Abstract
Background
We aimed to determine whether higher neutrophil counts (NC) and neutrophil-to-lymphocyte ratio (NLR) were independently predictive of worse in-hospital outcome in patients after acute ischemic stroke (IS).
Methods
A retrospective observational study with prospective manner of IS registration. Between April 2012 and August 2014, a total number of 1731 patients with post-IS were consecutively enrolled in the study. Blood samples were drawn upon admission. Primary endpoint was in-hospital mortality. Secondary endpoint was severe stroke (≥16 NIHSS).
Results
The NC progressively increased from mild (NIHSS ≤ 5) to moderate (NIHSS ≥ 6 < 16) and severe (NIHSS ≥ 16) stroke (p = 0.006). NLR was independently associated with in-hospital mortality (p = 0.002). Multiple stepwise linear regression analysis showed that NC (p = 0.001) and NLR (p = 0.002) were independently predictive of higher NIHSS. Multiple stepwise logistic regression analysis showed that NC was independently associated with severe stroke (p < 0.0001). The best discriminating factor for in-hospital mortality with respect to NLR was ≥3.20 (sensitivity 62.7%, specificity 60.3%, likelihood ratio: 12.2). Patients with NLR ≥3.20 had a 2.55-fold increased risk for in-hospital mortality (OR = 1.49–4.37) compared to patients with NLR <3.20. The best discriminating factor for severe stroke (≥16 NIHSS) with respect to NC was ≥74% (sensitivity 47.1%, specificity 74.0%, likelihood ratio: 29.0). Patients with NC >74% had a 2.54-fold increased risk of severe stroke (OR = 1.82–3.54) compared to patients with NC <74%.
Conclusion
NLR was independently associated with in-hospital mortality and higher NC was independently predictive of severe stroke.
Keywords: Acute ischemic stroke, In-hospital mortality, Discriminating factor, Severe stroke, Tissue plasminogen activator
At a glance commentary
Scientific background on the subject
This study tested the impact of higher neutrophil counts and neutrophil-to-lymphocyte ratio on prognostic outcome in patients after acute ischemic stroke.
What this study adds to the field
The results of the study demonstrated that higher neutrophil counts and neutrophil-to lymphocyte ratio were independently predictive of unfavorable prognostic outcome in patients with non-atrial fibrillation-caused ischemic stroke.
Stroke is one of the most common global causes of mortality and disability [1], [2]. Currently, acute ischemic stroke (IS) treatment is limited thrombolytic therapy utilizing tissue plasminogen activator (tPA) [3]. However, a rather large number of contraindications restrict its scope in clinical practice [3], [4], [5], [6]. Furthermore, tPA appears to have a relatively high incidence of intracranial bleeding complications [3], [4], [5], [6], [7]. Thus, currently, only a limited number of acute IS patients are candidates for tPA therapy. Therefore, conventional therapy (i.e., supportive care and rehabilitation) remains the mainstay treatment for the majority of patients after acute IS. Therefore, the discovery of a simple and accurate factor for risk-factor stratification and for daily clinical application for prediction of prognostic outcome after acute IS is of utmost importance to the multidisciplinary team who take care of IS patients.
The most commonly assessed markers of prognostic outcome after acute IS are age [8], [9], infarct volume [9], and baseline National Institutes of Health Stroke Scale (NIHSS) score [2], [3], [7], [9]. Interestingly, for purposes of more complete and accurate assessment of the prognostic outcome after acute IS, investigators have continuously identified new potential risk associated biomarkers such as leukocyte count [10], proteins and microRNA [11], [12], pro-inflammatory cytokines [13], [14], and inflammatory biomarkers [15], as well as platelet reactivity [16]. Although most of these biomarkers were shown to offer additional accessory prognostic information, limitations of these blood biomarkers included expense, amount of time needed to complete the examination, and effectiveness in the laboratory that does not translate well to clinical application.
It is well recognized that atherosclerosis is an inflammatory process, and inflammatory biomarkers have been identified as useful predictors of clinical outcome. The neutrophil–lymphocyte ratio (NLR) has previously been established as a systemic inflammatory biomarker [17], [18]. Interestingly, abundant data have shown that NLR is a useful biomarker predictive of untoward clinical outcome in the setting of acute myocardial infarction (AMI) [19], [20], [21]. On the other hand, ischemic heart disease (IHD) and cerebrovascular disease (CVD) comprise the majority of the same causal etiologies. Thus, IHD and CVD are two sides of the same coin. Surprisingly, while NLR has been displayed to be associated with increased risk of mortality in AMI patients undergoing primary percutaneous coronary intervention (PCI) [19], [20], [21], the prognostic value of NLR in IS patients has seldom been reported [22], [23], suggesting a need for further studies to address this issue. This study, therefore, tested the hypothesis that NLR might be a simple, inexpensive and readily available marker of prognosis in acute IS.
Methods
Patient population and data collection
According to the IS treatment program (i.e., Program for Acute Ischemic Stroke Registry) at Kaohsiung Chang Gang Memorial Hospital, since January 2010 patients of any age with acute IS must be completely registered. Thus all acute IS patients were prospectively identified, both detailed in-hospital and follow-up data including age, sex, coronary artery disease risk factors, NIHSS score on admission, time onset of IS, laboratory findings (i.e., complete blood count, differential count, biochemistry), computerized tomography, magnetic resonance imaging (MRI), duplex scanning of the carotid arteries, routine cardiac analysis by of 12-lead electrocardiogram and echocardiography, chest X-ray, carotid Doppler examination, drug history and family history, and any in-hospital adverse event as well as in-hospital mortality were obtained. These data were prospectively collected and entered into digital database.
This study was based on the Declaration of Helsinki (revised 2013). Written informed consent was obtained from all study participants. And this study was approved by the Institutional Review Committee on Human Research at Kaohsiung Chang Gung Memorial Hospital (IRB number: 102-1583A3).
