Abstract
Accumulating mutations may drive cells into the acquisition of abnormal phenotypes that are characteristic of cancer cells. Cancer cells feature profound alterations in proliferation programs that result in a new population of cells that overrides normal tissue construction and maintenance programs. To achieve this goal, cancer cells are endowed with up regulated survival signaling pathways. They also must counteract the cytotoxic effects of high levels of nitric oxide (NO) and of reactive oxygen species (ROS), which are by products of cancer cell growth. Accumulating experimental evidence associates cancer cell survival with their capacity to up-regulate antioxidant systems. Elevated expression of the antioxidant protein thioredoxin-1 (Trx1) has been correlated with cancer development. Trx1 has been characterized as a multifunctional protein, playing different roles in different cell compartments. Trx1 migrates to the nucleus in cells exposed to nitrosative/oxidative stress conditions. Trx1 nuclear migration has been related to the activation of transcription factors associated with cell survival and cell proliferation. There is a direct association between the p21Ras-ERK1/2 MAP Kinases survival signaling pathway and Trx1 nuclear migration under nitrosative stress. The expression of the cytoplasmic protein, the thioredoxin-interacting protein (Txnip), determines the change in Trx1 cellular compartmentalization. The anti-apoptotic actions of Trx1 and its denitrosylase activity occur in the cytoplasm and serve as important regulators of cell survival. Within this context, this review focuses on the participation of Trx1 in cells under nitrosative/oxidative stress in survival signaling pathways associated with cancer development.
Keywords: Thioredoxin-1, Nitric oxide, Survival signaling, S-nitrosylation, Denitrosylation, Cancer development

Thioredoxin-1 in the context of the thioredoxin system
Cytosolic thioredoxin-1 (Trx1) and mitochondrial thioredoxin-2 (Trx2) are 12 kDa multi-functional proteins with two conserved Cys residues (Cys32 and Cys35 for Trx1 and Cys31 and Cys34 for Trx2) at their redox active site. They play a major role in cellular redox balance and signaling in normal cells and tumor cells [1], [2], [3], [4], [5]. Thioredoxins are expressed in prokaryotic and eukaryotic cells and apparently are present in all living cells [1], [6].
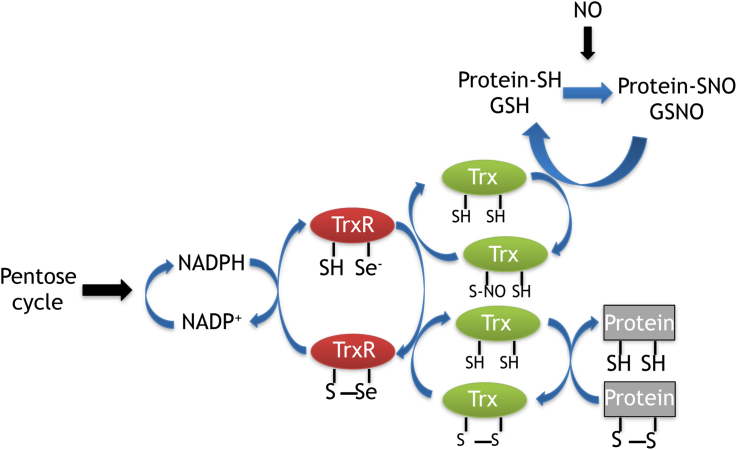
Trx1 and Trx2 (Trxs) are part of the Trx system, a major antioxidant system which is essential for maintenance of the intracellular redox status. The Trx system is formed by the Trxs, NADPH and the Trx reductases (TrxR). Mammalian TrxRs are selenoenzymes that operate as pyridine nucleotide disulfide oxidoreductases [5], [7], [8]. They maintain Trxs in their reduced state due to the presence of a selenocysteine residue in the active site of TrxRs [9], TrxRs are highly reactive proteins which consume NADPH and reduce the disulfide form of Trxs to a dithiol [Fig. 1].
Fig. 1.
The Trx system and its redox couples: *TrxRSSe/TrxR/SHSe and TrxSS/Trx(SH)2 are responsible for the delivery of reducing equivalents from NADPH and are essential for denitrosylase activities. *SHSe stands for Selenothiol and SSe stands for Selenylsulfide.
TrxRs comprise a group of three isoenzymes, the cytosolic TrxR1 [10], [11], the mitochondrial TrxR2 [12], [13], and the testis specific Trx glutathione reductase [14] . Expression and sub-cellular localization of the different members of the Trx system expands the number of their targets. Direct targets such as peroxiredoxins, which are essential for reduction of H2O2 and organic peroxides associated with intracellular redox signaling require disulfide reduction by Trx1 or Trx2 for their function [5]. Ribonucleotide reductase activity as an indirect target of the Trx system is of major importance for the supply of DNA precursors. The enzyme which is up regulated in tumor cells uses Trx1 as an electron donor for deoxyribonucleotide and DNA synthesis [2], [5], [15]. Elevated expression of TrxR1 is found in human and murine tumor cell lines [2], [3], [16], [17]. P53 mutations in glioblastomas are associated with increased expression of TrxRs and this expression is used for tumor grading in astrocytomas [18]. Inhibition of expression of TrxR1 in a mouse model of prostate cancer and in human hepatocellular carcinoma SMMC-7721 cells causes growth inhibition in both situations [19], [20].
In the absence of TrxRs, alternative pathways may be operative in maintaining intracellular redox status. The methionine sulfoxide pathway may be a possible alternative pathway. After generation of null-hepatocytes for both TrxR and GSH reductase genes in transgenic mice, though they were long-term viable, these mice were dependent on methionine supplementation in their diets needed for de novo synthesis of cysteine and GSH [21]. There are other reductases capable of redox-regulation of the system, but the methionine sulfoxide pathway is responsible for maintaining the redox environment when NADPH does not deliver the reducing power either to Trx or to GSH [22], [23], [24].
Among the Trxs the best characterized is Trx1 [5]. It regulates the activity of various signaling proteins and antioxidant enzymes within cells. Trx1 also acts as a positive regulator of survival related signaling pathways to enhance survival of tumor cells [25], [26]. Trx1 provides reducing equivalents to peroxiredoxins that in turn will reduce reactive oxygen species (ROS) [5], and directly inhibits pro apoptotic proteins such as the apoptosis signal-regulating kinase 1 (ASK-1) [27]. The intracellular location of Trx1 is a determining factor for its function as a mediator of signaling events occurring either in the cytoplasm or in the nucleus.
Trx1 plays a central role in signaling associated with the Trx system in normal and tumor cell development. This occurs in the regulation of a large number of transcription factors that are redox sensitive [28], [29], [30], [31], [32], [33], [34], the interaction with partners of components of the system, e.g. the Thioredoxin interacting protein – Txnip [35], [36] and the metabolism of low molecular weight S-nitrosothiols (SNO) [37], [38], [39], [40], [41], [42], [43], [44], [45]. All have in common the central role played by Trx1.
There is a growing body of experimental evidence on the role of the Trx system in tumor development. The reader is referred to other review and research articles to access this information [2], [3], [25], [26]. This review focuses on Trx1 intracellular compartmentalization under nitrosative/oxidative stress and its consequences on survival signaling pathways associated with tumor progression.
Thioredoxin-1 signaling and cellular compartmentalization
Signaling pathways are operative through reversible post-translational modifications of proteins and their intracellular localization. This is true for Trx1, which is predominantly a cytosolic protein that can be secreted from cells in two forms, a full-length and a truncated form [46]. In the extracellular milieu, both forms perform co-cytokine and chemokine activities [47], [48], [49]. Cytosolic Trx1 participates in anti-apoptotic signaling events related to the inhibition of the pro apoptotic protein kinase ASK-1 [27], while Trx1 migration from the cytosol to the nucleus is involved in signaling associated with cell survival, even though it does not have a nuclear localization sequence [48]. A number of different oxidative stress inducing agents such as H2O2, UV irradiation, and the chemotherapeutic agent cis-Diamine-dichloroplatinum (II) induce Trx1 nuclear migration. Intracellular generation or exposure of cells to NO also promotes Trx1 nuclear translocation [31], [48], [49].
