Abstract
Apoptosis-inducing factor (AIF) is emerging as a key protein in regulation of basic physiological processes including phagocytosis, mitophagy and regulation of the redox state. Recent evidences suggest that the enzymatic activity of AIF may play an active role in tumor progression controlling energy metabolism and redox balance. The present manuscript briefly describes the story of this protein from its initial discovery as caspase-independent apoptotic protein, throughout its role in oxidative phosphorylation and lately involvement in tumor progression. Understanding the dualistic nature of AIF is a critical starting point to clarify its contribution in tumor metabolic balance and to develop new AIF-specific therapeutic strategies.
Keywords: Mitochondria, Apoptosis-inducing factor, Phagocytosis, Mitophagy, Phosphatidylserine
Mitochondria have a dual role in cellular life and death as energy providers and as contributors to the apoptotic process. Apoptosis-inducing factor (AIF) was discovered as a mitochondrial protein that, like cytochrome c is released into the cytoplasm during the apoptotic process. While cytochrome c participates in the activation of caspases via the apoptosome complex formation, AIF has been showed to cause nuclear DNA damage by a caspase-independent mechanism. The key signal for the removal of apoptotic cells is the externalization of the major anionic phospholipid, phosphatidylserine (PS), which is recognized by a host of receptors on phagocytes. In the nematode C. elegans, the externalization of PS was proved to occur via WAH-1 (worm homolog of AIF)-dependent activation of the phospholipid scramblase, SCRM-1, a homolog of the mammalian phospholipid scramblases [1].
An unexpected role for AIF was discovered when a group at the Jackson Laboratory found that the Harlequin (Hq) mutant mice harbor a pro-viral insertion in the first intron of the Aif gene, leading to 80% reduction in AIF protein levels [2]. Cerebellar granule cells from the Hq mutant mice were more susceptible to peroxide-induced apoptosis than their wild type counterpart. This degeneration stems from a loss of AIF-mediated protection against cellular damage induced by oxidative stress. AIF promotes cell death yet also controls mitochondrial homeostasis and energy metabolism. The aim of future research is to clarify how these apparently conflicting activities are coordinated, and what is the impact of AIF upon human diseases particularly for tissues resistant to caspase-dependent apoptosis. For example, cardiomyocytes contain high levels of caspase inhibitors, suggesting that caspase-independent apoptosis may be amplified in the heart. The importance of an effective phagocytosis is strengthened by the fact that high levels of cell death occur within a tumor environment and the mechanisms through which dying tumor cells are removed can drastically influence tumor-specific immunity. Changing the phagocytes mitochondrial membrane potential significantly affected phagocytosis, with lower potential increasing engulfment capacity and higher membrane potential inhibiting the uptake of apoptotic cells [3]. Therefore, mitochondria are crucial actors in programmed cell clearance acting at both sides of the “phagocytic synapse”.
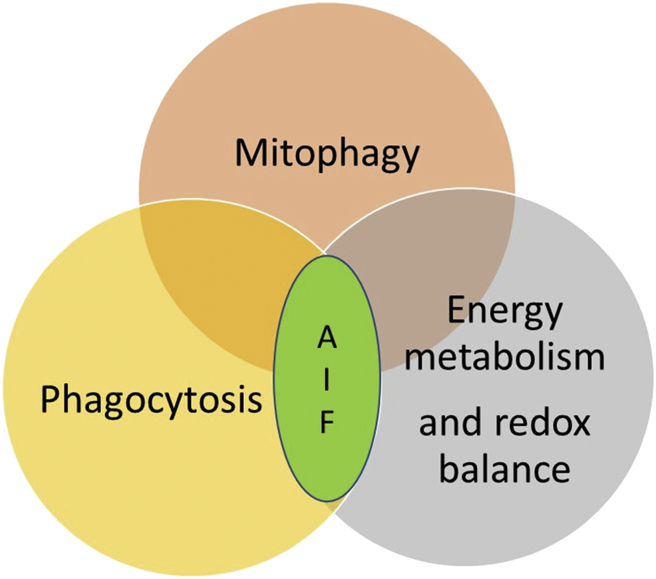
The enzymatic activity of AIF was shown to be critical for cancer progression through control of energy metabolism and redox balance [4]. Malignant transformation has been found to induce marked changes in mitochondria dynamic and structure. Mitochondrial turnover (mitophagy) is emerging as a key quality-control mechanism in cells. Mitophagy has always been recognized to play a double-faceted role in tumorigenesis, either supporting survival or promoting death, depending on the specific cellular context. A recent study aimed to identify new AIF binding partners revealed the mitochondrial protein PGAM5 as an AIF-associated factor. Transient overexpression of PGAM5 morphologically and biochemically resembles mitophagic cell death [5]. The widespread connection between development of cancer disease and alteration of mitochondrial dynamic together with the recent flood of new acquisitions on the role of AIF in phagocytosis, tumor progression and mitophagy [Fig. 1] suggest the importance to clarify how AIF influences tumor metabolic balance to develop novel specific therapeutic strategies.
Fig. 1.
AIF could influence tumor progression in a triple way: 1) The mechanisms through which dying tumor cells are removed can considerably influence tumor-specific immunity and recent evidences suggest an active role of AIF in the mechanism of phagocytosis. 2) Mitophagy is emerging as a key quality-control mechanism in cancer cells development and AIF was found to influence mitochondrial turnover by binding with specific regulatory proteins. 3) AIF through control of energy metabolism and redox balance was also demonstrated to directly promote tumor progression.
Conflicts of interest
There is no conflict of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Wang X., Wang J., Gengyo-Ando K., Gu L., Sun C.L., Yang C. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol. 2007;9:541–549. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- 2.Klein J.A., Longo-Guess C.M., Rossmann M.P., Seburn K.L., Hurd R.E., Frankel W.N. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002;419:367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 3.Park D., Han C.Z., Elliott M.R., Kinchen J.M., Trampont P.C., Das S. Continued clearance of apoptotic cells critically depends on the phagocyte Ucp2 protein. Nature. 2011;477:220–224. doi: 10.1038/nature10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis E.M., Wilkinson A.S., Jackson J.S., Mehra R., Varambally S., Chinnaiyan A.M. The enzymatic activity of apoptosis-inducing factor supports energy metabolism benefiting the growth and invasiveness of advanced prostate cancer cells. J Biol Chem. 2012;287:43862–43875. doi: 10.1074/jbc.M112.407650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenhausen A.M., Wilkinson A.S., Lewis E.M., Dailey K.M., Scott A.J., Khan S. Apoptosis inducing factor binding protein PGAM5 triggers mitophagic cell death that is inhibited by the ubiquitin ligase activity of X-linked inhibitor of apoptosis. Biochemistry. 2016;55:3285–3302. doi: 10.1021/acs.biochem.6b00306. [DOI] [PubMed] [Google Scholar]