Definition of IS, physical examination and neurological assessment
Stroke was defined as sudden onset of loss of global or focal cerebral function persisting for more than 24 h. Evaluation of physical function and neurological impairment of stroke patients was based on the National Institutes of Health Stroke Scale (NIHSS).
We stratified patients according to stroke severity using admission NIHSS scores into three groups: (1) Mild stroke (NIHSS < 6), (2) Moderate stroke (NIHSS ≥ 6 and <16, and (3) Severe stroke (NIHSS ≥ 16). This classification was based on the previously report [24] with some modification.
Patient enrollment, inclusion and exclusion criteria
For the purpose of this study, inclusion criteria were a score of ≥2 on the NIHSS and a time window of ≤48 h from onset of symptoms to blood sampling (at 48 h after IS). Patients with a history of the following were excluded from the study: intracranial hemorrhage, surgery or trauma within the preceding 3 months, hematology disorders, renal insufficiency [i.e., estimated glomerular filtration rate (eGFR) < 20 (mL/min/1.73 m2)], malignancy, febrile disorders, acute or chronic inflammatory disease at study entry, liver cirrhosis, atrial fibrillation, contraindications for MRI examination, no evidence of acute IS by MRI study, myeloproliferative disorder, or autoimmune disease.
Accordingly, between April 2012 and August 2014, a total number of 1731 consecutive patients were enrolled for the study after IS. Of these patients, 1055 were categorized into mild stroke (Group 1), 519 were categorized into moderate stroke (Group 2), and the remainder of 157 were categorized into severe stroke (Group 3) in this study.
Medications
Aspirin was the first choice for acute stroke patients unless they were allergic or intolerant to aspirin, such as having a history of peptic ulcer or upper gastro-intestinal tract bleeding during aspirin therapy. Clopidogrel was used in patients intolerant to aspirin therapy. Other commonly used drugs included statins, angiotensin converting enzyme inhibitors, calcium channel blocker agents and beta-blocker agents.
Statistical analysis
Categorical data among the three groups were compared using chi-square test and those of continuous variables among three groups were compared using one-way ANOVA followed by Tukey's multiple comparison procedure. The relationship between NIHSS score and prognostic variables was analyzed by linear regression. Multivariate logistic regression analysis with stepwise selection was utilized for identifying the independent predictors of prognostic outcomes. Statistical analysis was performed using SAS statistical software for Windows version 8.2 (SAS institute, Cary, NC). A value of P < 0.05 was considered statistically significant and all statistical tests were two-sided.
Results
Baseline characteristics among three groups [Table 1]
Table 1.
Baseline characteristics of 1731 study patients.
| Variables | Group 1 (n = 1055)a | Group 2 (n = 519)a | Group 3 (n = 157)a | p valuee |
|---|---|---|---|---|
| Age (y) | 66.4A ± 12.3 | 69.3B ± 12.5 | 74.7C ± 12.3 | <0.0001 |
| Male gender | 68.3% (720)A | 57.8% (300)B | 45.9% (72)C | <0.0001 |
| Diabetes mellitus | 40.4% (426) | 42.6% (221) | 36.9% (58) | 0.422 |
| Hypertension | 74.2% (783) | 76.3% (396) | 72.6% (114) | 0.550 |
| Smoking | 29.3% (309)A | 21.6% (112)B | 14.0% (22)C | <0.0001 |
| Systolic blood pressure (mmHg)b | 156.2 ± 27.8 | 156.6 ± 29.6 | 155.1 ± 31.2 | 0.852 |
| Diastolic blood pressure (mmHg)b | 87.2A ± 14.7 | 86.8A,B ± 18.2 | 83.7B ± 16.9 | 0.040 |
| Old stroke | 28.9% (305)A | 37.0% (192)B | 45.2% (71)C | <0.0001 |
| History of myocardial infarction | 3.2% (35) | 2.9% (15) | 2.6% (4) | 0.868 |
| Presence of PAOD | 1.3% (14) | 2.1% (11) | 0.6% (1) | 0.309 |
| History of obstructive CADc | 5.8% (61) | 4.4% (23) | 3.85 (6) | 0.377 |
| Congestive heart failured | 1.3% (14) | 2.5% (13) | 2.6% (4) | 0.192 |
| ACEI use | 7.9% (79) | 6.5% (36) | 8.3% (13) | 0.838 |
| ARB use | 34.0% (359) | 32.4% (168) | 24.8% (39) | 0.071 |
| Statin use | 41.9% (442)A | 43.4% (225)A | 31.9% (50)B | 0.033 |
| Peak blood flow velocity of LICA | 73.5 ± 41.2 | 71.7 ± 50.5 | 67.1 ± 41.8 | 0.248 |
| Peak blood flow velocity of LCCA | 75.9A ± 22.5 | 72.8B ± 26.8 | 67.9B ± 25.2 | 0.0002 |
| Ratio velocity of LICA/LCCA | 1.03 ± 0.71 | 1.05 ± 0.81 | 1.06 ± 0.82 | 0.861 |
| Peak blood flow velocity of RICA | 71.5 ± 43.9 | 69.9 ± 48.6 | 65.0 ± 32.4 | 0.244 |
| Peak blood flow velocity of RCCA | 70.8A ± 22.4 | 67.2B ± 23.3 | 65.0B ± 23.0 | 0.0008 |
| Ratio velocity of RICA/RCCA | 1.09 ± 0.95 | 1.12 ± 1.00 | 1.08 ± 0.65 | 0.876 |
Data are expressed as mean ± S.D. or % (n).
Abbreviations: PAOD: peripheral arterial obstructive disease; CAD: coronary artery disease; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin II type receptor blocker; LICA: left internal carotid artery; LCCA: left common carotid artery; RICA: right internal carotid artery; RCCA: right common carotid artery.
Group 1 = patients with National Institute of Health Stroke Scale (NIHSS) <6.0. Group 2 = patients with NIHSS ≥6.0 and <16.0. Group 3 = patients with NIHSS >16.0.