DNA binding activity of a number of transcription factors, including NFκB, AP-1, p53, and the glucocorticoid receptor (GR) [28], [29], [30], [31], [32] is regulated by Trx1 reducing activities of essential cysteine residues. Trx1-mediated reduction of Cys62 located in the DNA binding loop of the p50 subunit of NFκB facilitates its transcriptional activities [33]. Trx1, in association with reducing catalyst redox-factor-1 reduces conserved cysteine residues within the DNA-binding domains of Fos and Jun, promoting the transcriptional activity of AP-1 [29]. P53 transcriptional activity relies on Trx1-mediated redox regulation of a conserved DNA-binding domain that contains a zinc ion and essential cysteine residues [31].
Glucocorticoid receptor (GR), a ligand-inducible transcription factor, has its activity regulated by Trx1. Essential conserved cysteine residues at the DNA-binding domain and the ligand binding domain of GR are targets for Trx1 mediated redox regulation of GR. Trx1 preserves ligand binding activity of cytosolic GR [34] and promotes GR nuclear translocation and its DNA binding activity [32].
Thioredoxin-1, nitrosative/oxidative stress, and survival signaling
Endogenous NO production is derived from the oxidation of l-arginine catalyzed by the three NO synthases isoforms. An inducible isoform (iNOS) and two constitutive isoforms isolated initially in endothelial cells (eNOS) and in neuronal cells (nNOS) are well characterized [50]. The three isoforms are expressed in a variety of tumor cells [47], [48], [51], [52].
Nerve growth factor (NGF) stimulation of nNOS in rat pheochromocytoma PC12 cells raises intracellular NO levels, which promotes cell differentiation and survival accompanied by Trx1 nuclear migration. Inhibition of phosphorylation of the ERK1/2 MAP Kinases by the MEK inhibitor PD98059, prevents NGF/NO-induced Trx1 nuclear migration and decreases cell survival [49]. NO-mediated activation of p21Ras upstream to the ERK1/2 MAP Kinases is essential for neuronal survival [53].
A connection has been found between Trx1 nuclear migration, the p21Ras-ERK1/2 MAP Kinases signaling pathway, and signaling events related to cell survival under nitrosative stress conditions. Trx1 migrates to the nucleus in HeLa cells in the presence of increasing concentrations of an external source of NO, the low molecular weight SNO, s-nitroso-N-acetyl-penicillamine (SNAP) [48]. The nuclear migration occurs at SNAP concentrations that cause nitrosylation and activation of p21Ras, with downstream activation of the ERK1/2 MAP Kinases. This results in cell survival, while inhibition of the pathway results in apoptotic cell death. The participation of a major partner of Trx1 in the cytosol, the Thioredoxin-interacting protein (Txnip) is essential to the overall process [36]. Txnip expression levels are up regulated by increasing vitamin D3 levels. Its original name was Vitamin D(3)-Up-Regulated Protein-1 (VDUP-1) [54]. Txnip binds exclusively to the reduced form of Trx1 in vitro and in vivo. The catalytic center of Trx1 is important for the interaction of Trx1 and Txnip, since mutation of the two redox active cysteine residues and their substitution by serine residues prevents the interaction [35]. Txnip has been characterized as a negative regulator of the Trx1-mediated pro-survival signaling pathways in tumor tissue and tumor cell models [55].
Rat cardiomyocytes and rat pulmonary smooth muscle cells exposed to oxidative or nitrosative stress by using either H2O2 or S-nitrosoglutathione (GSNO), undergo down regulation of Txnip expression [56], [57]. Down regulation of Txnip expression and activation of the ERK1/2 MAP Kinases in HeLa cells incubated with SNAP or H2O2 results in ERK1/2 MAP Kinases and Trx1 migration to the nucleus [36]. Incubation of HeLa cells with MEK inhibitors or the ectopic expression of the ERK1/2 MAP kinases cytoplasmic anchor, PEA-15 (the 15 Kda phosphoprotein enriched in astrocytes), originally identified in astrocytes [58] and now identified in a variety of human and mouse tissues [59], [60].
PEA-15 inhibits the integrin-stimulated p21Ras/ERK1/2 MAP kinases signaling axis [61]. PEA-15 participates in a wide range of cellular processes including, glucose metabolism, cell proliferation and apoptosis. PEA-15 is regulated by the PKC-dependent phosphorylation of Ser104 and by Calcium Calmodulin-Kinase-II or Akt-dependent phosphorylation of Ser116 [57], [62], [63], [64]. Phosphorylation of PEA-15 determines its stability and its cellular compartmentalization. Non-phosphorylated PEA-15 binds to the ERK1/2 MAP Kinases anchoring these kinases in the cytoplasm [65].
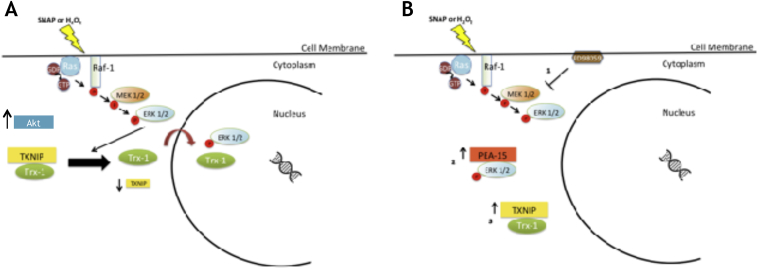
The ectopic expression of PEA-15 prevents nuclear migration of Trx1 and down regulation of Txnip expression levels [36]. Over expression of Txnip inhibits Trx1 nuclear migration under nitrosative/oxidative stress, whereas Txnip mRNA silencing facilitates Trx1 nuclear migration even in the absence of stress conditions. These findings indicate that compartmentalization, the phosphorylation status of the ERK1/2 MAP kinases and down regulation of the expression levels of Txnip, associated with stimulation of Trx1 nuclear migration under nitrosative/oxidative stress conditions allows for cell survival [36]. These observations are summarized in [Fig. 2].
Fig. 2.
The Trx1/p21Ras-ERK1/2 MAP kinases/PEA-15/Txnip compartmentalized signaling pathway is stimulated by nitrosative/oxidative stress and is associated with tumor development. (A) The Ras-Raf-MEK-ERK1/2 signaling axis is activated under nitrosative oxidative stress. Under these conditions expression of Akt is up regulated and expression of Txnip is down-regulated. Trx1 and the ERK1/2 MAP kinases migrate to the nuclear compartment. (B) Nuclear translocation of Trx1 and the ERK1/2 MAP kinases is prevented in three situations: (1) Cells pre-incubated with the MEK inhibitor PD98059; (2) Cells over-expressing the cytoplasmic anchor of ERK1/2 MAP kinases – PEA-15; (3) Cells over-expressing Txnip.
Trx1 compartmentalization, Txnip expression levels and cancer development
Oxidants inhibit the expression levels of Txnip [36], [56], [57]. Oxidative stress-induced knock down of Txnip expression facilitates Trx1 nuclear migration [36]. Txnip is down regulated in tumor cells which have high intracellular ROS levels. This is associated with the development of cancer [66]. Hypermethylation of the Txnip promoter associated with oxidative stress-induced renal carcinogenesis results in loss or decrease of Txnip levels [67]. Histone deacetylation mediates the repression of Txnip expression. The use of two histone deacetylase inhibitors, suberoylanilide hydroxamic acid – SAHA and depsipeptide - FK228, stimulate Txnip expression resulting in a decrease of Trx1 expression in cancer cells but not in normal cells [66].
Tumor stage development is a determining factor for Txnip expression. Thyroid tumor cells at different stages of development exhibit differential Txnip expression [68]. Metastatic differentiated thyroid cancer cells have high Txnip expression and anaplastic thyroid cancer cells have low or absent Txnip expression. It appears that Txnip down regulation is of major importance in the transition from differentiated to advanced poorly differentiated and non-differentiated thyroid cancer [68].
A data compilation on Txnip expression levels in tumor tissues and cell lines and their correlation with clinical importance has been done. Down regulation of Txnip expression is a consistent feature in tumor cell models, tumor animal models, and in tumor tissues obtained from patients [55].
