Abstract
Radiation therapy is one of the major therapeutic modalities for most solid tumors. The anti-tumor effect of radiation therapy consists of the direct tumor cell killing, as well as the modulation of tumor microenvironment and the activation of immune response against tumors. Radiation therapy has been shown to promote immunogenic cells death, activate dendritic cells and enhance tumor antigen presentation and anti-tumor T cell activation. Radiation therapy also programs innate immune cells such as macrophages that leads to either radiosensitization or radioresistance, according to different tumors and different radiation regimen studied. The mechanisms underlying radiation-induced macrophage activation remain largely elusive. Various molecular players such as NF-κB, MAPKs, p53, reactive oxygen species, inflammasomes have been involved in these processes. The skewing to a pro-inflammatory phenotype thus results in the activation of anti-tumor immune response and enhanced radiotherapy effect. Therefore, a comprehensive understanding of the mechanism of radiation-induced macrophage activation and its role in tumor response to radiation therapy is crucial for the development of new therapeutic strategies to enhance radiation therapy efficacy.
Keywords: Radiation therapy, Tumor, Immune response, Macrophage activation

Radiation therapy is one of the cornerstones of cancer therapies. By inducing lethal DNA damages (such as DNA single- and double-strand breaks), ionizing radiation (IR) may eliminate irradiated cells, but also non-irradiated neighboring cells (also known as by-stander effect) through distinct death modalities (including apoptosis, necrosis and mitotic catastrophe). Several studies recently highlighted that ionizing radiation may also impact the tumoral microenvironment, the associated immune system and modulate tumor response to radiation therapy [1]. For example, accumulating evidence demonstrates that radiation therapy can promote tumor immune response by eliciting immunogenic cell death, tumor antigen release and immune cell activations. In addition, the combination of radiation therapy with a variety of immune modulators also enhanced tumor regression outside the field of irradiation, also know as abscopal effect, confirming that the biological consequences of the ionizing radiation of tumoral microenvironment components (such as immune effectors) are key events in tumor response to radiotherapy that remain to be elucidated [2].
Despite the macrophages play important roles in organ development, in host defense against tissue insults and infections, and in maintaining tissue homeostasis, these myeloid cells also participate in metabolic disorders, immune diseases and cancer development [3]. Characterized by their functional plasticity and heterogeneity, these innate cells can be activated by a plethora of stimuli such as growth factors, cytokines, microbial products, nucleotides and many other modulators. In vitro stimulation of macrophages by interferon-γ (IFN-γ) or tumor necrosis factor-α (TNF-α) and/or microbial products such as lipopolysaccharides (LPS) induces classical (M1) macrophage activation, which is characterized by an IL-12highIL-23highIL-10low phenotype with elevated production of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, increased expression of inducible nitric oxide synthase (iNOS) and reactive oxygen species (ROS). Classical activated macrophages are proficient effectors in promoting Th-1 type immune response and in fighting against bacterial infections as well as malignant tumors. On the other hand, macrophages stimulated by Th-2 related cytokines (such as IL-4 or IL-13), IL-10, immune complexes, glucocorticoids are grouped as alternative activated (M2) macrophages with an IL-12lowIL-23lowIL-10highTGF-βhigh phenotype. Alternative activated macrophages express high level of arginase 1 (Arg1), mannose receptors, scavenger receptors, galactose-type receptors, and participate in the Th-2 type immune response, the resolution of inflammation, the tissue repair, the intracellular parasite clearance, the immune regulation, the angiogenesis and the tumor progression [4].
Macrophages also represent a major cellular component of the tumor stroma. These tumor-associated macrophages (TAMs) derived from blood monocytes that differentiate into macrophages after recruitment to the tumor area by tumor-derived cytokines and chemokines. In the majority of cases, TAMs acquire pro-tumorigenic phenotypes that contribute to tumor growth, tumor invasion, angiogenesis, and tumor metastasis, making them attractive targets for developing new anti-cancer strategies [5]. The interaction of ionizing radiation and macrophage activity is the subject of intensive investigation. This review summarizes recent findings with regard to the regulation of macrophage activities by ionizing radiation (IR) and their roles in tumor responses.
Biological consequences of ionizing radiation on macrophages
In vitro/ex vivo studies
Ionizing radiation is reported in many studies to affect the biological functions of stimulated macrophages. The physic characteristics of IR (such as type, dose and treatment schedules), basal activation states and host genetic factors impact the biological responses of macrophages to ionizing radiation.
Delivered doses dictate biological functions of macrophages
A large body of evidence indicated that low-dose (single dose ≤1.0 Gy) irradiation predominantly induced anti-inflammatory activation of macrophages while high-dose irradiation was more prone to enhance the pro-inflammatory properties of macrophages [6]. For example, earlier studies using murine resident macrophages or macrophage-like cell lines demonstrated that ionizing radiation activated macrophages, increased the production of iNOS and subsequent nitric oxide (NO) as well as the production of O2− [7], [8], [9], and induced the expression of several pro-inflammatory cytokines such IL-1β, IL-6 and TNF-α [10], [11], [12], [13]. However, ex vivo irradiation of LPS-activated BALB/c peritoneal macrophages with low dose (0.5 Gy) X-ray led to reduced secretion of pro-inflammatory cytokine IL-1β while increased secretion of anti-inflammatory cytokine TGF-β, indicating that low-dose irradiation promoted anti-inflammatory macrophage phenotype in this particular setting [14]. Low-dose X-ray irradiation at 0.5 or 0.7 Gy reduced the expression of pro-IL-1β and secretion of IL-1β from LPS- and monosodium urate crystals-stimulated THP1-differentiated macrophages without affecting cell viability. This IR-induced anti-inflammatory phenotype was associated with reduced nuclear translocation of RelA and the decreased amount of p38 and Akt kinases [15]. Low-dose but not high dose X-ray irradiation also reduced the oxidative burst in activated macrophages [16]. However, there are also reports showing that low to intermediate dose irradiation of mouse peritoneal macrophages induced an early production of pro-inflammatory IL-1β and IL-6 in a protein kinase C- and phosphatidylinositol 3-kinase-dependent manner [13].
When irradiated at a higher dose (≥1 Gy), macrophages tend to display a pro-inflammatory phenotype. For example, irradiation at 1–5 Gy potentiated the production of iNOS and NO in IFN-γ and LPS-stimulated J774.1 and RAW264.7 macrophages [17]. Interestingly, TNF-α was involved in this boost of pro-inflammatory mediator as TNF-α blocking antibody treatment before irradiation inhibited the induction of NO by IFN-γ [18]. Irradiation of RAW264.7 murine macrophages with gamma-ray at 2.5 Gy up to 20 Gy did not significantly induced the production of NO and IL-1β but strongly enhanced NO production and IL-1β expression in LPS-activated macrophages [19].
Effects of ionizing radiation on human monocytes/macrophages have also been evaluated. A single dose of 2 Gy irradiation significantly increased the production of IL-1α and IL-1β in human alveolar macrophages [20]. Another study failed to detect significant induction of IL-1β or TNF-α after 10 Gy of gamma-irradiation in human monocytes/macrophages [21]. Ex vivo X-ray irradiation at 20 Gy induced the expression of scavenger receptor CD36 in human blood monocyte-derived macrophages in a way dependent on JNK activation, leading to enhanced uptake of oxidized low-density lipoprotein and the formation of foam cells [22].
Intrinsic radiosensitivity of macrophages
The activation of macrophages by irradiation is also dependent on host genetic factors that control macrophage radiosensitivity. For example, macrophages from CBA/Ca mice are more prone to be pro-inflammatory and are more radiosensitive whereas those from C57BL/6 mice which exhibited anti-inflammatory activities and radioresistant properties [23]. Despite the in vitro direct irradiation of bone marrow-derived macrophages from both CBA/Ca and C57BL/6 mice had no clear effect on macrophage phenotype change, the in vivo irradiation enhanced the M2-like features of macrophages from C57BL/6 mice. While macrophages from CBA/Ca mice displayed an intrinsic M1-like phenotype and retained this pro-inflammatory feature after in vivo irradiation, suggesting that microenvironment reaction to irradiation may also affect macrophage activity [24].
