Abstract
In this issue of the Biomedical Journal, we explore the inner workings of tumor-associated macrophages and seek to understand how these cells can boost or limit the efficacy of radiotherapy, depending on the context. We also highlight a study revealing that staffing patterns in the intensive care unit may affect the outcome of patients with severe sepsis. Finally, we learn how an advanced imaging technique can improve endodontic treatment planning.
Keywords: Radiotherapy, Tumor-associated macrophages, Severe sepsis
Spotlight on reviews
Radiotherapy and the tumor microenvironment: the “macro” picture
Radiotherapy has for many years been a cornerstone of cancer treatment. But to fully understand its effects and maximize them requires zooming out from individual cancer cells and looking at the tumor as a whole. In this issue of the Biomedical Journal, Wu et al. [1] describe how ionizing radiation affects macrophages in the tumor and what this means for cancer therapy of the future.
Zapping tumors with high doses of IR normally amounts to a death sentence for highly proliferating cancer cells, but it also has profound effects on the tumor microenvironment, the support system of non-cancerous cells and stroma that play a major role in determining the outcome of malignancy. An important component of the tumor microenvironment is immune cells, in particular tumor associated macrophages (TAMs). These cells are typically thought to drive tumor progression by stimulating cell proliferation, metastasis and angiogenesis, and inhibiting the T cell-mediated anti-tumor immune response [2]. Thus, understanding how TAMs react to IR, for better or worse, has important implications for cancer therapy.
As Wu et al. point out however, the response of these cells is not easy to predict and depends on a range of factors. Whereas low-dose IR induces an anti-inflammatory “M2” like phenotype, doses above 1 Gy induce a pro-inflammatory “M1” like phenotype resulting in the production of nitric oxide and several pro-inflammatory cytokines [3], [4]. However, the exact response is also likely to depend on host genetic factors and age, with rodent macrophages exhibiting different responses to the same dose depending on the strain and age of the animal from which they were isolated [5], [6]. These findings are reflected in in vivo studies to some extent, although small doses of IR have also been reported to induce M1 phenotypes in some settings [7].
Thus, the multitude of factors that influence how macrophages respond to IR complicates the important question of what happens to irradiated or bystander TAMs in vivo and can we manipulate these effects to our advantage from a therapeutic perspective? It is not surprising that IR has been shown to induce either the pro-inflammatory and anti-inflammatory activation of TAMs, depending on the context. For example, in an oral cancer mouse model, IR caused the infiltration of M2-like TAMs that promoted vascularization and hence tumor progression [8]. However, in another cancer murine model, IR increased the abundance of nitric oxide-producing pro-inflammatory macrophages, which in this context, contributed to anti-tumor responses [9].
One result that emerges more clearly however is that IR seems to promote the recruitment of macrophages to tumors [10]. Since TAMs are thought to contribute to cancer progression in established tumors [11], compounds that block the macrophage recruitment pathways are an obvious adjuvant to radiotherapy. The colony stimulating factor 1 (CSF-1) is implicated in the recruitment of macrophages to tumors [12], and the CSF-1 receptor is exclusively expressed in monocytic cells, which makes the CSF-1/CSF-1R pathway an attractive target to interfere with TAMs. In a mouse model of glioblastoma, blockade of CSF-1R using a chemical inhibitor combined with irradiation significantly impaired the accumulation of M2-like cells in the tumor and led to improved tumor control and longer survival [13]. Likewise, similar results have been reported in other cancer models [14].