In contrast to the various studies involving Txnip expression and tumor development, only a few studies have dealt with the signaling events associated with a decrease in Txnip expression and its consequences on tumor development. Reduced levels of Txnip in A549 human lung cancer cells and in MDA-MB-231 triple negative human breast cancer cells promote the transcriptional activity in response to TGF-β, enhancing TGF-β-induced Smad2 phosphorylation [69]. Enhanced expression in A549 cells of Snail and Slug transcription factors are associated with TGF-β−mediated induction of the Epithelial-Mesenchymal-Transition (EMT). These cells show spindle-like morphology and low expression levels of E-Cadherin. The participation of other elements of the Trx1 compartmentalized survival signaling pathway in addition to Txnip, such as PEA-15 and the ERK1/2 MAP kinases, are involved in tumor development. Coordinated signaling events associated with increased PEA-15 expression or the pharmacological inhibition of the ERK1/2 MAP kinases results in the maintenance of Txnip expression in HeLa cells exposed to oxidative and nitrosative stress [36]. Regarding tumor development, PEA-15 and the ERK1/2 MAP kinases play opposite roles. Increased activity of the ERK1/2 MAP kinases is found in a variety of cancers. ERK1/2 MAP kinases-dependent transcription activities in the nucleus downstream of activated growth factor receptors and p21Ras influence cell survival and proliferation, two determining factors associated with cancer progression [70], [71], [72]. The presence of elevated PEA-15 expression results in an interaction with the ERK1/2 MAP kinases to prevent the kinases associated nuclear transcription activity [73], [74].
Two hundred fifty two samples of mammary epithelial tissue from breast cancer and normal subjects evenly divided have been examined for PEA-15 expression levels. PEA-15 protein is highly expressed in normal mammary epithelial tissue, and its expression is decreased in tissue obtained from invasive tumors, suggesting an inverse relationship between PEA-15 expression and tumor invasion [74]. The effect of PEA-15 on tumor invasion is related to the anchoring of the ERK1/2 MAP kinases in the cytoplasm, preventing its migration to the nucleus [74]. Colorectal cancer cells from patients displaying high PEA-15 expression levels are associated with a better clinical prognosis [74].
A combination of events involving down regulation of PEA-15 and/or Txnip expression promotes nuclear migration of Trx1 and the ERK1/2 MAP kinases. Such conditions are encountered in tumor cells at an advanced stage with several lines of evidence converging to suggest that the up regulation of Trx1 expression is associated with tumor development [25], [36], [75], [76], [77], [78].
Trx1 and TrxR1 expression levels are up regulated in cells from breast cancer patients compared to normal individuals [25]. These findings are indicative of a major role for Trx1 and the Trx system in compensating for the inhibition of the GSH antioxidant pathway which is detected in malignant tumors at an advanced stage [76]. This compensation may be translated into stimulation of survival signaling pathways related to enhanced levels of Trx1 in highly aggressive human prostate cancer PC3 cells. Stimulation of PC3 cells with androgen up regulated Trx1 expression, making the cells less sensitive to the cytotoxicity of pro-oxidant compounds [77]. Trx1 expression in prostate cancer cell nuclei of patients with high-grade prostatic adenocarcinoma and metastatic prostatic adenocarcinoma is high compared with those with low-grade prostate cancer as identified by nuclear staining for Trx1. Semi-quantitative analysis of immunohistochemical staining in human prostate tissue has been correlated with the intensity of nuclear Trx1 staining and prostate cancer progression [77].
The distribution of TrxR1 and Trx1 into cellular compartments has been correlated with the outcome in 38 patients with gallbladder carcinoma (GBC). In all samples analyzed, TrxR1 expression has been detected only in the cytoplasm. TrxR1 cytoplasmic expression has been detected in the invasion front in 72% of the samples, while Trx1 expression has been detected in 100% of the samples analyzed, with Trx1 nuclear location in 76% of these samples. Within this group, Trx1 nuclear expression is observed in the invasion front in 45% of the samples. This suggests that Trx1 nuclear expression in the invasion front is a significant marker of poor prognosis in GBC patients [78].
Thioredoxin-1 compartmentalization and survival signaling
Enhanced survival signaling pathways are associated with tumor development. Trx1 is distributed in the cytoplasm and in the nucleus upon changes in intracellular redox status, which is an essential feature of Trx1-mediated survival signaling. Trx1-mediated survival signaling involves the redox-mediated activation of the p21Ras-ERK1/2 MAP kinases signaling axis, the activation of the Akt protein, down regulation of PEA-15, and of Txnip expression [Fig. 2]. This combination of events permits the shift of Trx1 between the cytoplasm, where the protein inhibits apoptotic signaling pathways, and the nuclear compartment, where Trx1 stimulates cell proliferation promoting the activity of transcription factors. This has been observed in various tumor cell models, tumor animal models, and in tumor tissues obtained from patients, emphasizing the importance of the Trx1/p21Ras-ERK1/2 MAP kinases/PEA-15/Txnip signaling axis in tumor development [36].
Being part of an effective antioxidant defense system in tumor cells, Trx1 has been investigated as a therapeutic target. 1-methylpropyl 2-imidazolyl disulfide (PX-12) is a small-molecule inhibitor of Trx1 [79]. The PX-12 mechanism of action is not completely understood. PX-12 reversibly inhibits Trx1 by thioalkylation of Cys32 and Cys35 residues at the catalytic site [79]. Thioalkylation of Cys73 residue at the structural site by PX-12 irreversibly inhibits Trx1 [79].
PX-12 also affects cancer cell growth because it is a competitive inhibitor for TrxR1 in addition to its direct effects on Trx1 [79]. It also causes GSH depletion and cell cycle arrest leading to further cell death by apoptosis [80] and inhibits tubulin polymerization by cysteine oxidation [81].
The use of PX-12 in clinical trials include a phase I trial in the treatment of gastrointestinal cancer [82], and a phase II trial in the treatment of advanced pancreatic cancer [83]. Drug-resistant multiple myeloma may be treated with Trx1 inhibitors, including PX-12, which is effective therapeutically in refractory multiple myeloma [84].
Thioredoxin-1-mediated denitrosylation of proteins is associated with survival signaling
The covalent attachment of NO to the thiol group of a cysteine is recognized as a redox-based posttranslational modification known as s-nitrosylation [85]. The direct reaction between NO and a thiol is very slow and therefore considered biologically irrelevant. Several mechanisms have been proposed to circumvent this problem in explaining SNO formation in biological systems. Mg-mediated catalysis is involved in the reaction between NO and GSH [86]. The participation of iron dinitrosyl complexes as precursors in the formation of SNO under hypoxic conditions, is especially relevant in tumor cells [87]. Trans-nitrosylation, the transfer of a nitroso moiety from one thiol to another can be added to these mechanisms. The intracellular generation of SNO resulting from trans-nitrosylation reactions occurs after exposure of cells to exogenous low-molecular weight SNOs (SNAP, GSNO, and SNOCys) [37], [43], [88]. Trans-nitrosylation contributes to target specificity in s-nitrosylation reactions [89], [90].
S-nitrosylation of proteins belonging to the cytoskeletal organization, cellular metabolism, redox homeostasis and signal transduction pathways is found in normal and tumor cells [91], [92]. SNOs derived from peptides (GSH) or proteins might play a major role in human health and disease [93], [94].
There are at least 1000 s-nitrosylated mammalian proteins [91], [92], [93], [94], [95]. Among these s-nitrosylated signaling proteins are the small GTPase p21Ras, EGFR, and Src tyrosine kinases. S-nitrosylation of these signaling proteins trigger proliferation and survival signaling pathways in normal and tumor cells [96], [97], [98], [99], [100], [101].
Constitutively high levels of ROS and NO in tumor cells translate into a pro-oxidant intracellular environment, accompanied by oxidation of DNA, lipids, and proteins [102]. Protein oxidative modifications mediated by ROS and NO in normal and tumor cells are reversed by the actions of GSH and Trx1 [85], [103].
GSH and Trx1 reduce sulfenic acids, disulfides, and SNOs [4]. However, there are important differences regarding the reducing activities of both reducing agents. GSH is utilized by glutaredoxin to restore oxidized thiols in proteins [104], while Trx1 acts directly on the oxidative modifications of proteins reducing oxidized thiols. Trx1 and glutaredoxin display different electrostatic affinities for their protein targets thereby determining different targets for each protein [105].
