Abstract
Systemic lupus erythematosus is associated with numerous pleuropulmonary complications. Although uncommon, diffuse alveolar hemorrhage represents a life-threatening cause of acute respiratory failure among patients with lupus. Here, we present a 24-year-old woman with a history of lupus who developed hemoptysis and respiratory failure associated with diffuse radiographic infiltrates and anemia. Bronchoscopy confirmed diffuse alveolar hemorrhage. She was managed with supportive care, plasmapheresis, and immunosuppressive pharmacotherapy leading to sustained resolution of her pulmonary hemorrhage and respiratory failure. We then review the available literature on the pathophysiology and management of lupus-associated diffuse alveolar hemorrhage, which centers on supportive care, reversal of coagulopathy, and immunosuppressive measures.
Keywords: systemic lupus erythematosus, antiphospholipid syndrome, diffuse alveolar hemorrhage, capillaritis, respiratory failure
SUMMARY FOR TABLE OF CONTENTS
A 24-year-old woman with a history of systemic lupus erythematosus presented with hemoptysis, diffuse radiographic infiltrates, anemia, and respiratory failure; bronchoscopy confirmed diffuse alveolar hemorrhage. Her condition was managed with supportive care, plasmapheresis, and immunosuppression with glucocorticoids, cyclophosphamide, and mycophenolate mofetil. Diffuse alveolar hemorrhage represents an uncommon but life-threatening complication of lupus with a growing evidence base to support acute and chronic management strategies centered on immunosuppressive pharmacotherapy.
CASE PRESENTATION
A 24-year-old woman with a history of systemic lupus erythematosus complicated by lupus nephritis and antiphospholipid syndrome (APS) was admitted to the intensive care unit with acute hypoxemic respiratory failure requiring mechanical ventilation (MV). She reported fevers, dyspnea and productive cough with streaks of blood for the 2 days prior to admission. On presentation, her physical examination was notable for a temperature of 38.8 °C, blood pressure of 124/70 mm Hg, heart rate of 130 beats per minute, respiratory rate of 34 breaths per minute and oxygen saturation of 94% while breathing a 50% fraction of inspired oxygen with 10 cm H2O of positive-end expiratory pressure applied via MV. She was in severe respiratory distress. Her lung exam was notable for diffuse low-pitched wheezes. A loud P2 and systolic murmur were heard over the left upper sternal border. Blood tests showed a hemoglobin of 6.5 g/dl (from a baseline of 8 g/dl), a leukocyte count of 9,100 per cubic millimeter and a platelet count of 200,000 per cubic millimeter. Chest imaging revealed diffuse airspace opacities (Figure 1). A diagnostic bronchoscopy with serial bronchoalveolar lavages (BAL) of the right middle lobe revealed persistent-to-increased bloodiness with each aliquot returned (Figure 2). A PCR-based panel testing for 14 common respiratory viruses, Pneumocystis jiroveci direct immunofluorescence assay and respiratory bacterial and fungal cultures were negative. An echocardiogram revealed normal left ventricular systolic function, with an ejection fraction calculated as 70%, along with mildly decreased right ventricular systolic function and a small circumferential pericardial effusion. Based on these findings, the patient was diagnosed with SLE-associated diffuse alveolar hemorrhage.
Figure 1.
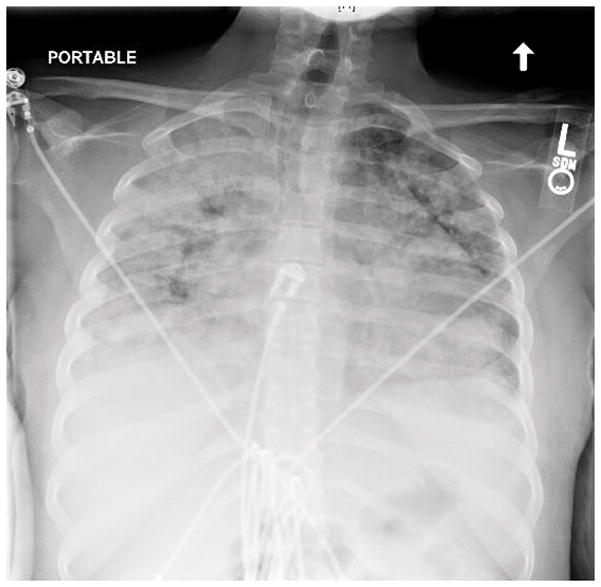
An anteroposterior chest radiograph showed diffuse airspace opacities.
