Neurophysiol, 67 (1992) 1185–1200. [DOI] [PubMed] [Google Scholar]
- [10].LoTurco JJ, Blanton MG, Kriegstein AR, Initial expression and endogenous activation of NMDA channels in early neocortical development, J. Neurosci, 11 (1991) 792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Behar TN, Scott CA, Greene CL, Wen X, Smith SV, Maric D, Liu QY, Colton CA, Barker JL, Glutamate acting at NMDA receptors stimulates embryonic cortical neuronal migration, J. Neurosci, 19 (1999) 4449–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hirasawa T, Wada H, Kohsaka S, Uchino S, Inhibition of NMDA receptors induces delayed neuronal maturation and sustained proliferation of progenitor cells during neocortical development, J. Neurosci. Res, 74 (2003) 676–687. [DOI] [PubMed] [Google Scholar]
- [13].Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A, Regulation of spine morphology and spine density by NMDA receptor signaling in vivo, Proc. Natl. Acad. Sci. U. S. A, 104 (2007) 19553–19558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hall BJ, Ripley B, Ghosh A, NR2B signaling regulates the development of synaptic AMPA receptor current, J. Neurosci, 27 (2007) 13446–13456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA, NMDA receptors inhibit synapse unsilencing during brain development, Proc. Natl. Acad. Sci. U. S. A, 105 (2008) 5597–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA, Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo, Neuron, 71 (2011) 1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lu W, Gray JA, Granger AJ, During MJ, Nicoll RA, Potentiation of synaptic AMPA receptors induced by the deletion of NMDA receptors requires the GluA2 subunit, J. Neurophysiol, 105 (2011) 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zweifel LS, Argilli E, Bonci A, Palmiter RD, Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors, Neuron, 59 (2008) 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Lujan R, Halbout B, Mameli M, Parlato R, Sprengel R, Luscher C, Schutz G, Spanagel R, Glutamate receptors on dopamine neurons control the persistence of cocaine seeking, Neuron, 59 (2008) 497–508. [DOI] [PubMed] [Google Scholar]
- [20].Chittajallu R, Wester JC, Craig MT, Barksdale E, Yuan XQ, Akgul G, Fang C, Collins D, Hunt S, Pelkey KA, McBain CJ, Afferent specific role of NMDA receptors for the circuit integration of hippocampal neurogliaform cells, Nat Commun, 8 (2017) 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mu Y, Zhao C, Toni N, Yao J, Gage FH, Distinct roles of NMDA receptors at different stages of granule cell development in the adult brain, Elife, 4 (2015) e07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lu W, Bushong EA, Shih TP, Ellisman MH, Nicoll RA, The cell-autonomous role of excitatory synaptic transmission in the regulation of neuronal structure and function, Neuron, 78 (2013) 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lu W, Bromley-Coolidge S, Li J, Regulation of GABAergic synapse development by postsynaptic membrane proteins, Brain Res. Bull, DOI 10.1016/j.brainresbull.2016.07.004(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gu X, Zhou L, Lu W, An NMDA Receptor-Dependent Mechanism Underlies Inhibitory Synapse Development, Cell Rep, 14 (2016) 471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Backman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC, Characterization of a mouse strain expressing Cre recombinase from the 3' untranslated region of the dopamine transporter locus, Genesis, 44 (2006) 383–390. [DOI] [PubMed] [Google Scholar]
- [26].Spruston N, Pyramidal neurons: dendritic structure and synaptic integration, Nat. Rev. Neurosci, 9 (2008) 206–221. [DOI] [PubMed] [Google Scholar]
- [27].Hutchison MA, Gu X, Adrover MF, Lee MR, Hnasko TS, Alvarez VA, Lu W, Genetic inhibition of neurotransmission reveals role of glutamatergic input to dopamine neurons in high-effort behavior, Mol. Psychiatry, DOI 10.1038/mp.2017.7(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, Ekker M, Rubenstein JL, Noebels JL, Rosenmund C, Zoghbi HY, Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes, Nature, 468 (2010) 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rubenstein JL, Three hypotheses for developmental defects that may underlie some forms of autism spectrum disorder, Curr. Opin. Neurol, 23 (2010) 118–123. [DOI] [PubMed] [Google Scholar]
- [30].Hines RM, Davies PA, Moss SJ, Maguire J, Functional regulation of GABAA receptors in nervous system pathologies, Curr. Opin. Neurobiol, 22 (2012) 552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Li J, Han W, Pelkey KA, Duan J, Mao X, Wang YX, Craig MT, Dong L, Petralia RS, McBain CJ, Lu W, Molecular Dissection of Neuroligin 2 and Slitrk3 Reveals an Essential Framework for GABAergic Synapse Development, Neuron, DOI 10.1016/j.neuron.2017.10.003(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F, Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin, Neuron, 63 (2009) 628–642. [DOI] [PubMed] [Google Scholar]