Similarly, ex vivo low-dose X-ray irradiations with 0.5 Gy or 0.7 Gy reduced the release of IL-1β and TNF-α in LPS-activated peritoneal macrophages from BALB/c mice that are more radiosensitive. However, same treatments failed to induce such anti-inflammatory response in peritoneal macrophages from C57BL/6 mice [25]. Another study performed on BALB/c and C57BL/6 mice showed that ten daily X-ray whole body irradiations at 0.01 Gy, 0.02 Gy or 0.1 Gy significantly enhanced cytotoxic activity and NO production of IFN-γ- and LPS-activated peritoneal macrophages from both strains. These responses that are different in their kinetics and their magnifications are associated with comparable reduced pulmonary tumor growths detected after intravenous injection of syngenic tumor cells. Notably, the authors reported that low-dose X-ray irradiated macrophages without further activation by IFN-γ or LPS only produced negligible amounts of NO as did unirradiated macrophages [26].
Host age seems also to affect macrophage activation in response to gamma-ray irradiation. Macrophages from aged rats were more radiosensitive than those from young rats as evidenced by irradiation-induced higher generation of superoxide anion, increased apoptosis and decreased cell viability [27].
Properties of irradiation
The physic and biologic properties of irradiation affect differentially macrophage functions. For example, at 2 Gy, low-linear energy transfer (LET) gamma-irradiation activated preferentially ERK kinase, while high LET carbon ion activated p38 and JNK kinases [28]. In another study, neither X-ray nor carbon ions irradiation up to 32 Gy induced pro-inflammatory cytokines such as TNF-α and IL-1β and NO production in RAW264.7 macrophages. Both rays did not affect TNF-α production in LPS-activated macrophages. However, carbon ions increased macrophage phagocytosis, NO production along with a decreased IL-1β production in LPS-activated macrophages whereas X-ray irradiation resulted in a reduction of NO at low doses and no significant effect on IL-1β expression or phagocytic activities [29].
In vivo studies
It has been demonstrated that low-dose radiation increased the serum level of IL-3, IL-4, monocyte chemoattractant protein (MCP)-1 and -5, macrophage inflammatory protein alpha (MIP-α) and vascular endothelial growth factor (VEGF) and decreased IL-12p70, IL-13, IL-17 and IFN-γ, suggesting that low-dose irradiation suppressed pro-inflammatory responses in mice [30]. In concert to these findings, low-dose (5 fractions of 1.0 Gy or 5 fractions of 0.5 Gy) X-ray irradiation in adjuvant-induced arthritis in rats significantly reduced the expression of iNOS and increased significantly HO-1 expression in macrophages, leading to reduced clinical symptoms and bone destruction [31].
Peritoneal macrophages
Although many studies have confirmed the anti-inflammatory properties of low-dose irradiation [32], some studies showed that low-dose irradiation enhanced pro-inflammatory properties of macrophages that contributed to tumor suppression. For example, 0.04 Gy in vivo irradiation induced enhanced NO and reactive oxygen species such as O2− in peritoneal resident macrophages [8]. Total body low dose (5 fractions of 0.04 Gy) gamma-ray irradiation enhanced macrophage phagocytosis and NO production along with increased CD8+ T cell response in C57BL/6 mice, suggesting an immune stimulatory role of low-dose irradiation in this setting [33]. Likewise, low-dose (0.01, 0.02, or 0.1 Gy) fractionated X-ray whole body irradiation (5 days/week for two weeks) in BALB/c mice increased the anti-tumor activities of macrophages and NK cells that were associated with suppressed formation of pulmonary tumor colonies. IR enhanced NO production, IL-1β, IL-12 and TNF-α secretion with increased cytotoxic activities in IFN-γ and LPS-stimulated mouse peritoneal macrophages [34], [35]. However, although irradiation alone still increased the cytotoxic activity of untreated peritoneal macrophages, it failed to induce the production of NO [34].
Lung macrophages
Thoracic X-ray irradiation at 12 Gy in C57BL/6 mice induced iNOS expression from 8 h until 72 h post-irradiation while an increase in Arg1 expression in a later phase at 16 weeks and 24 weeks post-irradiation, which are associated with the radiation-induced acute pneumonitis and chronic fibrosis, respectively [36]. Irradiation of lung macrophages caused p38-mediated inhibitory phosphorylation and proteasomal degradation of a TNF-α regulator, the tristetraprolin, leading to increased TNF-α expression that contributed to radiation-induced lung toxicity [37]. In BALB/c mice, thoracic irradiation using X-ray at 10 Gy caused an increase of macrophage infiltration and activation of pro-inflammatory macrophages as revealed by high iNOS and low Arg1 expression 18 weeks after irradiation [38].
Colon macrophages
Irradiation at 20 Gy or 27 Gy in rats caused a significant increase in macrophage recruitment in lamina propria with a shift of iNOS/Arg1 and CCR7/CD163 ratio toward higher iNOS and CCR7, respectively. Interestingly, irradiation increased TLR4+ and TLR5+ macrophage infiltration. Systemic administration of TLR4 agonist LPS or TLR5 agonist flagellin maintained this high macrophage infiltration but increased the expression of Arg1 and CD163. Accordingly, while irradiation without further treatment induced the expression of pro-inflammatory CXCL10, TNF-α and IL-1β in the mucosa, irradiation followed by LPS or flagellin treatment reduced the expression of these cytokines/chemokines but increased the expression of M2-macrophage-associated CCL22, TGF-β and IL-10 [39].
Brain macrophages
Brain irradiation led to increased myeloid cell infiltration dependent on CCR2 signaling pathway [40]. Cranial irradiation of C57BL/6J mice using gamma-ray at 10 Gy induced a significant accumulation of activated macrophages with increased levels of pro-inflammatory cytokines, chemokines [41].
Kidney macrophages
Higher dose (16 Gy) X-ray irradiation promoted macrophage infiltration into the kidney and the production of pro-inflammatory cytokines such as IL-1β and IL-6 in a way dependent on TGF-β co-receptor endoglin [42].
Mechanisms
Direct effects
IR may activate many signaling pathways and key transcription factors that are involved in macrophage activation. Additionally, in many studies, IR alone failed to affect the production of effector molecules or cytokines but either enhanced or inhibited the expression of these inflammatory mediators in IFN-γ or LPS-activated macrophages, suggesting that IR might also serve as a prime signal or work at a post-translational level to regulate macrophage functions [17], [43].
Toll-like receptor (TLR)- myeloid differentiation primary response 88 (MyD88) pathway
Shan YX et al. demonstrated that both low (0.075 Gy) and high (2 Gy) doses irradiation induced pro-inflammatory IL-12 and IL-18 production in mouse peritoneal macrophages. Irradiation induced a dose-dependent up-regulation of CD14 and TLR4-MD expression in macrophage surface and increased expression of MyD88 in macrophage cytoplasm, indicating an activation of TLR-MyD88 signaling pathway [44]. Another study showed that ex vivo low dose (0.05–0.1 Gy) X-ray irradiation of isolated human monocytes increased high mobility group box 1 protein (HMGB1) release and TLR4 and TLR9 expression, leading to increased MyD88-interleukin 1 receptor-associated kinase 1 (IRAK1) interaction and subsequent activation of NF-κB (at both 0.05 and 0.1 Gy) and MAPKs (ERK, JNK and p38; at 0.05 Gy) signaling pathway. However, at a higher dose of 1 Gy, although HMGB1 release remained increased, NF-κB activity was inhibited and the three MAPKs were down regulated [45].