GSH and Trx1 can mediate denitrosylation of proteins and peptides reviewed in [42]. GSH-mediated denitrosylation of intracellular SNOs generate GSNO [85]. GSNO is a major substrate of GSNO reductase, a ubiquitously expressed enzyme in eukaryotic and prokaryotic organisms [106]. In addition to GSNO reductase, denitrosylation of GSNO is effectively carried out by Trx1 [39], [43]. Trx1 serves as a denitrosylase, removing NO from s-nitrosylated cysteine residues of GSNO and signaling proteins, making it an important line of defense against nitrosative stress [85], [103]. Trx1 also catalyzes the trans-nitrosylation of its potential targets depending on the redox state of the dithiol at the active site [107].
Trx1-mediated cytoprotection is related to its denitrosylase/transnitrosylase activity. In vitro studies performed under nitrosative stress conditions demonstrate that Trx1 reduces two critical cysteine residues that are s-nitrosylated in the transcription factor AP-1, resulting in restoration of its DNA binding capacity [39], [108]. Trx1-mediated denitrosylation of a specific cysteine residue at the active site of caspase-3 promotes enzyme activity and apoptosis in human lymphocytes and endothelial cells [40], [41].
In addition to Trx1, other members of the Trx-fold protein family have been characterized as denitrosylases. Trx-related protein of 14 kDa (TRP14) is a member of the Trx-fold protein family which does not act on the same substrates as Trx1 [109]. TRP14 is a highly efficient l-cystine reductase and an efficient denitrosylase, displaying similar efficiency as Trx1 in denitrosylating GSNO and s-nitrosylated proteins in HEK293 cells [110].
Nucleoredoxin (NRX) is another member of the Trx family which is present in the nucleus and shows the same affinity for insulin as Trx1 [111]. NRX redox-regulates the Wnt/β-catenin signaling pathway. Over expression of NRX inhibits the Wnt-β-catenin pathway, whereas down regulation of NRX expression levels results in activation of the transcription factor TCF, acceleration of cell proliferation and stimulation of oncogenic transformation through cooperation with p21Ras or the protein kinase MEK [112].
Thioredoxin-1, S-nitrosylation, tyrosine nitration, and tumor development
Intracellular SNO levels in normal cells rise upon appropriate stimuli [85], while abnormally high levels of S-nitrosylated proteins are found in tumor cells [81], [113]. Elevated s-nitrosylation levels have been implicated in tumor progression [51], [81], [114], [115].
Chronic inflammatory processes are initiating factors in at least 25% of all cancer cases diagnosed worldwide [116]. Chronic inflammatory processes include high cell counts of activated macrophages which produce high NO levels derived from activated iNOS. Elevated levels of s-nitrosylated proteins are expected in cells exposed chronically to high concentrations of NO. Therefore, carcinogenesis may develop as a result of abnormal levels of s-nitrosylated proteins that constitute signaling pathways associated with cancer initiation and progression [51], [81], [114], [115].
S-nitrosylation of signaling proteins in relation to cancer progression implies the participation of the NOS isoforms as intracellular NO sources. The reactivity and abundance of NO and thiols determine the occurrence of s-nitrosylation at or in the vicinity of NOS. Formation of a signaling complex involving the protein target and NOS are essential in guaranteeing specificity and temporal regulation of s-nitrosylation reactions [117]. This nitrosylase heterotrimeric protein complex is composed of iNOS and two other proteins, S100A8 and S100A9 [118]. The nitrosylase complex conveys NO from iNOS to specific protein substrates, ensuring specific nitrosylation of cysteine residues located in an acid-base motif of these substrates [90]. Signaling events associated with breast cancer development are directly related to iNOS-mediated s-nitrosylation of p21ras, and of the protein tyrosine kinases, EGFR and Src [84], [119].
Elevated expression levels of iNOS are associated with different types of cancer and their outcome [120], [121]. This is observed in triple negative breast cancer cells from patients [122] that have high Trx1 expression that is associated with a poor prognosis [123]. Similar findings are found in the cells of gastric cancer patients with a poor prognosis, where a positive correlation between high expression levels of iNOS and Trx1 are associated [101], [124].
In addition to specificity, regulation of s-nitrosylation-mediated signaling pathways involves participation of denitrosylases. The Trx system and the GSH/GSNO reductase system are ubiquitous and physiologically important denitrosylases. While the relevance of the GSH/GSNO reductase system has been previously discussed [42], [89], here the emphasis is on the role of Trx1 in maintaining optimal intracellular SNO levels and cell viability.
Over nitrosylation of proteins is involved in cellular dysfunction and highly nitrosylated proteins have been detected in the cells of colon cancer patients [92]. Over nitrosylation of neuronal proteins has been associated with the progression of neurodegenerative diseases [125]. Optimal physiological levels of S-nitrosylated proteins are essential for regulating protein activity [85]. Over expression of Trx1 in cancer cells maintains optimal SNO levels in these cells by up regulating their survival signaling pathways [126].
The balance between nitrosylation-denitrosylation in cells may be affected by the expression levels of Txnip. Endogenously generated or exogenously added NO in HeLa cells inhibits Txnip expression and facilitates Trx1-mediated denitrosylation [36]. Over expression of Txnip in human embryonic kidney cells causes an increase in intracellular SNO levels and promotes cell death [127].
The effectiveness of Trx1 in removing SNO groups from s-nitrosylated proteins and in reducing low molecular weight SNO is needed for the maintenance of intracellular SNO homeostasis. A fine balance involving intracellular SNO homeostasis and nitrosative stress conditions must be kept [42], [89].
Elevated Trx1 expression is essential for maintaining the viability of HeLa cells exposed to pro-apoptotic concentrations of GSNO. Exposure of wild type HeLa cells to GSNO decreases Trx1 expression, activates caspase-3, and increases cell death. Ectopic over-expression of Trx1 in HeLa cells (HeLa-Trx1) partially attenuates caspase-3 activation and enhances cell viability upon GSNO treatment. Trx1 denitrosylase activity in HeLa-Trx1 cells mediates the reduction of intracellular SNO levels and enhances cell viability. Activation of ERK1/2 MAP kinases is critical for survival signaling. Basal phosphorylation levels of ERK1/2 MAP kinases in HeLa-Trx1 cells are higher and further increase after GSNO treatment. This suggests that the enhanced cell viability promoted by Trx1 correlates with its capacity to regulate the levels of intracellular SNO and up-regulate the survival signaling pathway mediated by the ERK1/2 MAP kinases [37], [38]. These findings are similar to those described in human THP1 monocytes exposed to nitrosative stress [128].
Formation of secondary products can occur from Trx1-mediated denitrosylation reactions after the initial step of trans-nitrosylation. Two reactions may follow trans-nitrosylation: 1) The S-N bond undergoes a heterolytic breakdown yielding nitroxyl and hydroxylamines; 2) The S-N bonds undergoes a homolytic breakdown yielding NO, O2−, and nitrite [39], [43]. A combination of NO and O2− generates another reactive species, peroxynitrite (ONOO-), a product of a diffusion controlled reaction between both radical species [129]. ONOO- promotes nitration of tyrosine residues and is an effective oxidant for thiols and lipids [130]. Increased intracellular tyrosine nitration levels are associated with redox signaling, nitrative stress, and cytotoxicity [131].
Increased denitrosylase activity in Hela-Trx1 cells is accompanied by elevation of intracellular nitrotyrosine levels. Elevated nitrotyrosine levels impact on redox regulated signaling pathways [37]. The redox-associated up regulation of the ERK1/2 MAP kinases activity is dependent on tyrosine nitration [132], [133]. Nitrotyrosine as a secondary product derived from Trx1-mediated denitrosylase activity in HeLa-Trx1 cells up regulates the ERK1/2 MAP kinases through nitration and/or oxidation of these kinases [37]. Activation of the ERK1/2 MAP kinases signaling pathway is a critical mechanism of cell survival. This activation is capable of delaying or inhibiting cell death [134], [135].