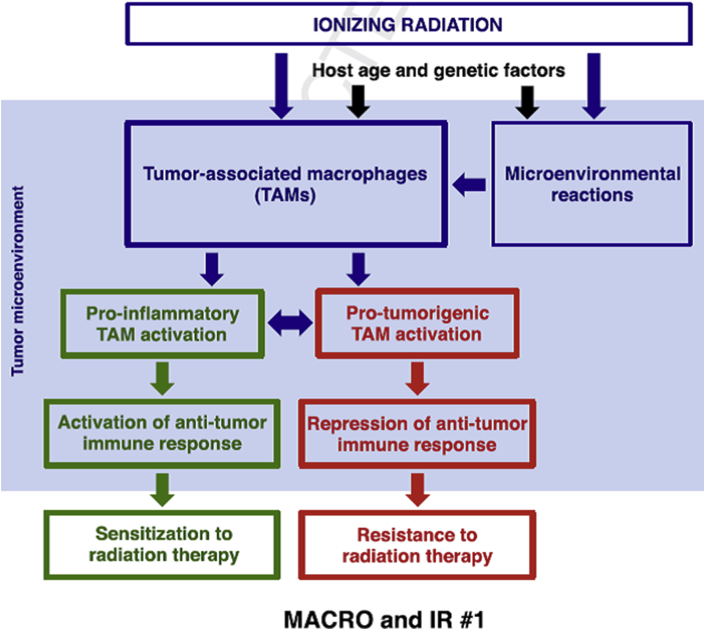
Although it may appear too soon to connect the dots and build a unified model for the influence of IR on TAMs [Fig. 1], manipulating TAM activity to our advantage, be it in the context of radiotherapy or not, is an exciting therapeutic avenue to explore. Ultimately, “macrophage reprogramming” therapies that polarize TAMs may one day provide an effective string to the bow in tackling tumor progression.
Fig. 1.
The effect of ionizing radiation on tumor-associated macrophages (TAMs). Depending on a multitude of factors, including dose, genetics and age, ionizing radiation may either promote a pro-inflammatory M1 like response or an anti-inflammatory M2 like response in tumor-associated macrophages (TAMs). As a result, TAMs may either promote or inhibit anti-tumor responses thus making the tumor sensitive or resistant to radiotherapy, respectively. Figure kindly provided by Wu et al. [1].
Spotlight on original articles
Intensivist wanted: staffing pattern and risk of sepsis-related death in the intensive care unit
Improving patient care is not just about providing better treatments, it is also about ensuring that our healthcare services are adequately staffed and optimally organized. In this issue of the Biomedical Journal, Lin et al. [15] investigate how staffing pattern in the intensive care unit (ICU) affects a patient's chance of succumbing to severe sepsis.
During infection, the body may launch a full-blown systemic inflammatory response designed to combat evading bacteria, but which may ultimately damage internal tissues and lead to organ failure. The result is called severe sepsis, a life-threatening condition that typically requires admission and treatment in an ICU. With an overall hospital mortality rate of 17.9–50% depending on the population [16], severe sepsis remains a challenge in modern medicine and international evidence-based guidelines - the Surviving Sepsis Campaign (SSC) – have been established for managing patients with the condition [17]. An essential component of these guidelines is the formulation of “bundles”, a set of evidence-based practices that when performed collectively and reliably have been shown to improve patient outcome beyond the effect of implementing each practice alone [18].
Besides better treatment, the organization of the ICU, in particular physician staffing, is a factor that influences ICU mortality [19]. In Taiwan, specialist training in pulmonary disorders and critical care are combined, and physicians choose whether to become a full-time critical care physician (intensivist) or manage patients with patients with lung disorders on the ward. Thus, depending on who is on duty, a patient with severe sepsis can be treated by either a “high care volume” physician (intensivist) or a “low care volume” physician (pulmonologist). In this setting, Lin et al. investigate whether this particular staffing model affects the outcome of patients with severe sepsis.
Their prospective observation study included 484 patients with severe sepsis treated at a single Taiwanese hospital over a three-year period. During this period, eight physicians rotated in the ICU, one of which treated more than half of the patients and could be described as a “high care volume” physician and the remainder as “low care volume” physicians. Various clinical data, in particular adherence to the SSC bundles were collected and patient outcome was examined. Statistical analysis revealed that patients treated by the high care volume physician had a lower risk of mortality than those treated by the low care volume physicians. Overall, the high care volume physician was more consistent than the low care volume physicians in performing several of the bundles therapies recommended by the SSC guidelines, including renal replacement therapy, administration of low-dose steroids for septic shock, prophylaxis of upper GI bleeding, and control of hyperglycemia.