Nuclear factor-κB (NF-κB) pathway
Fractionated ionizing radiation (2 Gy/fraction for 5 fractions) activates NF-κB with significant induction of pro-inflammatory markers such as CD80, CD86 and HLA-DR and inhibition of the expression of anti-inflammatory markers including CD163, macrophage mannose receptor 1 (MRC1), versican (VCAN) and IL-10 in human macrophages [46]. On the other hand and in agreement with the anti-inflammatory properties of low-dose irradiation in many studies, ex vivo irradiation of LPS-activated BALB/c peritoneal macrophages with low dose (0.5 Gy) X-ray showed that irradiation inhibited NF-κB activity in macrophages by impeding the nuclear translocation of NF-κB p65, leading to reduced expression of pro-inflammatory cytokines and increased production of anti-inflammatory cytokines [14], [15]. However, how ionizing radiation regulates NF-κB activity in macrophages is still unclear. Studies in HeLa and HEK293 cells demonstrated that gamma-ray irradiation activates IκB kinase (IKK) that phosphorylates IκBα at ser-32 and ser-36 leading to its proteasomal degradation and NF-κB activation [47]. DNA damages and reactive oxygen species also activate NF-κB [48], [49]. Whether and how these mechanisms are involved in irradiated-macrophages require further researches.
Mitogen-activated protein kinases (MAPKs) pathway
Both gamma-irradiation and carbon ion irradiation were shown to activate macrophages by activating MAPK signaling pathway (including ERK, JNK and p38) and the induction of NO and ROS generation [50]. Ex vivo gamma-ray irradiation at 2 Gy induced activation of all three MAPKs (ERK1/2, JNK and p38) in mouse peritoneal macrophages [51]. Low-dose gamma irradiation (0.1 Gy) was shown to up-regulate the expression of the transcription factor nuclear erythroid-derived 2-related factor (Nrf2) and promoted its nuclear translocation in RAW264.7 macrophages. An enhanced Nfr2 activity led to increased expression of heme-oxygenase-1 (HO-1) that is involved in antioxidant reaction in a way dependent on ERK1/2 pathway [52]. 2 Gy gamma-ray irradiation of LPS-activated peritoneal macrophages from Swiss mice showed enhanced production of iNOS and NO that was abrogated by JNK inhibitor [53]. Inversely, another study demonstrated that 0.5 Gy gamma-ray irradiation up-regulated the expression of MAPK phosphatase 1 (MKP-1), which dephosphorylated ERK1/2 and p38 MAPK, leading to inactivation of p38 MAPK and reduced production of TNF-α in LPS-activated RAW264.7 macrophages [54].
P53-dependent signaling pathways
Irradiation induces DNA damages in cells that lead to the activation of the transcription factor p53. P53 and NF-κB cooperatively regulate the expression of pro-inflammatory genes in human macrophages [55]. In addition, p53 was demonstrated to suppress anti-inflammatory macrophage activation [56]. Further more, it is reported that p53 promotes hepatic stellate cell (HSC) senescence, regulate senescence-associated secretory phenotype and activates HSC towards anti-tumoral M1 phenotype [57]. On the other hand, it is also shown that p53 can block pro-inflammatory activation of macrophages by inhibiting signal transducer and activator of transcription (STAT)-1 and p53−/− macrophages produced more pro-inflammatory cytokines as compared with p53+/+ cells [58]. Thus, p53 may play complex roles in macrophage activation. However, the direct role of p53 in IR-induced macrophage activation remains to be established.
Inflammasomes
Irradiation activated NLRP3 inflammasome in mouse macrophages in a dose-dependent manner [59]. This NLRP3 activation and subsequent increased secretion of IL-1β were involved in irradiation-induced lung inflammation [60]. In addition, irradiation also activated another inflammasome absent in melanoma 2 (AIM2) in macrophages and increased IL-1β expression that was associated with radiation pneumonitis [61].
Reactive oxygen species (ROS)
Ionizing radiation also exerts its biologic activities through the generation of ROS. ROS have been demonstrated in many studies to serve as secondary messenger molecules that regulate MAPK and NF-κB activity, leading to expression of pro-inflammatory genes and classical macrophage activation [62], [63]. Nevertheless, a recent study points out that ROS were also critical for M2 macrophage differentiation from monocytes. Pharmacological inhibition of superoxide blocks the differentiation of monocyte to M2 macrophages by the macrophage colony stimulating factor (M-CSF). While this inhibitory effect of ROS elimination in macrophage differentiation was overcome during M1 macrophage activation [64]. Another study also showed that ROS were induced by MCP-1 protein and were required for IL-4 induced M2 macrophage activation [65], suggesting that ROS may have more complicated roles in driving differential macrophage activations in different contexts.
Many other signaling pathways such as interferon-regulatory factors (IRFs), signal transducer and activator of transcription (STAT), and suppressors of cytokine signaling (SOCS) and microRNAs have been also involved in macrophage activation [62]. Although their roles in macrophage response to irradiation are still largely unknown, irradiation may directly or indirectly regulate these pathways and affect macrophage activation. It also should be noted that all these pathways are not mutually exclusive but rather may closely interact with each other and collectively contribute to distinct macrophage activation in a stimuli- and microenvironment-dependent manner.
Indirect effects
Irradiation might also affect macrophage functions indirectly, either through the release of soluble factors or through cell–cell interactions. Non-cell autonomous effects of irradiation on macrophages may depend on irradiation dose delivered. Lower dose irradiation induced apoptosis of tumor cells and stromal cells that are efficiently engulfed by macrophage leading to clearance of tumor antigens and production of anti-inflammatory mediators including TGF-β and IL-10. On the contrary, higher dose irradiation such as developped by stereotactic body radiation therapy released damage-associated molecular patterns (DAMPs) that induce the expression and the production of pro-inflammatory cytokines, chemokines and effector molecules [6].
Cell–cell interactions
Apoptotic cells promote the secretion of anti-inflammatory cytokine IL-10 and inhibit the production of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-12 in LPS-activated macrophages in a way that partially depends on the thrombospondin receptor (CD36) [66]. Basal macrophage activation state may influence the modulatory effect of apoptotic cells on macrophages. For example, engulfment of apoptotic cells by non-stimulated and M2 macrophages induces the expression of anti-inflammatory macrophage markers such as Arg1 and TGF-β, whereas such engulfment by M1 macrophages induced expression of pro-inflammatory markers such as iNOS and NO, superoxide, IL-6 and TNF-α [23]. Irradiated tumor cells also interact with macrophages to induce the expression of iNOS and the production of NO in a way dependent of the TLR 1 receptor which in turn promote tumor regrowth after irradiation [67]. However, another study demonstrated that co-culturing of BMDMs with irradiated prostate tumor cells revealed an increased expression of pro-tumorigenic factors such as Arg1, Fizz, CSF-1, CD206, MMP-9, VEGF-A, IL-1β and IL-10, as well as a decreased expression of pro-inflammatory genes such as iNOS and IL-12 [68].
Soluble mediators
Local high dose (15 Gy) irradiation induced IFN-γ expression in the tumor microenvironment that may contribute not only to anti-tumor T cell immune response but also promote pro-inflammatory activation [69] of tumor-associated macrophages that further enhance anti-tumor immunity. Irradiation also promotes immunogenic cell death to tumor cells that are characterized by the release of HMGB1, ATP and the membrane translocation of calreticulin [70], [71], [72], [73], [74]. HMGB1 upon ligation with TLR4 activated NF-κB signaling pathway [70], [75]. ATP binds to P2X7 purinergic receptor and activates the NLRP3 inflammasome [76], [77]. Both NF-κB and NLRP3 inflammasome activation contribute to expression and maturation of pro-inflammatory cytokines such as IL-1β [78], [79]. On the contrary, low-dose (0.3–0.7 Gy) irradiation was shown to inhibit peripheral blood monocyte recruitment and adhesion to endothelial cells due to increased TGF-β and decreased E-selectin expression in irradiation-stimulated endothelial cells [80], which may account for another mechanism of anti-inflammatory properties of low-dose irradiation.
Tumor-associated macrophages: preclinical studies
Irradiation induces tumor-associated monocytes/macrophages recruitment
Irradiation has been shown in many studies to induce monocyte/macrophage infiltration in tumors that in turn limits the efficacy of radiotherapy. Tumor hypoxia created by radiation-promoted vasculature disruption and enhanced activities of HIF-1/HIF-2 induced various cytokines/chemokines expression by residual tumor cells and tumor stromal. Several signaling pathways including colony stimulating factor 1 (CSF-1)/CSF-1R, stromal cell-derived factor 1 (SDF-1)/CXCR4 and VEGF/VEGFR are involved in radiation-induced macrophage recruitment. Recruited macrophages adopted an M2-like pro-tumoral phenotype with enhanced pro-survival and pro-angiogenic activities, often leading to tumor recurrence and treatment failure [81].