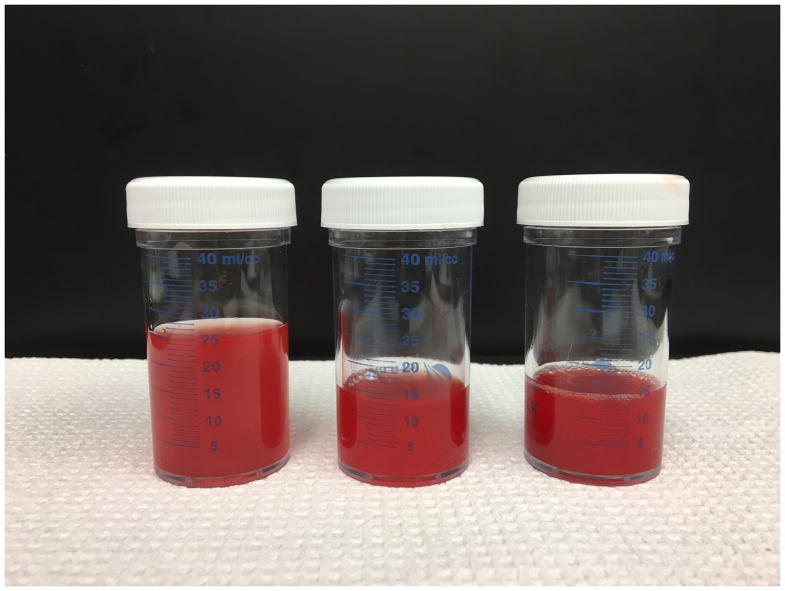
Figure 2.
Serial bronchoalveolar lavage fluid samples from the right middle lobe showed persistent-to-increasing bloodiness with each aliquot returned.
Patient outcome
The patient was supported with lung-protective mechanical ventilation. Empiric antibiotic therapy was initiated pending microbiological results from the BAL fluid. Methylprednisolone 1,000 mg/day for 3 days followed by a prednisone taper along with cyclophosphamide (750 mg/m2 based on adjusted body weight) were administered intravenously. Despite these interventions, she continued to require MV support and blood transfusions for ongoing bleeding. Therapeutic anticoagulation for APS was transitioned to prophylactic dosage to minimize bleeding. Plasmapheresis was performed for 3 days, after which she improved. She was extubated on hospital day 7 and discharged on day 11. No recurrent bleeding has been reported for the past 10 months and she remains on maintenance therapy with mycophenolate mofetil (MMF).
DISCUSSION
Systemic lupus erythematosus is a multisystem autoimmune disorder with protean clinical manifestations, including pleuropulmonary disease. DAH is an uncommon yet devastating complication of SLE, with a reported frequency ranging from 1 to 5.4%, and mortality up to 92% (1). DAH is typically defined and diagnosed by the presence of respiratory symptoms (dyspnea, cough, hemoptysis), diffuse lung infiltrates on chest imaging, acute hemoglobin loss (1–2 g/dl) and sequential increase in red blood cells on serial BAL. Chest radiography usually shows bilateral airspace opacities. If initial imaging is unrevealing but suspicion for DAH remains high, computed tomography (CT) could be warranted. Common CT findings include diffuse and patchy ground glass densities along with diffuse nodular opacities (2).
There is no known unifying pathogenic mechanism in the development of DAH. Instead, DAH can be associated with pulmonary capillaritis, diffuse alveolar damage and bland pulmonary hemorrhage, disorders characterized by distinct histopathological patterns. Pulmonary capillaritis is often associated with systemic vasculitides. Common features include neutrophilic infiltration and fibrinoid necrosis of interalveolar septa along with granular IgG/C3 deposits (3). DAD is characterized by hyaline membrane formation and protein-rich alveolar edema. Infection, drug toxicity and noninfectious complications of organ transplantation are among the leading causes of DAD. Bland hemorrhage is distinguished by the fact that bleeding is not accompanied by inflammation; it is often related to elevated left heart filling pressures or bleeding from coagulopathy or anticoagulation.