These findings highlight the importance of staffing models in the ICU. They are in line with the “practice makes perfect” concept put forward by a previous study showing that physician's care volume can predict mortality in ICU patients with pneumonia [20]. In other words, those with experience in managing complicated cases perform better in the ICU setting. It must be acknowledged however that only one physician was in high care volume group which raises the question of whether adherence to the SSC guidelines was a personal preference or that gained through experience. Nonetheless, the difference in bundle therapies between high and low volume groups sends a strong message: regardless of years of experience, stick to the guidelines.
Also in this issue
Review article
Thioredoxin: the “stress reliever” of prospective cancer cells?
Stern and Monteiro [21] upregulation of the anti-oxidant protein thioredoxin helps cancer cells to cope with the oxidative stress encountered during cancer development.
Original articles
Fighting tissue wasting and improving quality of life in end stage head and neck cancer
In the late stages of almost every major chronic illness, imbalance in the processes that degrade and build tissue can lead to devastating weight loss called cachexia. For many years progesterone derivatives, like megestrol acetate, have been used to improve appetite in cancer patients with cachexia, although it is questionable whether their use in isolation improves survival or quality of life [22]. Hung et al. [23] investigate the use of progesterone analogs in cachectic patients with nasopharyngeal carcinoma (NPC) patients. In the 80% of patients who gained weight following treatment, pain and quality of life scores were significantly improved and were correlated with control of EBV DNA load. These findings support the use of progesterone derivatives as part of arsenal of therapies specifically targeting cachexia as a separate, treatable disease.
Two images are better than one
Medical imaging is a cornerstone of all aspects of health care today; from public health and preventative medicine, to diagnosis, treatment, and prognostic monitoring. Different imaging modalities offer different advantages. Magnetic resonance imaging (MRI) can provide a detailed picture of soft tissues such as the heart and brain, whereas Positron Emission Tomography (PET) provides functional information of metabolism but at a lower resolution. Haddadpour et al. [24] propose a method to exploit the best of both, and applied Hilbert transform (2-D HT) and Intensity Hue Saturation (IHS) to combine and map MRI and PET images of the brain to a single, fused image. Their method preserved both the spatial and spectral features of input images and outperformed five other common image fusion methods tested.
Cataloging root canal variation in the Taiwanese
To avoid a trip back to the dentist, all root canal spaces must be identified, cleared and filled during endodontic therapy since undetected and subsequently unfilled spaces can become infected and lead to treatment failure. The maxillary molar is the tooth with the largest volume and most complex root and root canal anatomy and thus presents a challenge for treatment planning. Using cone beam computed tomography (CBCT), Lin et al. [25] obtain accurate three-dimensional images of the root canal of maxillary molars in 114 Taiwanese participants to catalog natural anatomical variation. Their study, the first of its kind in the Taiwanese population, reveals a spectrum of root canal configurations, with the biggest differences in the mesiobuccal root, and provides knowledge that will be very useful for endodontic treatment planning.
Correspondence
The many faces of apoptosis inducing factor
Apoptosis inducing factor (AIF) is released by mitochondria into the cytoplasm during cell death and causes DNA fragmentation through a caspase independent mechanism. More than just a trigger for destruction, Preta [26] et al. describe how AIF is emerging as an important factor regulating energy metabolism, redox balance and tumor progression.