Conclusion
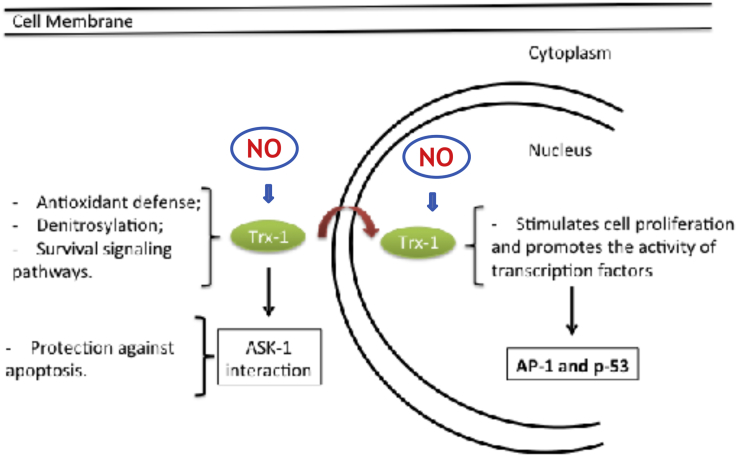
The intensified research efforts in understanding the NO-Trx1 interplay in cancer development may provide new therapeutic avenues to explore. Regarding the cross talk between NO and Trx1 in cancer development, control of the intracellular levels of s-nitrosylated proteins by Trx1-mediated denitrosylase activity and the activation of the ERK1/2 MAP kinases signaling pathway are essential for tumor survival. These signaling events are compartmentalized and occur in the cytoplasm and in the nucleus in a concerted manner. A general scheme illustrating the NO-Trx1 interaction in survival signaling is shown in [Fig. 3].
Fig. 3.
NO-Trx1 cross talk and the mediation of survival signaling events in the cytoplasm and in the nucleus.
Conflicts of interest
None of the authors have conflicts of interest.
Acknowledgments
The authors thank the financial support provided by the Brazilian Funding Institutions: Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) with grant numbers 2007/59617-6, 2009/52730-7 and 2012/10470-1 to HPM. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) with Grant number: 481154/2013-2 to HPM.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Holmgren A., Björnstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 2.Arnér E.S.J., Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Urig S., Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol. 2006;16:452–465. doi: 10.1016/j.semcancer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Hanschmann E.-M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19:1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu J., Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med. 2014;66:75–87. doi: 10.1016/j.freeradbiomed.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 6.Susanti D., Wong J.H., Vensel W.H., Loganathan U., DeSantis R., Schmitz R.A. Thioredoxin targets fundamental processes in a methane-producing archaeon Methanocaldococcus jannaschii. Proc Natl Acad Sci U S A. 2014;111:2608–2613. doi: 10.1073/pnas.1324240111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnér E.S., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 8.Mustacich D., Powis G. Thioredoxin reductase. Biochem J. 2000;346:1–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Böck A., Forchhammer K., Heider J., Leinfelder W., Sawers G., Veprek B. Selenocysteine: the 21st amino acid. Mol Microbiol. 1991;5:515–520. doi: 10.1111/j.1365-2958.1991.tb00722.x. [DOI] [PubMed] [Google Scholar]
- 10.Gasdaska P.Y., Gasdaska J.R., Cochran S., Powis G. Cloning and sequencing of a human thioredoxin reductase. FEBS Lett. 1995;373:5–9. doi: 10.1016/0014-5793(95)01003-w. [DOI] [PubMed] [Google Scholar]
- 11.Gasdaska P.Y., Berggren M.M., Berry M.J., Powis G. Cloning, sequencing and functional expression of a novel human thioredoxin reductase. FEBS Lett. 1999;442:105–111. doi: 10.1016/s0014-5793(98)01638-x. [DOI] [PubMed] [Google Scholar]
- 12.Miranda-Vizuete A., Damdimopoulos A.E., Pedrajas J.R., Gustafsson J.A., Spyrou G. Human mitochondrial thioredoxin reductase cDNA cloning, expression and genomic organization. Eur J Biochem. 1999;261:405–412. doi: 10.1046/j.1432-1327.1999.00286.x. [DOI] [PubMed] [Google Scholar]
- 13.Miranda-Vizuete A., Damdimopoulos A.E., Spyrou G. The mitochondrial thioredoxin system. Antioxid Redox Signal. 2000;2:801–810. doi: 10.1089/ars.2000.2.4-801. [DOI] [PubMed] [Google Scholar]
- 14.Miranda-Vizuete A., Sadek C.M., Jiménez A., Krause W.J., Sutovsky P., Oko R. The mammalian testis-specific thioredoxin system. Antioxid Redox Signal. 2004;6:25–40. doi: 10.1089/152308604771978327. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 16.Berggren M., Gallegos A., Gasdaska J.R., Gasdaska P.Y., Warneke J., Powis G. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 1996;16:3459–3466. [PubMed] [Google Scholar]
- 17.Lincoln D.T., Ali Emadi E.M., Tonissen K.F., Clarke F.M. The thioredoxin-thioredoxin reductase system: over-expression in human cancer. Anticancer Res. 2003;23:2425–2433. [PubMed] [Google Scholar]
- 18.Haapasalo H., Kylaniemi M., Paunul N., Kinnula V.L., Soini Y. Expression of antioxidant enzymes in astrocytic brain tumors. Brain Pathol. 2003;13:155–164. doi: 10.1111/j.1750-3639.2003.tb00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohler J.L., Morris T.L., Ford O.H., Alvey R.F., Sakamoto C., Gregory C.W. Identification of differentially expressed genes associated with androgen-independent growth of prostate cancer. Prostate. 2002;51:247–255. doi: 10.1002/pros.10086. [DOI] [PubMed] [Google Scholar]
- 20.Gan L., Yang X.L., Liu Q., Xu H.B. Inhibitory effects of thioredoxin reductase antisense RNA on the growth of human hepatocellular carcinoma cells. J Cell Biochem. 2005;96:653–664. doi: 10.1002/jcb.20585. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson S., Prigge J.R., Talago E.A., Arnér E.S.J., Schmidt E.E. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat Commun. 2015;6:6479. doi: 10.1038/ncomms7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noh M.R., Kim K.Y., Han S.J., Kim J.I., Kim H.Y., Park K.M. Methionine sulfoxide reductase a deficiency exacerbates cisplatin-induced nephrotoxicity via increased mitochondrial damage and renal cell death. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2016.6874. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Schmidt E.E. Interplay between cytosolic disulfide reductase systems and the Nrf2/Keap1 pathway. Biochem Soc Trans. 2015;43:632–638. doi: 10.1042/BST20150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh M.P., Kwak G.H., Kim K.Y., Kim H.Y. Methionine sulfoxide reductase A protects hepatocytes against acetaminophen-induced toxicity via regulation of thioredoxin reductase 1 expression. Biochem Biophys Res Commun. 2017;487:695–701. doi: 10.1016/j.bbrc.2017.04.119. [DOI] [PubMed] [Google Scholar]
- 25.Harris I.S., Treloar A.E., Inoue S., Sasaki M., Gorrini C., Lee K.C. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Benhar M., Shytaj I.L., Stamler J.S., Savarino A. Dual targeting of the thioredoxin and glutathione systems in cancer and HIV. J Clin Invest. 2016;126:1630–1639. doi: 10.1172/JCI85339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akamatsu Y., Ohno T., Hirota K., Kagoshima H., Yodoi J., Shigesada K. Redox regulation of the DNA binding activity in transcription factor PEBP2. The roles of two conserved cysteine residues. J Biol Chem. 1997;272:14497–14500. doi: 10.1074/jbc.272.23.14497. [DOI] [PubMed] [Google Scholar]
- 29.Hirota K., Matsui M., Iwata S., Nishiyama A., Mori K., Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci U S A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota K., Murata M., Sachi Y., Nakamura H., Takeuchi J., Mori K. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 31.Ueno M., Masutani H., Arai R.J., Yamauchi A., Hirota K., Sakai T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- 32.Makino Y., Yoshikawa N., Okamoto K., Hirota K., Yodoi J., Makino I. Direct association with thioredoxin allows redox regulation of glucocorticoid receptor function. J Biol Chem. 1999;274:3182–3188. doi: 10.1074/jbc.274.5.3182. [DOI] [PubMed] [Google Scholar]
- 33.Matthews J.R., Wakasugi N., Virelizier J.L., Yodoi J., Hay R.T. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grippo J.F., Holmgren A., Pratt W.B. Proof that the endogenous, heat-stable glucocorticoid receptor-activating factor is thioredoxin. J Biol Chem. 1985;260:93–97. [PubMed] [Google Scholar]