Defined as the blood pressure measured upon presentation.
Defined as patients having significant CAD and who received coronary intervention.
Defined as advanced congestive heart failure (≥New York Heart Association Functional Class III).
Indicated continuous data were analyzed using one-way ANOVA, and categorical data were analyzed by chi-square test. Different letters (A, B, C) indicated significant difference (at 0.05 level) by Tukey's multiple comparison procedure.
There were no significant differences in terms of diabetes mellitus, hypertension, systolic blood pressure, history of myocardial infarction, incidence of peripheral arterial occluded disease, and incidence of obstructive coronary artery disease undergoing percutaneous coronary artery intervention. Additionally, the incidences of congestive heart failure and utilizations of angiotensin converting enzyme inhibitor and angiotensin II type receptor blocker were similar among the three groups. Furthermore, carotid Doppler study showed the peak blood flow velocity of the left internal carotid artery (LICA), peak blood flow velocity of the right internal carotid artery (RICA), ratio velocity of LICA/left common carotid artery (LCCA), and ratio velocity of RICA/right common carotid artery (RCCA) did not differ among three groups.
However, age and incidence of old stroke were significantly higher in group 3 than in groups 1 and 2, and significantly higher in group 2 than in group 1, whereas male gender and history of smoking showed an opposite pattern of age among the three groups. Additionally, the incidence of statin use was significantly lower in group 3 than in groups 1 and 2, but it showed no difference between groups 1 and 2. Furthermore, the peak blood flow velocity of LCCA and the peak blood flow velocity of RCCA were significantly higher in group 1 than in groups 2 and 3, but they showed no difference between the latter two groups.
Laboratory findings and in-hospital death among 1731 study subjects [Table 2]
Table 2.
Laboratory findings and in-hospital mortality of 1731 study patients.
| Variables | Group 1 (n = 1055)a | Group 2 (n = 519)a | Group 3 (n = 157)a | p valueb |
|---|---|---|---|---|
| Total cholesterol level (mg/dL) | 177.6A ± 41.7 | 175.5A ± 43.2 | 161.7B ± 39.1 | <0.0001 |
| Low-density lipoprotein | 105.6A ± 36.6 | 106.3A ± 35.0 | 95.2B ± 34.4 | 0.002 |
| White blood cell count (×103)/mL | 9.8 ± 25.1 | 8.2 ± 3.4 | 8.6 ± 3.2 | 0.846 |
| Neutrophil (N) (%) | 65.2A ± 11.5 | 68.2B ± 11.8 | 71.2C ± 12.8 | <0.0001 |
| Lymphocyte (L) (%) | 26.2A ± 10.0 | 25.2A ± 29.6 | 20.9B ± 11.3 | 0.003 |
| Monocyte (M) (%) | 6.1 ± 4.7 | 5.8 ± 2.4 | 5.9 ± 2.3 | 0.413 |
| Platelet (P) count (×104)/mL | 213.0 ± 111.7 | 213.8 ± 75.8 | 211.5 ± 90.9 | 0.969 |
| Ratio of N to L | 3.5A ± 4.3 | 4.4B ± 6.7 | 5.9C ± 7.6 | <0.0001 |
| Ratio of N to M | 13.8 ± 12.3 | 16.7 ± 42.9 | 15.4 ± 13.2 | 0.113 |
| Ratio of P to S | 3.34 ± 1.59 | 3.24 ± 1.28 | 3.06 ± 1.40 | 0.054 |
| Hemoglobin (g/dL) | 13.6A ± 4.6 | 13.3A,B ± 2.2 | 12.7A ± 2.3 | 0.014 |
| Creatinine (mg/dL) | 1.6 ± 5.1 | 1.7 ± 2.3 | 1.2 ± 1.3 | 0.058 |
| eGFR (mL/min/1.73 m2) | 70.5 ± 29.7 | 66.7 ± 32.7 | 70.8 ± 33.4 | 0.512 |
| In-hospital mortality | 2.0% (21) | 3.1% (16) | 14.0% (22) | <0.0001 |
Data are expressed as mean ± S.D. or % (n).
Abbreviation: eGFR: estimated glomerular filtration rate.
Group 1 = patients with National Institute of Health Stroke Scale (NIHSS) <6.0. Group 2 = patients with NIHSS ≥6.0 and <16.0. Group 3 = patients with NIHSS >16.0.
Indicated continuous data were analyzed using one-way ANOVA, and categorical data were analyzed by chi-square test. Different letters (A, B, C) indicated significant difference (at 0.05 level) by Tukey's multiple comparison procedure.
Laboratory findings demonstrated that white blood cell count, platelet count, monocyte count, neutrophil-to-monocyte ratio, platelet-to-neutrophil ratio, eGFR and the creatinine level did not differ among the three groups. However, the levels of total cholesterol and low-density lipoprotein, and lymphocyte count were significantly higher in groups 1 and 2 than in group 3, but exhibited no difference between the former two groups. Additionally, the neutrophil count and the NLR (i.e., the ratio of neutrophils-to-lymphocytes) were significantly higher in group 3 than in groups 1 and 2, and significantly higher in group 2 than in group 1. Furthermore, the hemoglobin level was higher in group 2 than in group 3, but displayed no difference between groups 1 and 2 or between groups 1 and 3. Moreover, the in-hospital mortality rate was significantly higher in group 3 than in groups 1 and 2, but it showed no difference between groups 1 and 2.
Univariate and multiple stepwise logistic regression analyses of predictors for in-hospital mortality [Table 3, Table 4 and Fig. 1]
Table 3.
Univariate analysis of predictors of in-hospital mortality.