CSF-1/CSF-1R-dependent signaling pathways
In a mouse MMTV-PyMT–derived mammary tumor model, single dose of gamma-irradiation at 5 Gy promoted macrophage recruitment into tumor stromal, which displayed a mixed M1 and M2 activated phenotype. Macrophage accumulation after irradiation was associated with an increased CD4+ T cell infiltration. Different strategies, such as macrophage depletion with neutralizing mAb to CSF-1 or a competitive ATP inhibitor of the CSF-1 receptor kinase PLX3397, CD4+ T cell depletion with neutralizing mAb to CD4 and IL-4 neutralization using antibody to IL-4, all resulted in delayed tumor regrowth after irradiation, which is abrogated by CD8+ T cell depletion. This study suggests that irradiation promotes macrophage recruitment that created an immunosuppressive microenvironment with IL-4 expressed by CD4+ T cell and suppresses anti-tumor T cell response [82]. Similarly, in a murine prostate cancer models, irradiation was shown to promote CSF-1 expression in tumor cells in part via DNA damage-induced nuclear translocation of the Abelson murine leukemia viral oncogene homolog 1, ABL1. Increased CSF-1 induced recruitment of tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs), which could be abrogated by a selective CSF-1R inhibitor. Accordingly, combinatory treatment with irradiation and CSF-1R inhibitor significantly improved anti-tumor efficacy [68].
SDF-1/CXCR4-dependent signaling pathways
After radiation therapy, tumor-associated macrophages accumulated in tumor hypoxic regions. Using murine prostate adenocarcinoma, astrocytoma and glioma models, it was shown that CD11blow/F4/80+macrophages were mainly distributed at the junctions of central necrotic and surrounding hypoxic area whereas CD11blow/CD68+ macrophages were located in the hypoxic regions in irradiated tumors. Tumor hypoxia induced the expression of SDF-1α that recruited tumor-associated macrophages that expressed high level of M2-macrophage marker Arg1 and contribute to tumor regrowth after radiation therapy [83]. In a glioblastoma multiforme xenograft model, irradiation induced hypoxia inducible factor-1 (HIF-1)-dependent expression of SDF-1, which upon interaction with its receptor CXCR4, induced recruitment of macrophages that restored tumor vasculature and promoted tumor regrowth. Inhibition of HIF-1 or SDF-1/CXCR4 interaction efficiently abrogated tumor vasculogenesis and tumor regrowth [84]. In another murine ALTS1C1 glioblastoma multiforme (GBM) model, whole-brain irradiation at 8 Gy or 15 Gy led to shrinkage of tumor core, but increased the number of tumor infiltrating islands along with increased blood monocyte-derived tumor-associated macrophages. Similarly, irradiation reduced the microvascular density (MVD) in the primary tumor core but increased the MVD in the tumor invasion front. Both the IR-induced macrophage accumulation and MVD increase at the invasion front were suppressed by depletion of stromal-derived factor 1 (SDF-1) in tumor cells, which further resulted in decreased IR-induced tumor invasiveness [85]. Likewise, inhibition of SDF-1α receptor CXCR4 with AMD3100 was shown to significantly delayed xenograft lung tumor regrowth after radiation therapy [86].
Irradiation promotes anti-inflammatory activation of TAMs
In several different animal tumor models, irradiation induced a pro-tumor phenotype that suppress anti-tumor immune response and promote tumor growth via their pro-angiogenic activities. For instance, in a murine prostate cancer model, irradiation induced high expression level of Arg1, cyclooxygenase-2 and iNOS in tumor-associated macrophages that promoted tumor growth [87]. Macrophages from irradiated tumors also display low MHC class II expression, indicating their pro-tumor activities [68]. In an oral squamous cell carcinoma model, local irradiation (12 Gy) promoted the recruitment of CD11b+ myeloid cells into tumors. These myeloid cells differentiated into macrophages that were further activated in the tumor hypoxic microenvironment created by irradiation and acquired an M2-like phenotype. M2-like macrophages then promote tumor recurrence via their ability of promoting tumor vasculogenesis [88]. Radiation therapy upregulated VEGF expression in tumor-associated macrophages in a TNF-α dependent manner that contributed to tumor radioresistance [89].
Three fractions of 20 Gy of X-ray irradiation significantly increased myeloid cells and in particular tumor-associated macrophage infiltration in mouse pancreatic tumors, which although showed a transient up-regulation of several pro-inflammatory genes such as cd80, tnf, and tnfsf9 one day after irradiation, markedly increased the expression of M2 macrophage marker Arg1 and no expression of iNOS at 7 days after IR. In addition, macrophage co-cultured with irradiated tumor cells increased the expression of anti-inflammatory cytokine IL-10 following LPS stimulation in a way that depends on NF-κB p50 subunit as compared to those co-cultured with unirradiated tumor cells. In accordance with the findings that NF-κB p50 subunit is a key component in the orchestration of M2-macrophage activation and in inhibiting M1-driven inflammatory response [90], radiation therapy effectively controlled tumor growth in mice deficient of NF-κB p50 and led to improved animal survival. Interestingly, NF-κB p50 deficiency induced long-term specific anti-tumor immunity that protected animal from tumor rechallenge [91]. However, the changes of NF-κB p50 activity in tumor-associated macrophages following irradiation were not documented in these studies.
In another murine pancreatic ductal adenocarcinoma model, tumors receiving ionizing radiation (with doses ranging from 2 to 12 Gy) contain higher proportion of M2-like tumor-associated macrophages with increased Th2 and T regulatory (Treg) cells and fewer CD8+ T cells than do control tumors. As a result, radiation promotes higher frequency of advanced pancreatic intraepithelial lesions and invasive cancer and shortens the overall survival time of animals as compared to non-irradiated tumor-bearing control animals. Radiation induced the production of M-CSF by tumor cells, which not only recruited tumor-associated macrophages but also differentiated them into immune-suppressive macrophage phenotype. Thus, macrophage depletion or inactivation by anti-F4/80 or anti-M-CSF neutralizing antibody prevented radiation induced M2 macrophage phenotype and increased anti-tumor T cell response [92].
Irradiation also favors pro-inflammatory activation of TAMs
Given the plasticity and diversity of macrophage functions, it is not surprising that some other studies demonstrate that irradiation can also program TAMs towards pro-inflammatory phenotype that contributes to anti-tumor response. These discrepancies may rely on different doses and different tumor models used in the studies.
In a prime study on murine insulinoma, local low-dose irradiation as well as total body low-dose irradiation reprogrammed macrophages towards an iNOS+/M1 phenotype [93], [94]. Importantly, the skewed M1 macrophages by local low-dose irradiation contributed to vascular normalization and improved T cell immunotherapy [93]. Total body low-dose irradiation not only increased iNOS and the effector molecule NO and Th1 effector cytokines such as TNF-α, IL-12 (p70) and IFN-γ in peritoneal macrophages and tumor-associated macrophages but also significantly inhibited M2 macrophage markers such as Ym-1, Arg1 and Fizz-1. Although detailed mechanisms remain unclear, NF-κB, STAT3 and p38 MAPK were suggested to be involved [94]. Another study with murine breast cancer showed that matrix metalloproteinase (MMP) 14 inhibition using inhibitory antibody decreased the expression of TGF-β, and promoted macrophage activation towards pro-inflammatory phenotype as evidenced by an increased expression of iNOS. MMP14 blockade synergized with radiotherapy by improving tumor vascularization and reduction of tumor hypoxia [95].
The effect of irradiation on tumor-associated macrophage functions may vary during different phases. In the early phases, macrophages respond to dying tumor cells and DAMPs and differentiated into pro-inflammatory macrophages. However, at a later phase, following the vascular disruption, fibrosis, and tumor hypoxia, resident and recruited macrophages may skew to a regulatory phenotype that favors angiogenesis and tissue repair, leading to tumor regrowth [6].