In an effort to identify early predictors of DAH development, investigators have performed systematic retrospective reviews of SLE-associated DAH cases and have found significant predisposing risk factors. Multivariate analysis demonstrated, as independent risk factors in the development of DAH, history of thrombocytopenia and low C3 in one study (1) and coexisting neuropsychiatric lupus and high SLE-disease activity index (SLEDAI) in the second (4).
The heterogeneity of clinical findings and pathogenic mechanisms along with the difficulty and therefore paucity of randomized controlled trials further complicate the management of SLE-associated DAH (1, 5). Treatment is based upon the combined experience of multiple case series, expert opinion and extrapolation from controlled studies of DAH associated with other systemic vasculitides and management of lupus nephritis (6–8) (Table 1). Indeed, there are no therapies specifically FDA-approved for SLE-associated DAH. Acute management involves supportive care with mechanical ventilation in cases of severe hypoxemia, correction of coagulation abnormalities and initiation of antibiotic therapy if infection is suspected. Prompt initiation of high doses of glucocorticoids constitute the cornerstone of management along with additional immunosuppressive therapy to sustain remission (9–11). Traditionally, the alkylating agent cyclophosphamide has been the immunosuppressive agent of choice for managing life-threatening manifestations of systemic vasculitides (12). During the acute phase of DAH, immediate hemostasis can be achieved with bronchoscopic administration of intrapulmonary recombinant factor VIIa (rFVIIa) while awaiting the effect of immunosuppressive agents to take effect (13). Also, in cases of refractory hypoxemia, supportive care with extra-corporeal membrane oxygenation (ECMO) has been described as a temporizing measure while awaiting the effect of pharmacologic agents as a bridge to full recovery (14).
Table 1.
Management of systemic lupus erythematosus-associated diffuse alveolar hemorrhage.
| Therapeutic Agent | Mechanism of Action | Clinical Studies in DAH |
|---|---|---|
| Glucocorticoids | Suppression of peripheral T helper responses Regulation of immunoglobulin production and B cell differentiation Suppression of proinflammatory mediators |
Barile et al (11) Kazzaz et al (1) Santos-Ocampo et al (30) Zamora et al (10) Andrade et al (31) |
| Cyclophosphamide | Regulation of B cell activation, differentiation and suppression of B cell function Immunoregulatory effects on T cell subsets and activation markers (increased effector T cells and plasmacytoid dendritic cells) |
Kazzaz et al (1) Santos-Ocampo et al (30) Zamora et al (10) Badsha et al (32) Canas et al (33) Koh et al (34) |
| Rituximab | Elimination of CD20+ B cells Modulation of costimulatory molecules that mediate B and T cell interactions |
Narshi et al (17) Tse et al (19) Machado et al (35) Aakjaer et al (36) |
| Plasmapheresis | Direct removal of circulating immune complexes, antibodies and cytokines | Jones John et al (18) Claridge et al (37) |
| Azathioprine and mycophenolate mofetil | Reduction of de novo purine synthesis results in decreased T and B cell proliferation | Rashidi et al (24) Andrade et al (31) Zamora et al (10) Santos-Ocampo et al (30) |
| Immunoglobulin | Immunoregulatory effects including inhibition of B-cell proliferation, antibody production and growth factors and cytokines Anti-inflammatory effect through complement system inhibition and Fc receptor blockade on macrophages |
Andrade et al (31) Shen et al (38) Kwok et al (4) |
| Mesenchymal stem cells | Immunomodulatory effects through inhibition of T, B, NK and dendritic cell proliferation and activation | Liang et al (27) Shi et al (39) |
| Recombinant factor VIIa | Alveolar hemostasis through formation of FVIIa-tissue factor complex, which activates Factor X, ultimately leading to thrombin generation and clot formation | Heslet et al (13) Esper et al (40) |
| Extracorporeal membrane oxygenation | Management of refractory hypoxemia through increased mixed venous oxygen content | Patel et al (14) |
Recent advances in our understanding of the pathogenic mechanisms driving the development of DAH highlight the pivotal role played by B lymphocytes and humoral immunity (15). In a murine model of pristane-induced DAH, B cell knockout mice (B6 Igμ−/−) showed a significant protection compared with wild-type (B6) and T cell knockout mice (B6 TCR−/−). This protective phenotype disappeared upon adoptive transfer of wild-type CD19+ B cells, demonstrating a causal role for B cells in the pathogenesis of DAH (16). Accordingly, pharmacologic depletion of B cells with rituximab (anti-CD20 monoclonal antibody) or direct removal of circulating antibodies through plasmapheresis have emerged as therapeutic approaches in DAH (17–19). Glucocorticoids and cyclophosphamide affect B cell development, differentiation and function, thereby explaining their therapeutic efficacy in the management of systemic vasculitides (20–22). Interestingly, the most recently approved drug by the US FDA for the management of SLE is a human monoclonal antibody (belimumab) that neutralizes the B-cell survival factor B-lymphocyte stimulator (BLys), which promotes B cell proliferation and differentiation (23). The effect of belimumab specifically on SLE-associated DAH remains unknown.