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Age (yrs) | 1.06 | 1.04–1.09 | <0.0001 |
| Male gender | 0.85 | 0.50–1.44 | 0.543 |
| Diabetes mellitus | 0.93 | 0.55–1.58 | 0.781 |
| Hypertension | 0.99 | 0.55–1.80 | 0.983 |
| Smoking | 0.58 | 0.29–1.16 | 0.126 |
| Systolic blood pressure | 1.0 | 0.99–1.00 | 0.306 |
| Diastolic blood pressure | 1.0 | 0.97–1.01 | 0.213 |
| Old stroke | 2.03 | 1.21–3.42 | 0.008 |
| History of myocardial infarction | 1.74 | 0.53–5.74 | 0.365 |
| Presence of PAOD | <0.001 | <0.001–1000 | 0.985 |
| History of obstructive CAD | 0.31 | 0.04–2247 | 0.244 |
| Congestive heart failurea | 4.43 | 1.50–13.10 | 0.007 |
| Total cholesterol level | 1.0 | 0.99–1.002 | 0.147 |
| Low-density lipoprotein | 1.0 | 0.99–1.007 | 0.877 |
| White blood cell count | 1.0 | 0.99–1.01 | 0.700 |
| Neutrophil (N) | 1.03 | 1.01–1.05 | 0.011 |
| Lymphocyte (L) | 0.95 | 0.92–0.98 | 0.0001 |
| Monocyte (M) | 0.97 | 0.87–1.08 | 0.564 |
| Platelet (P) count | 1.0 | 0.995–1.0 | 0.606 |
| Ratio of N to L | 1.04 | 1.02–1.06 | 0.0006 |
| Ratio of N to M | 1.0 | 0.99–1.01 | 0.967 |
| Ratio of P to N | 0.87 | 0.69–1.10 | 0.250 |
| Hemoglobin | 0.79 | 0.71–0.87 | <0.0001 |
| Creatinine | 1.01 | 0.98–1.05 | 0.414 |
| eGFR | 0.99 | 0.99–1.0 | 0.176 |
Abbreviations: OR: odds ratio; CI: confidence intervals; PAOD: peripheral arterial obstructive disease; CAD: coronary artery disease; eGFR: estimated glomerular filtration rate (mL/min/1.73 m2).
Defined as advanced congestive heart failure (≥New York Heart Association Functional Class III).
Table 4.
Multiple stepwise logistic regression analysis of predictors for in-hospital mortality.
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Age | 1.06 | 1.03–1.09 | <0.0001 |
| Congestive heart failurea | 4.58 | 1.52–13.85 | 0.007 |
| Ratio N to L | 1.04 | 1.01–1.06 | 0.002 |
Abbreviations: OR: odds ratio; CI: confidence intervals; N: neutrophil; L: lymphocyte.
Defined as advanced congestive heart failure (≥New York Heart Association Functional Class III).
Fig. 1.
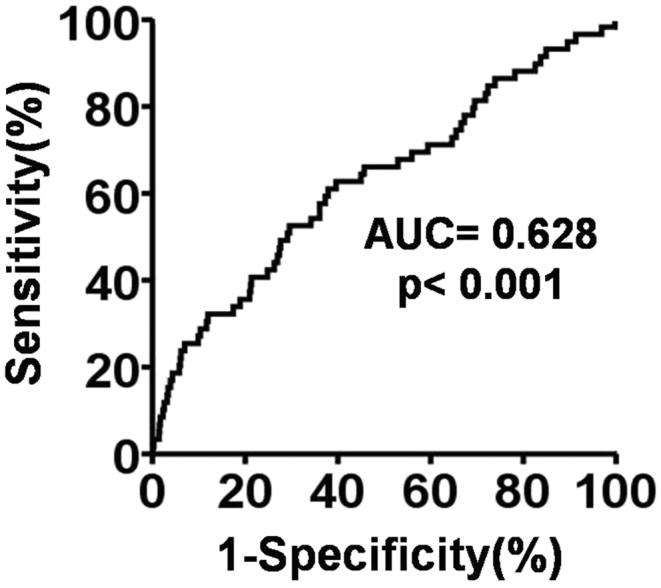
Receiver operating characteristics (ROC) curve analysis revealed that the neutrophil-to-lymphocyte ratio (NLR) ≥3.20 was the most powerful predictor of in-hospital mortality with a sensitivity 62.7%, a specificity 60.3%, p < 0.001; likelihood ratio 12.2.
Variables in Table 1, Table 2 were enrolled for univariate analysis for finding the significant predictors related to in-hospital mortality. As shown in Table 3, age, lymphocyte count and lower hemoglobin were strongly associated with in-hospital mortality. Additionally, old stroke, congestive heart failure, neutrophil count, and NLR were also significantly predictive of in-hospital mortality.
The multiple stepwise logistic regression analysis demonstrated that age was the most strongly independent predictor of in-hospital mortality. Additionally, NLR and congestive heart failure were correlated with in-hospital mortality, significantly and independently.
Receiver operating characteristics (ROC) curve analysis [Fig. 1] revealed that the best discriminating factor for in-hospital mortality with respect to NLR was ≥3.20 (sensitivity 62.7%, specificity 60.3%, likelihood ratio 12.2). Additionally, those of patients with NLR ≥3.20 had a 2.55-fold increased risk for in-hospital mortality (95% confidence interval; OR = 1.49–4.37) compared to patients with NLR <3.20.
Univariate linear regression and multiple stepwise linear regression analyses of relevant baseline variables for predicting higher NIHSS in study patients [Table 5, Table 6]
Table 5.