Conflicts of interest
The author declares that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Wu Q., Allouch A., Martins I., Modjtahedi N., Deutsch E., Perfettini J.L. Macrophage biology plays a central role during ionizing radiation-elicited tumor response activation of macrophages dictates immune response induced by ionizing radiation. Biomed J. 2017;40:200–211. doi: 10.1016/j.bj.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gough M.J., Young K., Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol. 2013;2013:281958. doi: 10.1155/2013/281958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibuki Y., Goto R. Enhancement of NO production from resident peritoneal macrophages by in vitro gammairradiation and its relationship to reactive oxygen intermediates. Free Radic Biol Med. 1997;22:1029–1035. doi: 10.1016/s0891-5849(96)00500-x. [DOI] [PubMed] [Google Scholar]
- 5.Rastogi S., Boylan M., Wright E.G., Coates P.J. Interactions of apoptotic cells with macrophages in radiation-induced bystander signaling. Radiat Res. 2013;179:135–145. doi: 10.1667/RR2969.1. [DOI] [PubMed] [Google Scholar]
- 6.Tasat D.R., Mancuso R., Evelson P., Polo J.M., Llesuy S., Molinari B. Radiation effects on oxidative metabolism in young and aged rat alveolar macrophages. Cell Mol Biol (Noisy-le-grand) 2002;48:529–535. [PubMed] [Google Scholar]
- 7.Pandey R., Shankar B.S., Sharma D., Sainis K.B. Low dose radiation induced immunomodulation: effect on macrophages and CD8þ T cells. Int J Radiat Biol. 2005;81:801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- 8.Okubo M., Kioi M., Nakashima H., Sugiura K., Mitsudo K., Aoki I. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep. 2016;6:27548. doi: 10.1038/srep27548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N. Low-dose irradiation programs macrophage differentiation to an iNOS(þ)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Russell J.S., Brown J.M. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery. Front Physiol. 2013;4:157. doi: 10.3389/fphys.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A., Marchesi F., Malesci A., Laghi L., Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serafini P., Borrello I., Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Stafford J.H., Hirai T., Deng L., Chernikova S.B., Urata K., West B.L. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18:797–806. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J., Escamilla J., Mok S., David J., Priceman S., West B. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin C.Y., Tseng J.C., Huang C.Y., Chu C.M., Wu H.P. Mortality of severe septic patients between physician's high and low care volumes. Biomed J. 2017;40:226–231. doi: 10.1016/j.bj.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr F.B., Yende S., Angus D.C. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellinger R.P., Carlet J.M., Masur H., Gerlach H., Calandra T., Cohen J. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32:858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 18.http://www.survivingsepsis.org/Bundles/Pages/default.aspx [accessed 17.07.05].
- 19.Wilcox M.E., Chong C.A., Niven D.J., Rubenfeld G.D., Rowan K.M., Wunsch H. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analyses. Crit Care Med. 2013;41:2253–2274. doi: 10.1097/CCM.0b013e318292313a. [DOI] [PubMed] [Google Scholar]
- 20.Lin H.C., Xirasagar S., Chen C.H., Hwang Y.T. Physician's case volume of intensive care unit pneumonia admissions and in-hospital mortality. Am J Respir Crit Care Med. 2008;177:989–994. doi: 10.1164/rccm.200706-813OC. [DOI] [PubMed] [Google Scholar]
- 21.Stern A., Monteiro H. Thioredoxin promotes survival signaling events under nitrosative/oxidative stress associated with cancer development. Biomed J. 2017;40:189–199. doi: 10.1016/j.bj.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Les’niak W., Bała M., Jaeschke R., Krzakowski M. Effects of megestrol acetate in patients with cancer anorexia-cachexia syndrome – a systematic review and meta-analysis. Pol Arch Med Wewn. 2008;118:636–644. [PubMed] [Google Scholar]
- 23.Hung C.Y., Lin T.L., Kuo Y.C., Hsieh C.H., Wang H.M., Hsu C.L. Progesterone analogues reduce plasma Epstein-Barr virus DNA load and improve pain control in recurrent/metastatic nasopharyngeal carcinoma patients under supportive care. Biomed J. 2017;40:212–218. doi: 10.1016/j.bj.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haddadpour M., Daneshvar S., Seyedarabi H. PET and MRI image fusion based on combination of 2-D Hilbert transform and IHS method. Biomed J. 2017;40:219–225. doi: 10.1016/j.bj.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin Y.H., Lin H.N., Chen C.C., Chen M.S. Evaluation of the root and canal systems of maxillary molars in Taiwanese patients: a cone beam computed tomography study. Biomed J. 2017;40:232–238. doi: 10.1016/j.bj.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preta G. Understanding the Dr. Jekyll and Mr. Hyde nature of apoptosis inducing factor: future perspectives. Biomed J. 2017;40:239–240. doi: 10.1016/j.bj.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]