- 35.Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 36.Ogata F.T., Batista W.L., Sartori A., Gesteira T.F., Masutani H., Arai R.J. Nitrosative/oxidative stress conditions regulate Thioredoxin-Interacting Protein (TXNIP) expression and thioredoxin-1 (TRX-1) nuclear localization. PLoS One. 2013;8:e84588. doi: 10.1371/journal.pone.0084588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arai R.J., Ogata F.T., Batista W.L., Masutani H., Yodoi J., Debbas V. Thioredoxin-1 promotes survival in cells exposed to S-nitrosoglutathione: correlation with reduction of intracellular levels of nitrosothiols and up-regulation of the ERK1/2 MAP kinases. Toxicol Appl Pharmacol. 2008;233:227–237. doi: 10.1016/j.taap.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Lulu S., Ziv T., Admon A., Weisman-Shomer P., Benhar M. A substrate trapping approach identifies proteins regulated by reversible s-nitrosylation. Mol Cell Proteom. 2014;13:2573–2583. doi: 10.1074/mcp.M114.038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoyanovsky D.A., Tyurina Y.Y., Tyurin V.A., Anand D., Mandavia D.N., Gius D. Thioredoxin and lipoic acid catalyze the denitrosation of low molecular weight and protein S-nitrosothiols. J Am Chem Soc. 2005;127:15815–15823. doi: 10.1021/ja0529135. [DOI] [PubMed] [Google Scholar]
- 40.Mannick J.B., Hausladen A., Liu L., Hess D.T., Zeng M., Miao Q.X. Fas induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann J., Haendeler J., Zeiher A.M., Dimmeler S. TNFα and oxLDL reduce protein S-nitrosylation in endothelial cells. J Biol Chem. 2001;276:41383–41387. doi: 10.1074/jbc.M107566200. [DOI] [PubMed] [Google Scholar]
- 42.Benhar M., Forrester M.T., Stamler J.S. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat Rev Mol Cell Biol. 2009;10:721–732. doi: 10.1038/nrm2764. [DOI] [PubMed] [Google Scholar]
- 43.Nikitovic D., Holmgren A. S-nitrosoglutathione is cleaved by the thioredoxin system with liberation of glutathione and redox regulating nitric oxide. J Biol Chem. 1996;271:19180–19185. doi: 10.1074/jbc.271.32.19180. [DOI] [PubMed] [Google Scholar]
- 44.Rubartelli A., Bajetto A., Allavena G., Wollman E., Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 45.Bertini R., Howard O.M., Dong H.F., Oppenheim J.J., Bizarri C., Sergi R. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pekkari K., Avila-Cariño J., Bengtsson Å., Gurunath R., Scheynius A., Holmgren A. Truncated thioredoxin. (Trx80) induces production of interleukin-12 and enhances CD14 expression in human monocytes. Blood. 2001;97:3184–3190. doi: 10.1182/blood.v97.10.3184. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura H., De Rosa S., Roederer M., Andersen M.T., Dubs J.G., Yodoi J. Elevation of plasma thioredoxin levels in HIV-infected individuals. Int Immunol. 1996;8:603–611. doi: 10.1093/intimm/8.4.603. [DOI] [PubMed] [Google Scholar]
- 48.Arai R.J., Masutani H., Yodoi J., Debbas V., Laurindo F.R., Stern A. Nitric oxide induces thioredoxin-1 nuclear translocation: possible association with the p21Ras survival pathway. Biochem Biophys Res Commun. 2006;348:1254–1260. doi: 10.1016/j.bbrc.2006.07.178. [DOI] [PubMed] [Google Scholar]
- 49.Bai J., Nakamura H., Kwon Y.-W., Hattori I., Yamaguchi Y., Kim Y.C. Critical roles of thioredoxin in nerve growth factor-mediated signal transduction and neurite outgrowth in PC12 cells. J Neurosci. 2003;23:503–509. doi: 10.1523/JNEUROSCI.23-02-00503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Forstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fukumura D., Kashiwagi S., Jain R.K. The role of nitric oxide in tumour progression. Nat Rev Cancer. 2006;6:521–534. doi: 10.1038/nrc1910. [DOI] [PubMed] [Google Scholar]
- 52.de Oliveira G.A., Rosa H., Reis A.K.C.A., Stern A., Monteiro H.P. A role for nitric oxide and for nitric oxide synthases in tumor biology. Immunopathol Dis Ther. 2012;3:169–182. [Google Scholar]
- 53.Carreira B.P., Morte M.I., Inácio A., Costa G., Rosmaninho-Salgado J., Agasse F. Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells. 2010;28:1219–1230. doi: 10.1002/stem.444. [DOI] [PubMed] [Google Scholar]
- 54.Chen K.S., DeLuca H.F. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 55.Zhou J., Yu Q., Chng W.-J. TXNIP (VDUP-1, TBP-2): a major redox regulator commonly suppressed in cancer by epigenetic mechanisms. Int J Biochem Cell Biol. 2011;43:1668–1673. doi: 10.1016/j.biocel.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., De Keulenaer G.W., Lee R.T. Vitamin D(3)-up-regulated protein-1 is a stress-responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem. 2002;277:26496–26500. doi: 10.1074/jbc.M202133200. [DOI] [PubMed] [Google Scholar]
- 57.Schulze P.C., Liu H., Choe E., Yoshioka J., Shalev A., Bloch K.D. Nitric oxide-dependent suppression of thioredoxin-interacting protein expression enhances thioredoxin activity. Arterioscler Thromb Vasc Biol. 2006;26:2666–2672. doi: 10.1161/01.ATV.0000248914.21018.f1. [DOI] [PubMed] [Google Scholar]
- 58.Araujo H., Danziger N., Cordier J., Glowinski J., Chneiweiss H. Characterization of PEA-15, a major substrate for protein kinase C in astrocytes. J Biol Chem. 1993;268:5911–5920. [PubMed] [Google Scholar]
- 59.Danziger N., Yokoyama M., Jay T., Cordier J., Glowinski J., Chneiweiss H. Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and protein kinase C substrate. J Neurochem. 1995;64:1016–1025. doi: 10.1046/j.1471-4159.1995.64031016.x. [DOI] [PubMed] [Google Scholar]
- 60.Krueger J., Chou F.L., Glading A., Schaefer E., Ginsberg M.H. Phosphorylation of phosphoprotein enriched in astrocytes (PEA-15) regulates extracellular signal-regulated kinase-dependent transcription and cell proliferation. Mol Biol Cell. 2005;16:3552–3561. doi: 10.1091/mbc.E04-11-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ramos J.W., Kojima T.K., Hughes P.E., Fenczik C.A., Ginsberg M.H. The death effector domain of PEA-15 is involved in its regulation of integrin activation. J Biol Chem. 1998;273:33897–33900. doi: 10.1074/jbc.273.51.33897. [DOI] [PubMed] [Google Scholar]
- 62.Kubes M., Cordier J., Glowinski J., Girault J.A., Chneiweiss H. Endothelin induces a calcium-dependent phosphorylation of PEA-15 in intact astrocytes: identification of Ser104 and Ser116 phosphorylated, respectively, by protein kinase C and calcium/calmodulin kinase II in vitro. J Neurochem. 1998;71:1307–1314. doi: 10.1046/j.1471-4159.1998.71031307.x. [DOI] [PubMed] [Google Scholar]
- 63.Estellés A., Yokoyama M., Nothias F., Vincent J.D., Glowinski J., Vernier P. The major astrocytic phosphoprotein PEA-15 is encoded by two mRNAs conserved on their full length in mouse and human. J Biol Chem. 1996;271:14800–14806. doi: 10.1074/jbc.271.25.14800. [DOI] [PubMed] [Google Scholar]
- 64.Trencia A., Perfetti A., Cassese A., Vigliotta G., Miele C., Oriente F. Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol Cell Biol. 2003;23:4511–4521. doi: 10.1128/MCB.23.13.4511-4521.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Formstecher E., Ramos J.W., Fauquet M., Calderwood D.A., Hsieh J.C., Canton B. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev Cell. 2001;1:239–250. doi: 10.1016/s1534-5807(01)00035-1. [DOI] [PubMed] [Google Scholar]
- 66.Butler L.M., Zhou X., Xu W.-S., Scher H.I., Rifkind R.A., Marks P.A. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci U S A. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dutta K.K., Nishinaka Y., Masutani H., Akatsuka S., Aung T.T., Shirase T. Two distinct mechanisms for loss of thioredoxin-binding protein-2 in oxidative stress-induced renal carcinogenesis. Lab Invest. 2005;8:798–807. doi: 10.1038/labinvest.3700280. [DOI] [PubMed] [Google Scholar]