Univariate linear regression analysis of relevant baseline variables for predicting higher NIHSS in study patients.
| Variables | Regression Coefficient (95% CI) | p value |
|---|---|---|
| Age | 0.098 (0.077, 0.120) | 0.008 |
| Male gender | −1.845 (−2.417, −1.273) | <0.0001 |
| Diabetes mellitus | −0.020 (−0.588, 0.548) | 0.945 |
| Hypertension | 0.143 (−0.499, 0.786) | 0.662 |
| Smoking | −1.480 (−2.116, −0.844) | <0.0001 |
| Systolic blood pressure | −0.002 (−0.012, 0.008) | 0.705 |
| Diastolic blood pressure | −0.025 (−0.042, −0.008) | 0.005 |
| Old stroke | 1.545 (0.955, 2.135) | <0.0001 |
| History of myocardial infarction | −0.416 (−2.036, 1.205) | 0.615 |
| Presence of PAOD | −0.917 (−3.213, 1.378) | 0.433 |
| History of obstructive CAD | −0.725 (−1.982, 0.532) | 0.258 |
| Congestive heart failurea | 0.876 (−1.229, 2.981) | 0.414 |
| Peak blood flow velocity of LICA | −0.005 (−0.011, 0.001) | 0.094 |
| Peak blood flow velocity of LCCA | −0.024 (−0.035, −0.013) | <0.0001 |
| Ratio velocity of LICA/LCCA | 0.066 (−0.298, 0.429) | 0.723 |
| Peak blood flow velocity of RICA | −0.004 (−0.010, 0.002) | 0.163 |
| Peak blood flow velocity of RCCA | −0.020 (−0.032, −0.008) | 0.001 |
| Ratio velocity of RICA/RCCA | 0.054 (−0.233, 0.343) | 0.705 |
| Total cholesterol level | −0.013 (0.019, −0.006) | 0.0002 |
| Neutrophil (N), | 0.081 (0.057, 0.104) | <0.0001 |
| Lymphocyte (L) | −0.029 (−0.044, −0.014) | 0.0002 |
| Monocyte (M) | −0.023 (−0.094, 0.047) | 0.512 |
| Platelet (P) count | −0.001 (−0.003, 0.003) | 0.943 |
| Ratio of N to L | 0.148 (0.098, 0.198) | <0.0001 |
| Ratio of N to M | 0.006 (−0.004, 0.017) | 0.275 |
| Ratio of P to N | −0.221 (−0.409, −0.034) | 0.021 |
| Hemoglobin | −0.087 (−0.159, −0.015) | 0.017 |
| Creatinine | −0.023 (−0.089, 0.043) | 0.496 |
| Egfr | −0.004 (−0.013, 0.005) | 0.400 |
Abbreviations: NIHSS: National Institutes of Health Stroke Scale; PAOD: peripheral arterial obstructive disease; CAD: coronary artery disease; LICA: left internal carotid artery; LCCA: left common carotid artery; RICA: right internal carotid artery; RCCA: right common carotid artery; eGFR: estimated glomerular filtration rate (mL/min/1.73 m2); CI: confidence intervals.
Defined as advanced congestive heart failure (≥New York Heart Association Functional Class III).
Table 6.
Multiple stepwise linear regression analysis of relevant baseline variables for predicting higher NIHSS in study patients.
| Variables | Regression coefficient (95% CI) | p value |
|---|---|---|
| Intercept | −0.244 (−2.887, 2.400) | 0.857 |
| Age (yrs) | 0.073 (0.051, 0.095) | <0.0001 |
| Male gender | −1.736 (−2.300, −1.172) | <0.0001 |
| Old stroke | 1.200 (0.624, 1.776) | <0.0001 |
| Neutrophil (N) | 0.051 (0.026, 0.077) | 0.000 |
| Ratio of N to L | 0.077 (0.022, 0.133) | 0.007 |
| Total cholesterol level (mg/dL) | −0.008 (−0.015, −0.002) | 0.016 |
Abbreviations: NIHSS: National Institutes of Health Stroke Scale; CI: confidence intervals.
Variables in Table 1, Table 2 were enrolled for univariate linear regression analysis for finding the significant predictors correlated with higher NIHSS. As shown in Table 5, old stroke, neutrophil count and NLR were the most powerful predictors of higher NIHSS. Additionally, age was also significantly predictive of higher NIHSS. On the other hand, male gender, smoking (i.e., smoking was notably increased in younger than in elder patients) and peak blood flow velocity of LCCA were the most powerful predictors of freedom from higher NIHSS. Besides, diastolic blood pressure, peak blood flow velocity of RCCA, total cholesterol level (an indicator of nutrient status), lymphocyte count, ratio of platelet-to-neutrophil and hemoglobin level were significantly associated with freedom from higher NIHSS.
The multiple stepwise linear regression analysis showed that age and old stroke were the most strongly and independently predictive of higher NIHSS. Additionally, neutrophil count and NLR were significantly and independently predictive of higher NIHSS. In contrast, male gender and total cholesterol level were significantly and independently predictive of freedom from higher NIHSS.
Univariate analysis and multiple stepwise logistic regression analysis of predictors for severe NIHSS (i.e., ≥16) [Table 7, Table 8 and Fig. 2]
Table 7.
Univariate analysis of significant predictors for severe NIHSS.a
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Age | 1.06 | 1.04–1.07 | <0.0001 |
| Male gender | 0.46 | 0.33–0.64 | <0.0001 |
| Smoking | 0.45 | 0.28–0.71 | 0.0007 |
| Diastolic blood pressure | 0.99 | 0.98–0.997 | 0.012 |
| Old stroke | 1.79 | 1.28–2.49 | 0.0006 |
| Total cholesterol level | 0.99 | 0.99–0.995 | <0.0001 |
| Low-density lipoprotein | 0.99 | 0.99–0.996 | 0.0005 |
| Neutrophil (N) | 1.04 | 1.02–1.05 | <0.0001 |
| Lymphocyte (L) | 0.96 | 0.94–0.98 | <0.0001 |
| Platelet (P) count | 1.0 | 0.998–1.00 | 0.206 |
| Ratio of S to L | 1.04 | 1.02–1.06 | 0.0004 |
| Ratio of P to N | 0.84 | 0.73–0.98 | 0.026 |
| Hemoglobin | 0.87 | 0.81–0.93 | 0.0001 |
Abbreviations: OR: odds ratio; CI: confidence intervals; PAOD: peripheral arterial obstructive disease; CAD: coronary artery disease.