Tumor-associated macrophages: clinical researches
In light of their central role in cancer biology and cancer therapies in pre-clinical studies, tumor-associated macrophages have attracted enormous attention in clinical researches.
Brachytherapy of uveal melanoma also induced increased infiltration of tumor-associated macrophages [96]. Another study showed an increased serum level of CSF-1 in prostate cancer patients that received radiotherapy, which might be involved in tumor-associated macrophage recruitment [68]. Baseline CD163 expression in tumor-associated macrophages is a poor predicting marker of clinical outcome in chemo-radiation-treated head and neck squamous cell carcinoma patients [97]. Surprisingly, a study from 29 locally advanced rectal patients undergoing preoperative short-course radiotherapy showed that the HLA-DR+ M1 macrophage score was inversely related to tumor response to radiotherapy whereas CD163+ M2 macrophage score was not significantly correlated [98]. These results should be further validated in more patients before drawing a definitive conclusion. In a study of 73 primary advanced cervical squamous cell carcinoma patients undergoing radiotherapy, it was shown that the hypoxia inducible factor HIF-2α expression in tumor-associated macrophages was inversely related to disease-free survival and an higher HIF-1α positive tumor-associated macrophage ratio was associated with increased risk of local recurrence [99].
Although these scattered pieces of evidence demonstrate critical roles of tumor-associated macrophages in tumor responses to radiation therapy, more effort is required in disclosing the mechanisms and in developing new strategies to promote anti-tumor activities while prevent pro-tumor features of TAMs.
Targeting macrophages to enhance radiation therapy efficacy
As CSF-1/CSF-1R and SDF-1/CXCR4 are important pathways involved in irradiation-induced macrophage recruitment, blocking these pathways has shown promising tumor-suppressing activities and synergized effects with radiation therapy. For example, CSF-1R inhibition using PLX3397 inhibitor combined with irradiation significantly reduced the number of CD11b+ myeloid cells and blocked the differentiation of pro-tumorigenic tumor-associated macrophages, leading to improved tumor control and prolonged animal survival [100]. CSF-1R inhibitor in combination with irradiation not only profoundly decrease the infiltration of tumor-associated macrophages and pro-tumoral myeloid-derived suppressor cells but also significantly reduced the expression of IR-induced CSF-1, Arg1, MMP9 and CCL2, suggesting a reprogramming to anti-tumor phenotype [68]. Similarly, CXCR4 inhibition with AMD3100 significantly delayed xenograft lung tumor regrowth after radiation therapy [86]. Other strategies such as blocking CD11b [101] and MCP-1 [102] using neutralizing antibodies also yielded enhanced anti-tumor effect of radiation therapy.
Macrophage reprogramming represents a more attractive and perhaps less toxic strategy. The effects of different radiotherapy regimens such as hyper-fractionated versus hypo-fractionated radiation therapy, conventional radiotherapy versus accelerated radiotherapy, low-dose radiotherapy versus high-low radiosurgery, on the modulation of macrophage functions and on the tumor control, will be extremely interesting subjects of research. In addition, combined strategies with radiotherapy and other methods to boost the anti-tumor phenotype of macrophages and anti-tumor immunities also provide opportunities towards efficient tumor treatment. For example, irradiation when combined with the glycolytic inhibitor 2-deoxy-d-glucose was shown to activate macrophages and promoted the production of various pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-12 and IL-2, while inhibited the expression of anti-inflammatory mediators such as IL-10 and TGF-β, demonstrating a skewing towards M1 phenotype [103]. Combined treatment with radiation and hyperthermia of tumor cells increased HSP70 surface exposure and extracellular release that enhanced phagocytic activity and production of inflammatory cytokine IL-8 in macrophages [104]. It would be also interesting to ask whether ex vivo activation of blood monocyte-derived macrophages by irradiation or by other methods and autologous transfusion of these classical activated macrophages might improve radiation therapy effect.
Immune checkpoint blockade has shown significant advance in recent years and has led to spectacular anti-tumor effect both in pre-clinical models and in many clinical studies [105]. IR was shown to up-regulate the expression of PD-L1 in tumor cells, dendritic cells and TAMs that limit the anti-tumor effect of radiotherapy. However IR-induced PD-L1 up-regulation in TAMs thus represents an important obstacle for efficient tumor control. Combined therapy of irradiation and anti-PD-L1 resulted in activation of cytotoxic T cells and synergistic elimination of MDSCs by T cell-generated TNF, which is associated with delayed tumor growth [92], [106].
Furthermore, deeper studies of the mechanisms of irradiation-induced macrophage and a more comprehensive understanding of irradiation impacts on macrophages functions such as MHC molecules expression and antigen presentation, CD47-signal regulatory protein alpha and phagocytosis [107], might provide more tools to modulate macrophage activities and enhance tumor treatment effect.
Concluding remarks
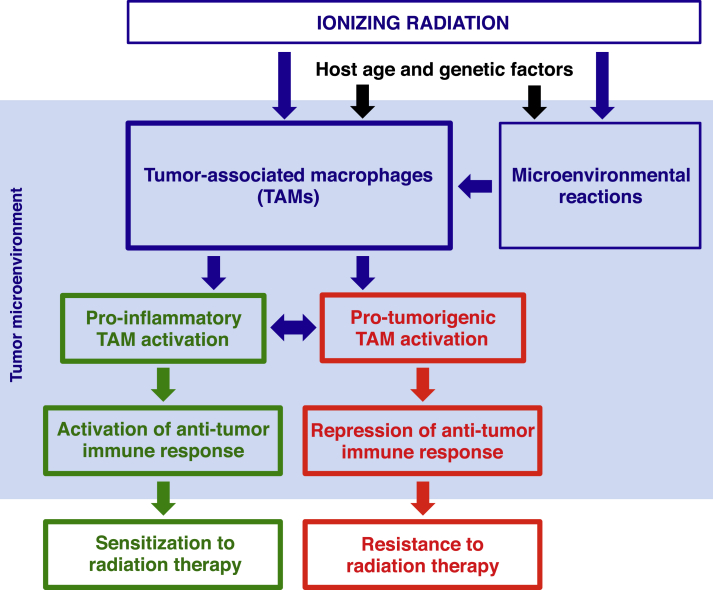
Apart from its tumor-killing role, irradiation profoundly impacts on tumor microenvironment. In particular, irradiation significantly affects the functions of tumor-associated macrophages [Fig. 1]. Although data from different studies using variant models and irradiation strategies did not provide a uniform scenario and a definitive conclusion with respect to the effects of irradiation on TAMs activation, our knowledge of radiobiology in immune system at both cellular and molecular levels has significantly improved. On going studies on molecular mechanisms and exploration on different combinatory strategies will certainly pave ways to more effective and less toxic ant-tumor treatments and bring more benefits to cancer patients.
Fig. 1.