- 68.Morrison J.A., Pike L.A., Sams S.B., Sharma V., Zhou Q., Severson J.J. Thioredoxin interacting protein (TXNIP) is a novel tumor suppressor in thyroid cancer. Mol Cancer. 2014;13:62. doi: 10.1186/1476-4598-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masaki S., Masutani H., Yoshihara E., Yodoi J. Deficiency of thioredoxin binding protein-2 (TBP-2) enhances TGF-β signaling and promotes epithelial to mesenchymal transition. PLoS One. 2012;7:e39900. doi: 10.1371/journal.pone.0039900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weijman J.F., Riedl S.J., Mace P.D. Springer; New York: 2017. Structural studies of ERK2 protein complexes; pp. 53–63. (Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 71.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5:a006098. doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 73.Mace P.D., Wallez Y., Egger M.F., Dobaczewska M.K., Robinson H., Pasquale E.B. Structure of ERK2 bound to PEA-15 reveals a mechanism for rapid release of activated MAPK. Nat Commun. 2013;4:1681. doi: 10.1038/ncomms2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glading A., Koziol J.A., Krueger J., Ginsberg M.H. PEA-15 inhibits tumor cell invasion by binding to extracellular signal-regulated kinase 1/2. Cancer Res. 2007;67:1536–1544. doi: 10.1158/0008-5472.CAN-06-1378. [DOI] [PubMed] [Google Scholar]
- 75.Funke V., Lehmann-Koch J., Bickeböller M., Benner A., Taqscherer K.E., Grund K. The PEA-15/PED protein regulates cellular survival and invasiveness in colorectal carcinomas. Cancer Lett. 2013;335:431–440. doi: 10.1016/j.canlet.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 76.Koboldt D.C., Fulton R.S., McLellan M.D., Schmidt H., Kalicki-Veizer J., McMichael J.F. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shan W., Zhong W., Zhao R., Oberley T.D. Thioredoxin 1 as a subcellular biomarker of redox imbalance in human prostate cancer progression. Free Radic Biol Med. 2010;49:2078–2087. doi: 10.1016/j.freeradbiomed.2010.10.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagano M., Hatakeyama K., Kai M., Nakamura H., Yodoi J., Asada Y. Nuclear expression of thioredoxin-1 in the invasion front is associated with outcome in patients with gallbladder carcinoma. HPB. 2012;14:573–582. doi: 10.1111/j.1477-2574.2012.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirkpatrick D.L., Kuperus M., Dowdeswell M., Potier N., Donald L.J., Kunkel N. Mechanism of inhibition of the thioredoxin growth factor system by antitumor 2-imidazolyl disulfides. Biochem Pharmacol. 1998;55:987–994. doi: 10.1016/s0006-2952(97)00597-2. [DOI] [PubMed] [Google Scholar]
- 80.Shin H.R., You B.R., Park W.H. PX-12-induced HeLa cell death is associated with oxidative stress and GSH depletion. Oncol Lett. 2013;6:1804–1810. doi: 10.3892/ol.2013.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huber K., Patel P., Zhang L., Evans H., Westwell A.D., Fischer P.M. 2-[(1-methylpropyl) dithio-1H-imidazole inhibits tubulin polymerization through cysteine oxidation. Mol Cancer Ther. 2008;7:143–151. doi: 10.1158/1535-7163.MCT-07-0486. [DOI] [PubMed] [Google Scholar]
- 82.Galmarini C.M. Drug evaluation: the thioredoxin inhibitor PX-12 in the treatment of cancer. Curr Opin Investig Drugs. 2006;7:1108–1115. [PubMed] [Google Scholar]
- 83.Ramanathan R.K., Abbruzzese J., Dragovich T., Kirkpatrick L., Guillen J.M., Baker A.F. A randomized phase II study of PX-12, an inhibitor of thioredoxin in patients with advanced cancer of the pancreas following progression after a gemcitabine-containing combination. Cancer Chemother Pharmacol. 2011;67:503–509. doi: 10.1007/s00280-010-1343-8. [DOI] [PubMed] [Google Scholar]
- 84.Raninga P.V., Di Trapani G., Vuckovic S., Bhatia M., Tonissen K.F. Inhibition of thioredoxin 1 leads to apoptosis in drug-resistant multiple myeloma. Oncotarget. 2015;6:15410–15424. doi: 10.18632/oncotarget.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hess D.T., Matsumoto A., Kim S.-O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 86.Kolesnik B., Heine C.L., Schmidt R., Schmidt K., Mayer B., Gorren A.C.F. Aerobic nitric oxide-induced thiol nitrosation in the presence and absence of magnesium cations. Free Radic Biol Med. 2014;76:286–298. doi: 10.1016/j.freeradbiomed.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hickok J.R., Sahni S., Shen H., Arvind A., Antoniou C., Fung L.W. Dinitrosyliron complexes are the most abundant nitric oxide-derived cellular adduct: biological parameters of assembly and disappearance. Free Radic Biol Med. 2011;51:1558–1566. doi: 10.1016/j.freeradbiomed.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsumoto A., Gow A.J. Membrane transfer of S-nitrosothiols. Nitric Oxide. 2011;25:102–107. doi: 10.1016/j.niox.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anand P., Stamler J.S. Enzymatic mechanisms regulating protein s-nitrosylation: implications in health and disease. J Mol Med. 2012;90:233–244. doi: 10.1007/s00109-012-0878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marino S.M., Gladyshev V.N. Structural analysis of cysteine S-Nitrosylation: a modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395:844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seth D., Stamler J.S. The SNO-proteome: causation and classifications. Curr Opin Chem Biol. 2011;15:129–136. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen Y.J., Ching W.C., Chen J.S., Lee T.Y., Lu C.T., Chou H.C. Decoding the S-nitrosoproteomic atlas in individualized human colorectal cancer tissues using a label-free quantitation strategy. J Proteome Res. 2014;13:4942–4958. doi: 10.1021/pr5002675. [DOI] [PubMed] [Google Scholar]
- 93.Foster M.W., Hess D.T., Stamler J.S. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maron B.A., Tang S.S., Loscalzo J. S-nitrosothiols and the S-nitrosoproteome of the cardiovascular system. Antioxid Redox Signal. 2013;18:270–287. doi: 10.1089/ars.2012.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y.J., Lu C.T., Su M.G., Huang K.Y., Ching W.C., Yang H.H. DbSNO 2.0: a resource for exploring structural environment, functional and disease association and regulatory network of protein S-nitrosylation. Nucleic Acids Res. 2015;43:D503–D511. doi: 10.1093/nar/gku1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lander H.M., Hajjar D.P., Hempstead B.L., Mirza U.A., Chait B.T., Campbell S. A molecular redox switch on p21(ras). Structural basis for the nitric oxide-p21(ras) interaction. J Biol Chem. 1997;272:4323–4326. doi: 10.1074/jbc.272.7.4323. [DOI] [PubMed] [Google Scholar]
- 97.Oliveira C.J.R., Curcio M.F., Moraes M.S., Tsujita M., Travassos L.R., Stern A. The low molecular weight S-nitrosothiol, S-nitroso-N-acetylpenicillamine, promotes cell cycle progression in rabbit aortic endothelial cells. Nitric Oxide. 2008;18:241–255. doi: 10.1016/j.niox.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Moraes M.S., Costa P.E., Batista W.L., Paschoalin T., Curcio M.F., Borges R.E. Endothelium-derived nitric oxide (NO) activates the NO-epidermal growth factor receptor-mediated signaling pathway in bradykinin-stimulated angiogenesis. Arch Biochem Biophys. 2014;558:14–27. doi: 10.1016/j.abb.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 99.Curcio M.F., Batista W.L., Linares E., Nascimento F.D., Moraes M.S., Borges R.E. Regulatory effects of nitric oxide on Src kinase, FAK, p130Cas, and receptor protein tyrosine phosphatase alpha (PTP-alpha): a role for the cellular redox environment. Antioxid Redox Signal. 2010;13:109–125. doi: 10.1089/ars.2009.2534. [DOI] [PubMed] [Google Scholar]