Severe NIHSS defined as ≥16 National Institute of Health Stroke Scale (NIHSS).
Table 8.
Multiple stepwise logistic regression analysis of predictors for severe NIHSS.a
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Age | 1.04 | 1.03–1.06 | <0.0001 |
| Male gender | 0.45 | 0.32–0.64 | <0.0001 |
| Old stroke | 1.50 | 1.06–2.14 | 0.0231 |
| Neutrophil | 1.03 | 1.02–1.05 | <0.0001 |
| Total cholesterol level | 0.99 | 0.987–0.996 | 0.0006 |
Abbreviations: OR: odds ratio; CI: confidence intervals.
Severe NIHSS defined as ≥16 National Institute of Health Stroke Scale (NIHSS).
Fig. 2.
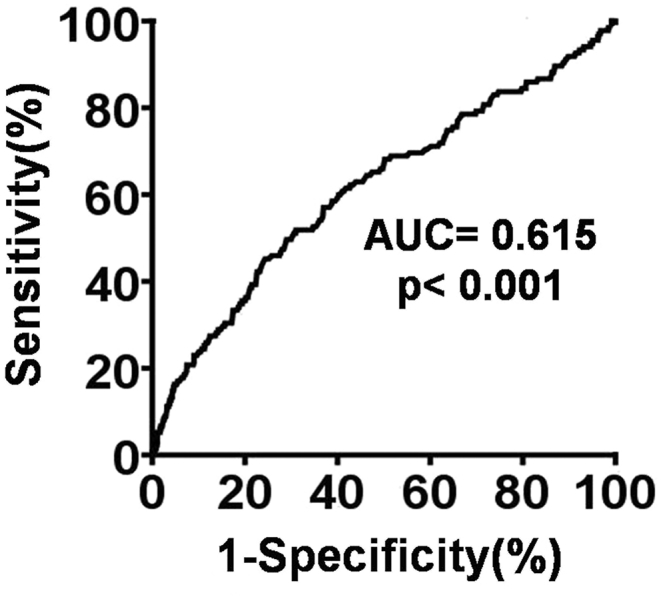
Receiver operating characteristics (ROC) curve analysis revealed that the neutrophil count (NC) ≥74% was the most powerful predictor of severe stroke (i.e., NIHSS ≥ 16) with a sensitivity 47.1%, a specificity 74.0%, p < 0.001; likelihood ratio 29.0.
Variables in Table 1, Table 2 were enrolled for univariate analysis for identifying the significant predictors that were associated with severe NIHSS. As displayed in Table 7, age and neutrophil count were the most powerful predictors of severe NIHSS. Additionally, old stroke and the NLR were also significantly associated with severe NIHSS. On the other hand, male gender, total cholesterol level, lymphocyte count and hemoglobin level were the most powerful predictors of freedom from severe NIHSS. In addition, smoking, diastolic blood pressure, low-density lipoprotein and the ratio of platelets-to-neutrophils were also significantly predictive of freedom from severe NIHSS.
The multiple stepwise logistic regression analysis showed that age and new neutrophil count were most strongly and independently predictive of severe NIHSS. Additionally, old stroke was also found to be significantly and independently predictive of severe NIHSS. On the other hand, male gender and total cholesterol level were significantly and independently predictive of freedom from severe NIHSS.
The ROC curve analysis [Fig. 2] revealed that the best discriminating factor for severe stroke (defined as ≥16 NIHSS) with respect to NC was ≥74% (sensitivity 47.1%, specificity 74.0%, likelihood ratio 29.0). Additionally, those patients with NC >74% had a 2.54-fold increased risk for severe stroke (95% confidence interval; OR = 1.82–3.54) compared to patients with NC <74%.
Discussion
This study investigated the impact of NC and NLR on predicting unfavorable outcome in in-hospital patients after IS had several clinical implications. First, the NLR was established to be strongly and significantly predictive of in-hospital mortality, suggesting that NLR should be seriously considered as an accessory biomarker for risk stratification in the setting of acute IS. Second, the NC and NLR were identified to be strongly associated with higher NIHSS, implying that both these two parameters may be useful as supplementary biomarkers for dividing IS patients into high-risk and low-risk subgroups for optimal and individualized care. Third, NC was displayed to be the strongest predictor of severe NIHSS, highlighting that NC should be considered as a useful auxiliary biomarker for more intensive care and pharmacomodulation as well as the application of beneficially invasive intervention for improving neurological function and clinical outcome.
Abundant data have shown that NC and NLR are stronger inflammatory mediators than white blood cells (WBC) in various diseases [17], [18], [25], [26], [27]. Of interest, NC and NLR are not only inflammatory mediators but have also emerged as important biomarkers for predicting adverse clinical outcomes in various clinical settings, such as in solid tumors [27], atrial fibrillation [28], non-ST segment elevation myocardial infarction (non-STEMI) [29] and STEMI [19], [20], [21] undergoing primary PCI. Surprisingly, on the other hand, there are only a few studies [22], [23] that have investigated the impact of NC/NLR on prognostic outcome in acute IS patients. The chief concern of these studies is their sample size [22], [23], which was quite small and might not determine the true impact of these biomarkers on predicting the short-term and long-term prognostic outcome in patients after IS.
To the best of our knowledge, our present study is the largest cohort study to investigate the impact of NC and NLR on prediction of worse clinical outcome in patients after acute IS. The most important finding in the present study was that the NLR was significantly and independently predictive of in-hospital mortality in patients after IS. Our finding, in addition to supporting two recent articles [22], [23] that reported that NLR was significantly correlated with unfavorable clinical outcome in patients after IS, highlights that NLR is a simple inexpensive and readily available marker of prognosis in acute IS [22].