Given that proliferation of both T and B lymphocytes is critically dependent on de novo purine synthesis, evidence supporting the use of antimetabolites such as azathioprine and MMF has emerged as an alternative therapeutic approach in the management of DAH, although antimetabolites are mostly limited to maintenance therapy once remission has been achieved (24–26). Based on the immunomodulatory properties of both intravenous immunoglobulin (IVIG) and mesenchymal stem cells, investigators have applied these therapies to manage different autoimmune diseases and their respective complications, including DAH (27). Through production of inhibitory cytokines and cell contact-mediated inhibitory molecules (e.g., interleukin 10 and cytotoxic T lymphocyte antigen 4), regulatory T (Treg) cells play a key role in the mitigation of autoimmunity (28). The number and suppressive function of this important subset of CD4+ T cells is commonly affected in patients with autoimmune diseases. Therefore, novel therapies that replenish the Treg cell pool or enhance their function might be of therapeutic benefit. Finally, epigenetic mechanisms (such as DNA methylation and histone modifications) modulate T and B lymphocyte development, differentiation and function, suggesting that epigenetic-based therapies (histone deacetylase inhibitors and DNA methyltransferase inhibitors) could mitigate and manage autoimmune disease-related complications (29).
CONCLUSIONS
DAH is an uncommon but potentially life-threatening complication of SLE. Emerging data suggest that outcomes can be improved with the use of supportive care and immunosuppressive pharmacotherapy, including glucocorticoids and steroid-sparing agents of multiple classes. Ongoing studies into the pathophysiology of SLE-associated DAH may help refine management strategies to limit drug toxicities from these agents while effectively inducing and sustaining remission.
Footnotes
Conflicts of interest and source of funding: The authors declare that they have no conflicts of interest with the contents of this article. BDS was supported by NIH award K08HL128867 and the Francis Family Foundation’s Parker B. Francis Research Opportunity Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Francis Family Foundation.
References
- 1.Kazzaz NM, Coit P, Lewis EE, et al. Systemic lupus erythematosus complicated by diffuse alveolar haemorrhage: Risk factors, therapy and survival. Lupus Sci Med. 2015;2:e000117. doi: 10.1136/lupus-2015-000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheah FK, Sheppard MN, Hansell DM. Computed tomography of diffuse pulmonary haemorrhage with pathological correlation. Clin Radiol. 1993;48:89–93. doi: 10.1016/s0009-9260(05)81078-5. [DOI] [PubMed] [Google Scholar]
- 3.Franks TJ, Koss MN. Pulmonary capillaritis. Curr Opin Pulm Med. 2000;6:430–435. doi: 10.1097/00063198-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Kwok SK, Moon SJ, Ju JH, et al. Diffuse alveolar hemorrhage in systemic lupus erythematosus: Risk factors and clinical outcome: Results from affiliated hospitals of catholic university of korea. Lupus. 2011;20:102–107. doi: 10.1177/0961203310381511. [DOI] [PubMed] [Google Scholar]