- [33].Woo J, Kwon SK, Nam J, Choi S, Takahashi H, Krueger D, Park J, Lee Y, Bae JY, Lee D, Ko J, Kim H, Kim MH, Bae YC, Chang S, Craig AM, Kim E, The adhesion protein IgSF9b is coupled to neuroligin 2 via S-SCAM to promote inhibitory synapse development, J. Cell Biol, 201 (2013) 929–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Panzanelli P, Fruh S, Fritschy JM, Differential role of GABAA receptors and neuroligin 2 for perisomatic GABAergic synapse formation in the hippocampus, Brain Struct Funct, DOI 10.1007/s00429-017-1462-7(2017). [DOI] [PubMed] [Google Scholar]
- [35].Lee K, Kim Y, Lee SJ, Qiang Y, Lee D, Lee HW, Kim H, Je HS, Sudhof TC, Ko J, MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development, Proc. Natl. Acad. Sci. U. S. A, 110 (2013) 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pettem KL, Yokomaku D, Takahashi H, Ge Y, Craig AM, Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development, J. Cell Biol, 200 (2013) 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Takahashi H, Katayama K, Sohya K, Miyamoto H, Prasad T, Matsumoto Y, Ota M, Yasuda H, Tsumoto T, Aruga J, Craig AM, Selective control of inhibitory synapse development by Slitrk3-PTPdelta trans-synaptic interaction, Nat. Neurosci, 15 (2012) 389–398, S381–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yim YS, Kwon Y, Nam J, Yoon HI, Lee K, Kim DG, Kim E, Kim CH, Ko J, Slitrks control excitatory and inhibitory synapse formation with LAR receptor protein tyrosine phosphatases, Proc. Natl. Acad. Sci. U. S. A, 110 (2013) 4057–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kuzirian MS, Moore AR, Staudenmaier EK, Friedel RH, Paradis S, The class 4 semaphorin Sema4D promotes the rapid assembly of GABAergic synapses in rodent hippocampus, J. Neurosci, 33 (2013) 8961–8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brown LE, Nicholson MW, Arama JE, Mercer A, Thomson AM, Jovanovic JN, gamma-Aminobutyric Acid Type A (GABAA) Receptor Subunits Play a Direct Structural Role in Synaptic Contact Formation via Their N-terminal Extracellular Domains, J. Biol. Chem, 291 (2016) 13926–13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA, Siddiqui TJ, Kawabe H, Chen F, Zhang L, Rudenko G, Wang YT, Brose N, Craig AM, The specific alpha-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development, Neuron, 80 (2013) 113–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Um JW, Pramanik G, Ko JS, Song MY, Lee D, Kim H, Park KS, Sudhof TC, Tabuchi K, Ko J, Calsyntenins function as synaptogenic adhesion molecules in concert with neurexins, Cell Rep, 6 (2014) 1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tsien JZ, Huerta PT, Tonegawa S, The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory, Cell, 87 (1996) 1327–1338. [DOI] [PubMed] [Google Scholar]
- [44].Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S, NMDA receptors, place cells and hippocampal spatial memory, Nat. Rev. Neurosci, 5 (2004) 361–372. [DOI] [PubMed] [Google Scholar]
- [45].Ohtsuka N, Tansky MF, Kuang H, Kourrich S, Thomas MJ, Rubenstein JL, Ekker M, Leeman SE, Tsien JZ, Functional disturbances in the striatum by region-specific ablation of NMDA receptors, Proc. Natl. Acad. Sci. U. S. A, 105 (2008) 12961–12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ, NMDA receptors in dopaminergic neurons are crucial for habit learning, Neuron, 72 (2011) 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K, Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes, Nat. Neurosci, 13 (2010) 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, Moore CI, Tsai LH, A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior, Mol. Psychiatry, 17 (2012) 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Beutler LR, Wanat MJ, Quintana A, Sanz E, Bamford NS, Zweifel LS, Palmiter RD, Balanced NMDA receptor activity in dopamine D1 receptor (D1R)- and D2R-expressing medium spiny neurons is required for amphetamine sensitization, Proc. Natl. Acad. Sci. U. S. A, 108 (2011) 4206–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Glass MJ, Hegarty DM, Oselkin M, Quimson L, South SM, Xu Q, Pickel VM, Inturrisi CE, Conditional deletion of the NMDA-NR1 receptor subunit gene in the central nucleus of the amygdala inhibits naloxone-induced conditioned place aversion in morphine-dependent mice, Exp. Neurol, 213 (2008) 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]