Another important finding was that this study demonstrated a strong correlation between higher NIHSS and NC and NLR. Interestingly, strong correlation between NLR and unfavorable neurological status and symptomatic intracerebral hemorrhage after thrombolytic therapy in acute IS patients has been established by a recent study [30]. In this way, our finding corroborated with the findings of the recent study [30], and suggests that identification of two mediators could provide accurate stratification of IS patients into high-risk and low-risk subgroups with new targets for neuroprotection [30].
Interestingly, the link between an increase in inflammation and poorer prognostic outcome in patients after acute IS has been extensively investigated previously [13], [14], [15]. Additionally, neutrophils and lymphocytes, i.e., the major components of white blood cell leukocyte count, and NLR have been well recognized as indicators of inflammation [26], [27], [31] for predicting prognostic outcome in various diseases. The principal finding in the present study is that NC in circulation is a powerful predictor of severe stroke. Accordingly, our finding in addition to strengthening the findings of recent study [26], [27], [31], encourage the use of NC as a useful auxiliary biomarker for predicting neurological function and clinical outcome in patients after IS.
Old age, old stroke and congestive heart failure have been fully investigated as three powerful traditional risk factors for predicting prognostic outcome in the setting of IHD [32], [33], [34] and CVD [8], [9], [16], [35]. The present study identified that age was one of most powerful predictors of in-hospital mortality, higher NIHSS and severe stroke. Additionally, history of old stroke was significantly and independently correlated to higher NIHSS and severe stroke. Besides, congestive heart failure was found to be strongly associated with in-hospital mortality. In this way, our findings corroborated with the findings of previous studies [8], [9], [16], [35].
An intriguing finding in the present study was that male gender was significantly correlated with free from higher NIHSS and severe stroke. These findings may implicate that the female gender may be a factor contributing to poor prognostic outcome after acute IS. Comparably, previous study has shown that female gender is a strong predictor of poor prognostic outcome after AMI [33]. Our finding was supported by the findings of previous study [33]. Another intriguing finding in the present study was that high level of total cholesterol was found to be significantly and independently predictive of freedom from higher NIHSS and severe stroke. We remain uncertain why such an unusual result emerged in the present study. Perhaps, it could reflect old age accompanied malnutrition, which has been identified as a risk factor for poorer prognostic outcome in the present study.
This study has limitations. First, although the predictive value of NC and NLR on short-term prognostic outcome in patients after acute IS is attractive and promising, the predictive value of these biomarkers on long-term prognostic outcome after IS was not investigated by the present study. Second, this study did not measure the patients' brain infarct volume. Therefore, we did not provide the correlation between the brain infarct volume and circulating levels of NC and NLR. Third, this study did not measure the correlation between subtype of IS and the level of NLR.
In conclusion, the results of our study demonstrated that NC and NLR are credible biomarkers with the advantage of being inexpensive and readily available for predicting prognostic outcome in patients after acute IS.
Conflicts of interest
The authors have no conflicts of interest relevant to this article.
Acknowledgment
This work was supported by the Chang Gung Memorial Hospital Research Project, Taiwan [CMRPG8E1241 and CMRPG8E0681].
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Feigin V.L., Forouzanfar M.H., Krishnamurthi R., Mensah G.A. Global burden of stroke: an underestimate - authors' reply. Lancet. 2014;383:1205–1206. doi: 10.1016/S0140-6736(14)60596-1. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahlgren N., Ahmed N., Davalos A., Ford G.A., Grond M., Hacke W. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y., Wang A., Liu G., Zhao X., Meng X., Zhao K. Cost-effectiveness of clopidogrel-aspirin versus aspirin alone for acute transient ischemic attack and minor stroke. J Am Heart Assoc. 2014;3:e000912. doi: 10.1161/JAHA.114.000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stecksen A., Asplund K., Appelros P., Glader E.L., Norrving B., Eriksson M. Thrombolytic therapy rates and stroke severity: an analysis of data from the Swedish stroke register (Riks-Stroke) 2007–2010. Stroke; J Cereb Circ. 2012;43:536–538. doi: 10.1161/STROKEAHA.111.630590. [DOI] [PubMed] [Google Scholar]
- 6.Yeo L.L., Paliwal P., Teoh H.L., Seet R.C., Chan B.P., Liang S. Timing of recanalization after intravenous thrombolysis and functional outcomes after acute ischemic stroke. JAMA Neurol. 2013;70:353–358. doi: 10.1001/2013.jamaneurol.547. [DOI] [PubMed] [Google Scholar]
- 7.Strbian D., Sairanen T., Meretoja A., Pitkaniemi J., Putaala J., Salonen O. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology. 2011;77:341–348. doi: 10.1212/WNL.0b013e3182267b8c. [DOI] [PubMed] [Google Scholar]
- 8.Yoo A.J., Chaudhry Z.A., Nogueira R.G., Lev M.H., Schaefer P.W., Schwamm L.H. Infarct volume is a pivotal biomarker after intra-arterial stroke therapy. Stroke. 2012;43:1323–1330. doi: 10.1161/STROKEAHA.111.639401. [DOI] [PubMed] [Google Scholar]
- 9.Munsch F., Sagnier S., Asselineau J., Bigourdan A., Guttmann C.R., Debruxelles S. Stroke location is an independent predictor of cognitive outcome. Stroke. 2016;47:66–73. doi: 10.1161/STROKEAHA.115.011242. [DOI] [PubMed] [Google Scholar]