- 5.Merrill JT, Manzi S, Aranow C, et al. Lupus community panel proposals for optimising clinical trials: 2018. Lupus Sci Med. 2018;5:e000258. doi: 10.1136/lupus-2018-000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn BH, McMahon M, Wilkinson A, et al. American college of rheumatology guidelines for screening, case definition, treatment and management of lupus nephritis. Arthritis Care Res (Hoboken) 2012;64:797–808. doi: 10.1002/acr.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guillevin L, Pagnoux C, Karras A, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–1780. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Martinez MU, Abud-Mendoza C. Diffuse alveolar hemorrhage in patients with systemic lupus erythematosus. Clinical manifestations, treatment, and prognosis. Reumatol Clin. 2014;10:248–253. doi: 10.1016/j.reuma.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Zamora MR, Warner ML, Tuder R, et al. Diffuse alveolar hemorrhage and systemic lupus erythematosus: Clinical presentation, histology, survival, and outcome. Medicine. 1997;76:192–202. doi: 10.1097/00005792-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Barile LA, Jara LJ, Medina-Rodriguez F, et al. Pulmonary hemorrhage in systemic lupus erythematosus. Lupus. 1997;6:445–448. doi: 10.1177/096120339700600506. [DOI] [PubMed] [Google Scholar]
- 12.Ednalino C, Yip J, Carsons SE. Systematic review of diffuse alveolar hemorrhage in systemic lupus erythematosus: Focus on outcome and therapy. J Clin Rheumatol. 2015;21:305–310. doi: 10.1097/RHU.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 13.Heslet L, Nielsen JD, Levi M, et al. Successful pulmonary administration of activated recombinant factor VII in diffuse alveolar hemorrhage. Crit Care. 2006;10:R177. doi: 10.1186/cc5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel JJ, Lipchik RJ. Systemic lupus–induced diffuse alveolar hemorrhage treated with extracorporeal membrane oxygenation: A case report and review of the literature. J Intensive Care Med. 2012;29:104–109. doi: 10.1177/0885066612464335. [DOI] [PubMed] [Google Scholar]
- 15.Anderton SM, Fillatreau S. Activated B cells in autoimmune diseases: The case for a regulatory role. Nat Clin Pract Rheumatol. 2008;4:657–666. doi: 10.1038/ncprheum0950. [DOI] [PubMed] [Google Scholar]
- 16.Barker TT, Lee PY, Kelly-Scumpia KM, et al. Pathogenic role of B cells in the development of diffuse alveolar hemorrhage induced by pristane. Lab Invest. 2011;91:1540–1550. doi: 10.1038/labinvest.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narshi CB, Haider S, Ford CM, et al. Rituximab as early therapy for pulmonary haemorrhage in systemic lupus erythematosus. Rheumatology. 2010;49:392–394. doi: 10.1093/rheumatology/kep356. [DOI] [PubMed] [Google Scholar]
- 18.Jones John V, Robinson Michael F, Parciany Rosanne K, et al. Therapeutic plasmapheresis in systemic lupus erythematosus. Effect on immune complexes and antibodies to DNA. Arthritis Rheum. 1981;24:1113–1120. doi: 10.1002/art.1780240901. [DOI] [PubMed] [Google Scholar]
- 19.Tse JR, Schwab KE, McMahon M, et al. Rituximab: An emerging treatment for recurrent diffuse alveolar hemorrhage in systemic lupus erythematosus. Lupus. 2015;24:756–759. doi: 10.1177/0961203314564235. [DOI] [PubMed] [Google Scholar]
- 20.Saxon A, Stevens RH, Ramer SJ, et al. Glucocorticoids administered in vivo inhibit human suppressor T lymphocyte function and diminish B lymphocyte responsiveness in in vitro immunoglobulin synthesis. J Clin Invest. 1978;61:922–930. doi: 10.1172/JCI109017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu LP, Cupps TR, Whalen G, et al. Selective effects of cyclophosphamide therapy on activation, proliferation, and differentiation of human B cells. J Clin Invest. 1987;79:1082–1090. doi: 10.1172/JCI112922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amano H, Morimoto S, Kaneko H, et al. Effect of intravenous cyclophosphamide in systemic lupus erythematosus: Relation to lymphocyte subsets and activation markers. Lupus. 2000;9:26–32. doi: 10.1177/096120330000900106. [DOI] [PubMed] [Google Scholar]