Ionizing radiation dictates tumor-associated macrophage activation and anti-tumor response.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Lauber K., Ernst A., Orth M., Herrmann M., Belka C. Dying cell clearance and its impact on the outcome of tumor radiotherapy. Front Oncol. 2012;2:116. doi: 10.3389/fonc.2012.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaur P., Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2012;2:191. doi: 10.3389/fonc.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez F.O., Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gough M.J., Young K., Crittenden M. The impact of the myeloid response to radiation therapy. Clin Dev Immunol. 2013;2013:281958. doi: 10.1155/2013/281958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibuki Y., Goto R. Enhancement of NO production from resident peritoneal macrophages by in vitro gamma-irradiation and its relationship to reactive oxygen intermediates. Free Radic Biol Med. 1997;22:1029–1035. doi: 10.1016/s0891-5849(96)00500-x. [DOI] [PubMed] [Google Scholar]
- 8.Ibuki Y., Goto R. Enhancement of O2− production from resident peritoneal macrophages by low-dose in vivo gamma-irradiation. Biol Pharm Bull. 2000;23:1094–1096. doi: 10.1248/bpb.23.1094. [DOI] [PubMed] [Google Scholar]
- 9.Ibuki Y., Goto R. Ionizing radiation-induced macrophage activation: augmentation of nitric oxide production and its significance. Cell Mol Biol (Noisy-le-grand) 2004;50 Online Pub:OL617-26. [PubMed] [Google Scholar]
- 10.Iwamoto K.S., McBride W.H. Production of 13-hydroxyoctadecadienoic acid and tumor necrosis factor-alpha by murine peritoneal macrophages in response to irradiation. Radiat Res. 1994;139:103–108. [PubMed] [Google Scholar]
- 11.Ishihara H., Tanaka I., Nemoto K., Tsuneoka K., Cheeramakara C., Yoshida K. Immediate-early, transient induction of the interleukin-1 beta gene in mouse spleen macrophages by ionizing radiation. J Radiat Res. 1995;36:112–124. doi: 10.1269/jrr.36.112. [DOI] [PubMed] [Google Scholar]
- 12.Ibuki Y., Goto R. Contribution of inflammatory cytokine release to activation of resident peritoneal macrophages after in vivo low-dose gamma-irradiation. J Radiat Res. 1999;40:253–262. doi: 10.1269/jrr.40.253. [DOI] [PubMed] [Google Scholar]
- 13.Hosoi Y., Miyachi H., Matsumoto Y., Enomoto A., Nakagawa K., Suzuki N. Induction of interleukin-1beta and interleukin-6 mRNA by low doses of ionizing radiation in macrophages. Int J Cancer. 2001;96:270–276. doi: 10.1002/ijc.1030. [DOI] [PubMed] [Google Scholar]
- 14.Wunderlich R., Ernst A., Rodel F., Fietkau R., Ott O., Lauber K. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin Exp Immunol. 2015;179:50–61. doi: 10.1111/cei.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodermann B., Wunderlich R., Frey S., Schorn C., Stangl S., Rodel F. Low dose ionising radiation leads to a NF-kappaB dependent decreased secretion of active IL-1beta by activated macrophages with a discontinuous dose-dependency. Int J Radiat Biol. 2012;88:727–734. doi: 10.3109/09553002.2012.689464. [DOI] [PubMed] [Google Scholar]
- 16.Schaue D., Marples B., Trott K.R. The effects of low-dose X-irradiation on the oxidative burst in stimulated macrophages. Int J Radiat Biol. 2002;78:567–576. doi: 10.1080/09553000210126457. [DOI] [PubMed] [Google Scholar]
- 17.McKinney L.C., Aquilla E.M., Coffin D., Wink D.A., Vodovotz Y. Ionizing radiation potentiates the induction of nitric oxide synthase by interferon-gamma and/or lipopolysaccharide in murine macrophage cell lines. Role of tumor necrosis factor-alpha. Ann N Y Acad Sci. 2000;899:61–68. doi: 10.1111/j.1749-6632.2000.tb06176.x. [DOI] [PubMed] [Google Scholar]
- 18.McKinney L.C., Aquilla E.M., Coffin D., Wink D.A., Vodovotz Y. Ionizing radiation potentiates the induction of nitric oxide synthase by IFN-gamma and/or LPS in murine macrophage cell lines: role of TNF-alpha. J Leukoc Biol. 1998;64:459–466. doi: 10.1002/jlb.64.4.459. [DOI] [PubMed] [Google Scholar]
- 19.Lee Y.J., Han J.Y., Lee C.G., Heo K., Park S.I., Park Y.S. Korean Red Ginseng saponin fraction modulates radiation effects on lipopolysaccharide-stimulated nitric oxide production in RAW264.7 macrophage cells. J Ginseng Res. 2014;38:208–214. doi: 10.1016/j.jgr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien-Ladner A., Nelson M.E., Kimler B.F., Wesselius L.J. Release of interleukin-1 by human alveolar macrophages after in vitro irradiation. Radiat Res. 1993;136:37–41. [PubMed] [Google Scholar]
- 21.Pons I., Gras G., Courberand S., Benveniste O., Dormont D. Consequences of gamma-irradiation on inflammatory cytokine regulation in human monocytes/macrophages. Int J Radiat Biol. 1997;71:157–166. doi: 10.1080/095530097144274. [DOI] [PubMed] [Google Scholar]
- 22.Katayama I., Hotokezaka Y., Matsuyama T., Sumi T., Nakamura T. Ionizing radiation induces macrophage foam cell formation and aggregation through JNK-dependent activation of CD36 scavenger receptors. Int J Radiat Oncol Biol Phys. 2008;70:835–846. doi: 10.1016/j.ijrobp.2007.10.058. [DOI] [PubMed] [Google Scholar]
- 23.Rastogi S., Boylan M., Wright E.G., Coates P.J. Interactions of apoptotic cells with macrophages in radiation-induced bystander signaling. Radiat Res. 2013;179:135–145. doi: 10.1667/RR2969.1. [DOI] [PubMed] [Google Scholar]
- 24.Coates P.J., Rundle J.K., Lorimore S.A., Wright E.G. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 2008;68:450–456. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- 25.Frischholz B., Wunderlich R., Ruhle P.F., Schorn C., Rodel F., Keilholz L. Reduced secretion of the inflammatory cytokine IL-1beta by stimulated peritoneal macrophages of radiosensitive Balb/c mice after exposure to 0.5 or 0.7 Gy of ionizing radiation. Autoimmunity. 2013;46:323–328. doi: 10.3109/08916934.2012.747522. [DOI] [PubMed] [Google Scholar]
- 26.Nowosielska E.M., Cheda A., Wrembel-Wargocka J., Janiak M.K. Effect of low doses of low-let radiation on the innate anti-tumor reactions in radioresistant and radiosensitive mice. Dose Response. 2012;10:500–515. doi: 10.2203/dose-response.12-018.Nowosielska. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasat D.R., Mancuso R., Evelson P., Polo J.M., Llesuy S., Molinari B. Radiation effects on oxidative metabolism in young and aged rat alveolar macrophages. Cell Mol Biol (Noisy-le-grand) 2002;48:529–535. [PubMed] [Google Scholar]
- 28.Narang H., Bhat N., Gupta S.K., Santra S., Choudhary R.K., Kailash S. Differential activation of mitogen-activated protein kinases following high and low LET radiation in murine macrophage cell line. Mol Cell Biochem. 2009;324:85–91. doi: 10.1007/s11010-008-9987-y. [DOI] [PubMed] [Google Scholar]
- 29.Conrad S., Ritter S., Fournier C., Nixdorff K. Differential effects of irradiation with carbon ions and x-rays on macrophage function. J Radiat Res. 2009;50:223–231. doi: 10.1269/jrr.08115. [DOI] [PubMed] [Google Scholar]
- 30.Shin S.C., Lee K.M., Kang Y.M., Kim K., Kim C.S., Yang K.H. Alteration of cytokine profiles in mice exposed to chronic low-dose ionizing radiation. Biochem Biophys Res Commun. 2010;397:644–649. doi: 10.1016/j.bbrc.2010.05.121. [DOI] [PubMed] [Google Scholar]
- 31.Hildebrandt G., Radlingmayr A., Rosenthal S., Rothe R., Jahns J., Hindemith M. Low-dose radiotherapy (LD-RT) and the modulation of iNOS expression in adjuvant-induced arthritis in rats. Int J Radiat Biol. 2003;79:993–1001. doi: 10.1080/09553000310001636639. [DOI] [PubMed] [Google Scholar]
- 32.Rodel F., Frey B., Gaipl U., Keilholz L., Fournier C., Manda K. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem. 2012;19:1741–1750. doi: 10.2174/092986712800099866. [DOI] [PubMed] [Google Scholar]
- 33.Pandey R., Shankar B.S., Sharma D., Sainis K.B. Low dose radiation induced immunomodulation: effect on macrophages and CD8+ T cells. Int J Radiat Biol. 2005;81:801–812. doi: 10.1080/09553000500531886. [DOI] [PubMed] [Google Scholar]