- 10.Elkind M.S., Cheng J., Rundek T., Boden-Albala B., Sacco R.L. Leukocyte count predicts outcome after ischemic stroke: the Northern Manhattan Stroke Study. J Stroke Cerebrovasc Dis. 2004;13:220–227. doi: 10.1016/j.jstrokecerebrovasdis.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Jickling G.C., Sharp F.R. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8:349–360. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia L., Hao F., Wang W., Qu Y. Circulating miR-145 is associated with plasma high-sensitivity C-reactive protein in acute ischemic stroke patients. Cell Biochem Funct. 2015;33:314–319. doi: 10.1002/cbf.3116. [DOI] [PubMed] [Google Scholar]
- 13.Yuen C.M., Chiu C.A., Chang L.T., Liou C.W., Lu C.H., Youssef A.A. Level and value of interleukin-18 after acute ischemic stroke. Circ J. 2007;71:1691–1696. doi: 10.1253/circj.71.1691. [DOI] [PubMed] [Google Scholar]
- 14.Chang L.T., Yuen C.M., Liou C.W., Lu C.H., Chang W.N., Youssef A.A. Link between interleukin-10 level and outcome after ischemic stroke. Neuroimmunomodulation. 2010;17:223–228. doi: 10.1159/000290038. [DOI] [PubMed] [Google Scholar]
- 15.Yeh K.H., Tsai T.H., Chai H.T., Leu S., Chung S.Y., Chua S. Comparison of acute versus convalescent stage high-sensitivity C-reactive protein level in predicting clinical outcome after acute ischemic stroke and impact of erythropoietin. J Transl Med. 2012;10:6. doi: 10.1186/1479-5876-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yip H.K., Liou C.W., Chang H.W., Lan M.Y., Liu J.S., Chen M.C. Link between platelet activity and outcomes after an ischemic stroke. Cerebrovasc Dis. 2005;20:120–128. doi: 10.1159/000086802. [DOI] [PubMed] [Google Scholar]
- 17.Walsh S.R., Cook E.J., Goulder F., Justin T.A., Keeling N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 18.Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 19.Akpek M., Kaya M.G., Lam Y.Y., Sahin O., Elcik D., Celik T. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–627. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 20.Park J.J., Jang H.J., Oh I.Y., Yoon C.H., Suh J.W., Cho Y.S. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2013;111:636–642. doi: 10.1016/j.amjcard.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 21.Gazi E., Bayram B., Gazi S., Temiz A., Kirilmaz B., Altun B. Prognostic value of the neutrophil-lymphocyte ratio in patients with ST-elevated acute myocardial infarction. Clin Appl Thromb Hemost. 2015;21:155–159. doi: 10.1177/1076029613492011. [DOI] [PubMed] [Google Scholar]
- 22.Celikbilek A., Ismailogullari S., Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. 2014;28:27–31. doi: 10.1002/jcla.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokgoz S., Keskin S., Kayrak M., Seyithanoglu A., Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J Stroke Cerebrovasc Dis. 2014;23:2163–2168. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Briggs D.E., Felberg R.A., Malkoff M.D., Bratina P., Grotta J.C. Should mild or moderate stroke patients be admitted to an intensive care unit? Stroke. 2001;32:871–876. doi: 10.1161/01.str.32.4.871. [DOI] [PubMed] [Google Scholar]
- 25.Thakore A.H., Guo C.Y., Larson M.G., Corey D., Wang T.J., Vasan R.S. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study) Am J Cardiol. 2007;99:1598–1602. doi: 10.1016/j.amjcard.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 26.de Jager C.P., van Wijk P.T., Mathoera R.B., de Jongh-Leuvenink J., van der Poll T., Wever P.C. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care. 2010;14:R192. doi: 10.1186/cc9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Templeton A.J., McNamara M.G., Seruga B., Vera-Badillo F.E., Aneja P., Ocana A. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 28.Shao Q., Chen K., Rha S.W., Lim H.E., Li G., Liu T. Usefulness of neutrophil/lymphocyte ratio as a predictor of atrial fibrillation: a meta-analysis. Arch Med Res. 2015;46:199–206. doi: 10.1016/j.arcmed.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Yilmaz M., Tenekecioglu E., Arslan B., Bekler A., Ozluk O.A., Karaagac K. White blood cell subtypes and neutrophil-lymphocyte ratio in prediction of coronary thrombus formation in non-ST-segment elevated acute coronary syndrome. Clin Appl Thromb Hemost. 2015;21:446–452. doi: 10.1177/1076029613507337. [DOI] [PubMed] [Google Scholar]
- 30.Maestrini I., Strbian D., Gautier S., Haapaniemi E., Moulin S., Sairanen T. Higher neutrophil counts before thrombolysis for cerebral ischemia predict worse outcomes. Neurology. 2015;85:1408–1416. doi: 10.1212/WNL.0000000000002029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ertas G., Sonmez O., Turfan M., Kul S., Erdogan E., Tasal A. Neutrophil/lymphocyte ratio is associated with thromboembolic stroke in patients with non-valvular atrial fibrillation. J Neurol Sci. 2013;324:49–52. doi: 10.1016/j.jns.2012.09.032. [DOI] [PubMed] [Google Scholar]
- 32.Yip H.K., Wu C.J., Chang H.W., Hang C.L., Fang C.Y., Hsieh Y.K. Comparison of primary angioplasty and conservative treatment on short- and long-term outcome in octogenarian or older patients with acute myocardial infarction. Jpn Heart J. 2002;43:463–474. doi: 10.1536/jhj.43.463. [DOI] [PubMed] [Google Scholar]
- 33.Yip H.K., Wu C.J., Chang H.W., Wang C.P., Cheng C.I., Chua S. Cardiac rupture complicating acute myocardial infarction in the direct percutaneous coronary intervention reperfusion era. Chest. 2003;124:565–571. doi: 10.1378/chest.124.2.565. [DOI] [PubMed] [Google Scholar]
- 34.Tsai T.H., Chua S., Hussein H., Leu S., Wu C.J., Hang C.L. Outcomes of patients with Killip class III acute myocardial infarction after primary percutaneous coronary intervention. Crit Care Med. 2011;39:436–442. doi: 10.1097/CCM.0b013e318206ccc3. [DOI] [PubMed] [Google Scholar]
- 35.Yip H.K., Chang L.T., Chang W.N., Lu C.H., Liou C.W., Lan M.Y. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]