- 23.Stohl W, Hilbert DM. The discovery and development of belimumab: The anti-blys–lupus connection. Nat Biotechnol. 2012;30:69–77. doi: 10.1038/nbt.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rashidi AA, Alajmi M, Hegazi MO. Mycophenolate mofetil as a maintenance therapy for lupus-related diffuse alveolar hemorrhage: A case report. Lupus. 2011;20:1551–1553. doi: 10.1177/0961203311411353. [DOI] [PubMed] [Google Scholar]
- 25.Pagnoux C, Mahr A, Hamidou MA, et al. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med. 2008;359:2790–2803. doi: 10.1056/NEJMoa0802311. [DOI] [PubMed] [Google Scholar]
- 26.Hiemstra TF, Walsh M, Mahr A, et al. Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibody–associated vasculitis: A randomized controlled trial. JAMA. 2010;304:2381–2388. doi: 10.1001/jama.2010.1658. [DOI] [PubMed] [Google Scholar]
- 27.Liang J, Gu F, Wang H, et al. Mesenchymal stem cell transplantation for diffuse alveolar hemorrhage in SLE. Nat Rev Rheumatol. 2010;6:486–489. doi: 10.1038/nrrheum.2010.80. [DOI] [PubMed] [Google Scholar]
- 28.Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol. 2014;5:46. doi: 10.3389/fimmu.2014.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busslinger M, Tarakhovsky A. Epigenetic control of immunity. Cold Spring Harb Perspect Biol. 2014:6. doi: 10.1101/cshperspect.a019307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos-Ocampo AS, Mandell BF, Fessler BJ. Alveolar hemorrhage in systemic lupus erythematosus: Presentation and management. Chest. 2000;118:1083–1090. doi: 10.1378/chest.118.4.1083. [DOI] [PubMed] [Google Scholar]
- 31.Andrade C, Mendonca T, Farinha F, et al. Alveolar hemorrhage in systemic lupus erythematosus: A cohort review. Lupus. 2016;25:75–80. doi: 10.1177/0961203315605365. [DOI] [PubMed] [Google Scholar]
- 32.Badsha H, Teh CL, Kong KO, et al. Pulmonary hemorrhage in systemic lupus erythematosus. Semin Arthritis Rheum. 2004;33:414–421. doi: 10.1016/j.semarthrit.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Canas C, Tobon GJ, Granados M, et al. Diffuse alveolar hemorrhage in Colombian patients with systemic lupus erythematosus. Clin Rheumatol. 2007;26:1947–1949. doi: 10.1007/s10067-007-0576-3. [DOI] [PubMed] [Google Scholar]
- 34.Koh WH, Thumboo J, Boey ML. Pulmonary haemorrhage in oriental patients with systemic lupus erythematosus. Lupus. 1997;6:713–716. doi: 10.1177/096120339700600906. [DOI] [PubMed] [Google Scholar]
- 35.Machado RIL, Scheinberg MA, Queiroz MYCFd, et al. Use of rituximab as a treatment for systemic lupus erythematosus: Retrospective review. Einstein (São Paulo) 2014;12:36–41. doi: 10.1590/S1679-45082014AO2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aakjaer S, Bendstrup E, Ivarsen P, et al. Continous rituximab treatment for recurrent diffuse alveolar hemorrhage in a patient with systemic lupus erythematosus and antiphosholipid syndrome. Respir Med Case Rep. 2017;22:263–265. doi: 10.1016/j.rmcr.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claridge S, Das P, Dorling A, et al. Plasmapheresis as rescue therapy for systemic lupus erthyematosus-associated diffuse alveolar haemorrhage. BMJ Case Rep. 2011;2011:bcr0220113893. doi: 10.1136/bcr.02.2011.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen M, Zeng X, Tian X, et al. Diffuse alveolar hemorrhage in systemic lupus erythematosus: A retrospective study in China. Lupus. 2010;19:1326–1330. doi: 10.1177/0961203310373106. [DOI] [PubMed] [Google Scholar]
- 39.Shi D, Wang D, Li X, et al. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin Rheumatol. 2012;31:841–846. doi: 10.1007/s10067-012-1943-2. [DOI] [PubMed] [Google Scholar]
- 40.Esper RC, Estrada IE, de la Torre Leon T, et al. Treatment of diffuse alveolar hemorrhage secondary to lupus erythematosus with recombinant activated factor VII administered with a jet nebulizer. J Intensive Care. 2014;2:47. doi: 10.1186/s40560-014-0047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]