- 34.Nowosielska E.M., Wrembel-Wargocka J., Cheda A., Lisiak E., Janiak M.K. Enhanced cytotoxic activity of macrophages and suppressed tumor metastases in mice irradiated with low doses of X-rays. J Radiat Res. 2006;47:229–236. doi: 10.1269/jrr.0572. [DOI] [PubMed] [Google Scholar]
- 35.Nowosielska E.M., Cheda A., Wrembel-Wargocka J., Janiak M.K. Anti-neoplastic and immunostimulatory effects of low-dose X-ray fractions in mice. Int J Radiat Biol. 2011;87:202–212. doi: 10.3109/09553002.2010.519422. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Han G., Liu H., Chen J., Ji X., Zhou F. The development of classically and alternatively activated macrophages has different effects on the varied stages of radiation-induced pulmonary injury in mice. J Radiat Res. 2011;52:717–726. doi: 10.1269/jrr.11054. [DOI] [PubMed] [Google Scholar]
- 37.Ray D., Shukla S., Allam U.S., Helman A., Ramanand S.G., Tran L. Tristetraprolin mediates radiation-induced TNF-alpha production in lung macrophages. PLoS One. 2013;8:e57290. doi: 10.1371/journal.pone.0057290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abernathy L.M., Fountain M.D., Rothstein S.E., David J.M., Yunker C.K., Rakowski J. Soy isoflavones promote radioprotection of normal lung tissue by inhibition of radiation-induced activation of macrophages and neutrophils. J Thorac Oncol. 2015;10:1703–1712. doi: 10.1097/JTO.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lacave-Lapalun J.V., Benderitter M., Linard C. Flagellin or lipopolysaccharide treatment modified macrophage populations after colorectal radiation of rats. J Pharmacol Exp Ther. 2013;346:75–85. doi: 10.1124/jpet.113.204040. [DOI] [PubMed] [Google Scholar]
- 40.Moravan M.J., Olschowka J.A., Williams J.P., O'Banion M.K. Brain radiation injury leads to a dose- and time-dependent recruitment of peripheral myeloid cells that depends on CCR2 signaling. J Neuroinflammation. 2016;13:30. doi: 10.1186/s12974-016-0496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morganti J.M., Jopson T.D., Liu S., Gupta N., Rosi S. Cranial irradiation alters the brain's microenvironment and permits CCR2+ macrophage infiltration. PLoS One. 2014;9:e93650. doi: 10.1371/journal.pone.0093650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharpfenecker M., Floot B., Russell N.S., Stewart F.A. The TGF-beta co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. Radiother Oncol. 2012;105:313–320. doi: 10.1016/j.radonc.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Hildebrandt G., Loppnow G., Jahns J., Hindemith M., Anderegg U., Saalbach A. Inhibition of the iNOS pathway in inflammatory macrophages by low-dose X-irradiation in vitro. Is there a time dependence? Strahlenther Onkol. 2003;179:158–166. doi: 10.1007/s00066-003-1044-x. [DOI] [PubMed] [Google Scholar]
- 44.Shan Y.X., Jin S.Z., Liu X.D., Liu Y., Liu S.Z. Ionizing radiation stimulates secretion of pro-inflammatory cytokines: dose-response relationship, mechanisms and implications. Radiat Environ Biophys. 2007;46:21–29. doi: 10.1007/s00411-006-0076-x. [DOI] [PubMed] [Google Scholar]
- 45.El-Saghire H., Michaux A., Thierens H., Baatout S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 2013;32:1407–1414. doi: 10.3892/ijmm.2013.1514. [DOI] [PubMed] [Google Scholar]
- 46.Teresa Pinto A., Laranjeiro Pinto M., Patricia Cardoso A., Monteiro C., Teixeira Pinto M., Filipe Maia A. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep. 2016;6:18765. doi: 10.1038/srep18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li N., Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee S.J., Dimtchev A., Lavin M.F., Dritschilo A., Jung M. A novel ionizing radiation-induced signaling pathway that activates the transcription factor NF-kappaB. Oncogene. 1998;17:1821–1826. doi: 10.1038/sj.onc.1202088. [DOI] [PubMed] [Google Scholar]
- 49.Gloire G., Legrand-Poels S., Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 50.Dong C., He M., Ren R., Xie Y., Yuan D., Dang B. Role of the MAPK pathway in the observed bystander effect in lymphocytes co-cultured with macrophages irradiated with gamma-rays or carbon ions. Life Sci. 2015;127:19–25. doi: 10.1016/j.lfs.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Narang H., Krishna M. Effect of nitric oxide donor and gamma irradiation on MAPK signaling in murine peritoneal macrophages. J Cell Biochem. 2008;103:576–587. doi: 10.1002/jcb.21429. [DOI] [PubMed] [Google Scholar]
- 52.Tsukimoto M., Tamaishi N., Homma T., Kojima S. Low-dose gamma-ray irradiation induces translocation of Nrf2 into nuclear in mouse macrophage RAW264.7 cells. J Radiat Res. 2010;51:349–353. doi: 10.1269/jrr.10002. [DOI] [PubMed] [Google Scholar]
- 53.Narang H., Krishna M. Inhibition of radiation induced nitration by curcumin and nicotinamide in mouse macrophages. Mol Cell Biochem. 2005;276:7–13. doi: 10.1007/s11010-005-2241-y. [DOI] [PubMed] [Google Scholar]
- 54.Tsukimoto M., Homma T., Mutou Y., Kojima S. 0.5 Gy gamma radiation suppresses production of TNF-alpha through up-regulation of MKP-1 in mouse macrophage RAW264.7 cells. Radiat Res. 2009;171:219–224. doi: 10.1667/RR1351.1. [DOI] [PubMed] [Google Scholar]
- 55.Lowe J.M., Menendez D., Bushel P.R., Shatz M., Kirk E.L., Troester M.A. p53 and NF-kappaB coregulate proinflammatory gene responses in human macrophages. Cancer Res. 2014;74:2182–2192. doi: 10.1158/0008-5472.CAN-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L., Ng D.S., Mah W.C., Almeida F.F., Rahmat S.A., Rao V.K. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ. 2015;22:1081–1093. doi: 10.1038/cdd.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lujambio A., Akkari L., Simon J., Grace D., Tschaharganeh D.F., Bolden J.E. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng S.J., Lamhamedi-Cherradi S.E., Wang P., Xu L., Chen Y.H. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes. 2005;54:1423–1428. doi: 10.2337/diabetes.54.5.1423. [DOI] [PubMed] [Google Scholar]
- 59.Stoecklein V.M., Osuka A., Ishikawa S., Lederer M.R., Wanke-Jellinek L., Lederer J.A. Radiation exposure induces inflammasome pathway activation in immune cells. J Immunol. 2015;194:1178–1189. doi: 10.4049/jimmunol.1303051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sohn S.H., Lee J.M., Park S., Yoo H., Kang J.W., Shin D. The inflammasome accelerates radiation-induced lung inflammation and fibrosis in mice. Environ Toxicol Pharmacol. 2015;39:917–926. doi: 10.1016/j.etap.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q., Hu Q., Chu Y., Xu B., Song Q. The influence of radiotherapy on AIM2 inflammasome in radiation pneumonitis. Inflammation. 2016;39:1827–1834. doi: 10.1007/s10753-016-0419-y. [DOI] [PubMed] [Google Scholar]
- 62.Wang N., Liang H., Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galvan-Pena S., O'Neill L.A. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y., Choksi S., Chen K., Pobezinskaya Y., Linnoila I., Liu Z.G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23:898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapoor N., Niu J., Saad Y., Kumar S., Sirakova T., Becerra E. Transcription factors STAT6 and KLF4 implement macrophage polarization via the dual catalytic powers of MCPIP. J Immunol. 2015;194:6011–6023. doi: 10.4049/jimmunol.1402797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 67.Ryu Y.K., Lee M.H., Lee J., Lee J.W., Jang S.J., Kang J.H. gamma-Irradiated cancer cells promote tumor growth by activation of Toll-like receptor 1-mediated inducible nitric oxide synthase in macrophages. J Leukoc Biol. 2015;97:711–721. doi: 10.1189/jlb.3A0114-055R. [DOI] [PubMed] [Google Scholar]
- 68.Xu J., Escamilla J., Mok S., David J., Priceman S., West B. CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer. Cancer Res. 2013;73:2782–2794. doi: 10.1158/0008-5472.CAN-12-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lugade A.A., Sorensen E.W., Gerber S.A., Moran J.P., Frelinger J.G., Lord E.M. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. 2008;180:3132–3139. doi: 10.4049/jimmunol.180.5.3132. [DOI] [PubMed] [Google Scholar]
- 70.Apetoh L., Ghiringhelli F., Tesniere A., Criollo A., Ortiz C., Lidereau R. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 71.Tesniere A., Apetoh L., Ghiringhelli F., Joza N., Panaretakis T., Kepp O. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 72.Tesniere A., Panaretakis T., Kepp O., Apetoh L., Ghiringhelli F., Zitvogel L. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ. 2008;15:3–12. doi: 10.1038/sj.cdd.4402269. [DOI] [PubMed] [Google Scholar]
- 73.Obeid M., Tesniere A., Ghiringhelli F., Fimia G.M., Apetoh L., Perfettini J.L. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 74.Martins I., Wang Y., Michaud M., Ma Y., Sukkurwala A.Q., Shen S. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ. 2014;21:79–91. doi: 10.1038/cdd.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Apetoh L., Ghiringhelli F., Tesniere A., Obeid M., Ortiz C., Criollo A. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 76.Michaud M., Martins I., Sukkurwala A.Q., Adjemian S., Ma Y., Pellegatti P. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 77.Ghiringhelli F., Apetoh L., Tesniere A., Aymeric L., Ma Y., Ortiz C. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 78.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franchi L., Eigenbrod T., Munoz-Planillo R., Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roedel F., Kley N., Beuscher H.U., Hildebrandt G., Keilholz L., Kern P. Anti-inflammatory effect of low-dose X-irradiation and the involvement of a TGF-beta1-induced down-regulation of leukocyte/endothelial cell adhesion. Int J Radiat Biol. 2002;78:711–719. doi: 10.1080/09553000210137671. [DOI] [PubMed] [Google Scholar]
- 81.Russell J.S., Brown J.M. The irradiated tumor microenvironment: role of tumor-associated macrophages in vascular recovery. Front Physiol. 2013;4:157. doi: 10.3389/fphys.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiao S.L., Ruffell B., DeNardo D.G., Faddegon B.A., Park C.C., Coussens L.M. TH2-Polarized CD4(+) T cells and macrophages limit efficacy of radiotherapy. Cancer Immunol Res. 2015;3:518–525. doi: 10.1158/2326-6066.CIR-14-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chiang C.S., Fu S.Y., Wang S.C., Yu C.F., Chen F.H., Lin C.M. Irradiation promotes an m2 macrophage phenotype in tumor hypoxia. Front Oncol. 2012;2:89. doi: 10.3389/fonc.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kioi M., Vogel H., Schultz G., Hoffman R.M., Harsh G.R., Brown J.M. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Investig. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang S.C., Yu C.F., Hong J.H., Tsai C.S., Chiang C.S. Radiation therapy-induced tumor invasiveness is associated with SDF-1-regulated macrophage mobilization and vasculogenesis. PLoS One. 2013;8:e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kozin S.V., Kamoun W.S., Huang Y., Dawson M.R., Jain R.K., Duda D.G. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–5685. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsai C.S., Chen F.H., Wang C.C., Huang H.L., Jung S.M., Wu C.J. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. Int J Radiat Oncol Biol Phys. 2007;68:499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 88.Okubo M., Kioi M., Nakashima H., Sugiura K., Mitsudo K., Aoki I. M2-polarized macrophages contribute to neovasculogenesis, leading to relapse of oral cancer following radiation. Sci Rep. 2016;6:27548. doi: 10.1038/srep27548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meng Y., Beckett M.A., Liang H., Mauceri H.J., van Rooijen N., Cohen K.S. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70:1534–1543. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Porta C., Rimoldi M., Raes G., Brys L., Ghezzi P., Di Liberto D. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crittenden M.R., Cottam B., Savage T., Nguyen C., Newell P., Gough M.J. Expression of NF-kappaB p50 in tumor stroma limits the control of tumors by radiation therapy. PLoS One. 2012;7:e39295. doi: 10.1371/journal.pone.0039295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Seifert L., Werba G., Tiwari S., Giao Ly N.N., Nguy S., Alothman S. Radiation therapy induces macrophages to suppress T-cell responses against pancreatic tumors in mice. Gastroenterology. 2016;150 doi: 10.1053/j.gastro.2016.02.070. 1659–72 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klug F., Prakash H., Huber P.E., Seibel T., Bender N., Halama N. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Prakash H., Klug F., Nadella V., Mazumdar V., Schmitz-Winnenthal H., Umansky L. Low doses of gamma irradiation potentially modifies immunosuppressive tumor microenvironment by retuning tumor-associated macrophages: lesson from insulinoma. Carcinogenesis. 2016;37:301–313. doi: 10.1093/carcin/bgw007. [DOI] [PubMed] [Google Scholar]
- 95.Ager E.I., Kozin S.V., Kirkpatrick N.D., Seano G., Kodack D.P., Askoxylakis V. Blockade of MMP14 activity in murine breast carcinomas: implications for macrophages, vessels, and radiotherapy. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/djv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toivonen P., Kivela T. Infiltrating macrophages in extratumoural tissues after brachytherapy of uveal melanoma. Acta Ophthalmol. 2012;90:341–349. doi: 10.1111/j.1755-3768.2010.01985.x. [DOI] [PubMed] [Google Scholar]
- 97.Balermpas P., Rodel F., Liberz R., Oppermann J., Wagenblast J., Ghanaati S. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br J Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaikh S., Noshirwani A., West N., Perry S., Jayne D. Can macrophages within the microenvironment of locally invasive rectal cancers predict response to radiotherapy? Lancet. 2015;385:S87. doi: 10.1016/S0140-6736(15)60402-0. [DOI] [PubMed] [Google Scholar]
- 99.Kawanaka T., Kubo A., Ikushima H., Sano T., Takegawa Y., Nishitani H. Prognostic significance of HIF-2alpha expression on tumor infiltrating macrophages in patients with uterine cervical cancer undergoing radiotherapy. J Med Invest. 2008;55:78–86. doi: 10.2152/jmi.55.78. [DOI] [PubMed] [Google Scholar]
- 100.Stafford J.H., Hirai T., Deng L., Chernikova S.B., Urata K., West B.L. Colony stimulating factor 1 receptor inhibition delays recurrence of glioblastoma after radiation by altering myeloid cell recruitment and polarization. Neuro Oncol. 2016;18:797–806. doi: 10.1093/neuonc/nov272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ahn G.O., Tseng D., Liao C.H., Dorie M.J., Czechowicz A., Brown J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim T.D., Li G., Song K.S., Kim J.M., Kim J.S., Kim J.S. Radiation-induced thymidine phosphorylase upregulation in rectal cancer is mediated by tumor-associated macrophages by monocyte chemoattractant protein-1 from cancer cells. Int J Radiat Oncol Biol Phys. 2009;73:853–860. doi: 10.1016/j.ijrobp.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 103.Farooque A., Afrin F., Adhikari J.S., Dwarakanath B.S. Polarization of macrophages towards M1 phenotype by a combination of 2-deoxy-D-glucose and radiation: implications for tumor therapy. Immunobiology. 2016;221:269–281. doi: 10.1016/j.imbio.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 104.Schildkopf P., Frey B., Ott O.J., Rubner Y., Multhoff G., Sauer R. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother Oncol. 2011;101:109–115. doi: 10.1016/j.radonc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 105.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng L., Liang H., Burnette B., Beckett M., Darga T., Weichselbaum R.R. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Investig. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vermeer D.W., Spanos W.C., Vermeer P.D., Bruns A.M., Lee K.M., Lee J.H. Radiation-induced loss of cell surface CD47 enhances immune-mediated clearance of human papillomavirus-positive cancer. Int J Cancer. 2013;133:120–129. doi: 10.1002/ijc.28015. [DOI] [PMC free article] [PubMed] [Google Scholar]