- 100.Rahman M.A., Senga T., Ito S., Hyodo T., Hasegawa H., Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J Biol Chem. 2010;285:3806–3814. doi: 10.1074/jbc.M109.059782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Oliveira G.A., Cheng R.Y.S., Ridnour L.A., Basudhar D., Somasundaram V., McVicar D.W. Inducible nitric oxide synthase in the carcinogenesis of gastrointestinal cancers. Antioxid Redox Signal. 2017;20:1059–1077. doi: 10.1089/ars.2016.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hussain S.P., Hofseth L.J., Harris C.C. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 103.Lillig C.H., Holmgren A. Thioredoxin and related molecules–from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 104.Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979;254:3672–3678. [PubMed] [Google Scholar]
- 105.Berndt C., Schwenn J.-D., Lillig C.H. The specificity of thioredoxins and glutaredoxins is determined by electrostatic and geometric complementarity. Chem Sci. 2015;6:7049–7058. doi: 10.1039/c5sc01501d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Staab C.A., Ålander J., Brandt M., Lengqvist J., Morgenstern R., Grafström R.C. Reduction of S-nitrosoglutathione by alcohol dehydrogenase 3 is facilitated by substrate alcohols via direct cofactor recycling and leads to GSH-controlled formation of glutathione transferase inhibitors. Biochem J. 2008;413:493–504. doi: 10.1042/BJ20071666. [DOI] [PubMed] [Google Scholar]
- 107.Wu C., Liu T., Chen W., Oka S., Fu C., Jain M.R. Redox regulatory mechanism of transnitrosylation by thioredoxin. Mol Cell Proteom. 2010;9:2262–2275. doi: 10.1074/mcp.M110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nikitovic D., Holmgren A., Spyrou G. Inhibition of AP-1 DNA binding by nitric oxide involving conserved cysteine residues in Jun and Fos. Biochem Biophys Res Commun. 1998;242:109–112. doi: 10.1006/bbrc.1997.7930. [DOI] [PubMed] [Google Scholar]
- 109.Jeong W., Yoon H.W., Lee S.-R., Rhee S.G. Identification and characterization of TRP14, a thioredoxin-related protein of 14 kDa. New insights into the specificity of thioredoxin function. J Biol Chem. 2004;279:3142–3150. doi: 10.1074/jbc.M307932200. [DOI] [PubMed] [Google Scholar]
- 110.Pader I., Sengupta R., Cebula M., Xu J., Lundberg J.O., Homgren A. Thioredoxin-related protein of 14 kDa is na efficient L-cystine reductase and s-denitrosylase. Proc Natl Acad Sci U S A. 2014;111:6964–6969. doi: 10.1073/pnas.1317320111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kurooka H., Kato K., Minoguchi S., Takahashi Y., Ikeda J., Habu S. Cloning and characterization of the nucleoredoxin gene that encodes a novel nuclear protein related to thioredoxin. Genomics. 1997;39:331–339. doi: 10.1006/geno.1996.4493. [DOI] [PubMed] [Google Scholar]
- 112.Funato Y., Miki H. Nucleoredoxin, a novel thioredoxin family member involved in cell growth and differentiation. Antioxid Redox Signal. 2007;9:1035–1058. doi: 10.1089/ars.2007.1550. [DOI] [PubMed] [Google Scholar]
- 113.Cañas A., López-Sánchez L.M., Valverde-Estepa A., Hernández V., Fuentes E., Muñoz-Castañeda J.R. Maintenance of S-nitrosothiol homeostasis plays an important role in growth suppression of estrogen receptor-positive breast tumors. Breast Cancer Res. 2012;14:R15. doi: 10.1186/bcr3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z. Protein S-nitrosylation and cancer. Cancer Lett. 2012;320:123–129. doi: 10.1016/j.canlet.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 115.Benhar M. Emerging roles of protein s-nitrosylation in macrophages and cancer cells. Curr Med Chem. 2016;23:2602–2617. doi: 10.2174/0929867323666160627114839. [DOI] [PubMed] [Google Scholar]
- 116.Weinberg R.A. 2nd ed. 2013. The biology of cancer. [Google Scholar]
- 117.Martínez-Ruiz A., Araújo I.M., Izquierdo-Álvarez A., Hernansanz-Agustín P., Lamas S., Serrador J.M. Specificity in S-nitrosylation: a short-range mechanism for NO signaling? Antioxid Redox Signal. 2013;19:1220–1235. doi: 10.1089/ars.2012.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jia J., Arif A., Terenzi F., Willard B., Plow E.F., Hazen S.L. Target-selective protein S-nitrosylation by sequence motif recognition. Cell. 2014;159:623–634. doi: 10.1016/j.cell.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Switzer C.H., Glynn S.A., Cheng R.Y., Ridnour L.A., Green J.E., Ambs S. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res. 2012;10:1203–1215. doi: 10.1158/1541-7786.MCR-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lechner M., Lirk P., Rieder J. Inducible nitric oxide synthase (iNOS) in tumor biology: the two sides of the same coin. Semin Cancer Biol. 2005;15:277–289. doi: 10.1016/j.semcancer.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 121.Vannini F., Kashfi K., Nath N. The dual role of iNOS in cancer. Redox Biol. 2015;6:334–343. doi: 10.1016/j.redox.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Glynn S.A., Boersma B.J., Dorsey T.H., Yi M., Yfantis H.G., Ridnour L.A. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest. 2010;120:3843–3854. doi: 10.1172/JCI42059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bhatia M., McGrath K.L., Di Trapani G., Charoentong P., Shah F., King M.M. The thioredoxin system in breast cancer cell invasion and migration. Redox Biol. 2015;8:68–78. doi: 10.1016/j.redox.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grogan T.M., Fenoglio-Prieser C., Zeheb R., Bellamy W., Frutiger Y., Vela E. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol. 2000;31:475–481. doi: 10.1053/hp.2000.6546. [DOI] [PubMed] [Google Scholar]
- 125.Shahani N., Sawa A. Nitric oxide signaling and nitrosative stress in neurons: role for s-nitrosylation. Antioxid Redox Signal. 2011;14:1493–1504. doi: 10.1089/ars.2010.3580. [DOI] [PubMed] [Google Scholar]
- 126.Benhar M. Nitric oxide and the thioredoxin system: a complex interplay in redox regulation. Biochim Biophys Acta. 2015;1850:2476–2484. doi: 10.1016/j.bbagen.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 127.Forrester M.T., Seth D., Hausladen A., Eyler C.E., Foster M.W., Matsumoto A. Thioredoxin-interacting protein (Txnip) is a feedback regulator of S-nitrosylation. J Biol Chem. 2009;284:36160–36166. doi: 10.1074/jbc.M109.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferret P.J., Soum E., Negre O., Wollman E.E., Fradelizi D. Protective effect of thioredoxin upon NO-mediated cell injury in THP1 monocytic human cells. Biochem J. 2000;346:759–765. [PMC free article] [PubMed] [Google Scholar]
- 129.Kissner R., Nauser T., Bugnon P., Lye P.G., Koppenol W.H. formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;11:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 130.Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Monteiro H.P., Arai R.J., Travassos L.R. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid Redox Signal. 2008;10:843–889. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- 132.Pesse B., Levrand S., Feihl F., Waeber B., Gavillet B., Pacher P. Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. J Mol Cell Cardiol. 2005;38:765–775. doi: 10.1016/j.yjmcc.2005.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pinzar E., Wang T., Garrido M.R., Xu W., Levy P., Bottari S.P. Angiotensin II induces tyrosine nitration and activation of ERK1/2 in vascular smooth muscle cells. FEBS Lett. 2005;579:5100–5104. doi: 10.1016/j.febslet.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 134.Callsen D., Brune B. Role of mitogen-activated protein kinases in S-nitrosoglutathione-induced macrophage apoptosis. Biochemistry. 1999;38:2279–2286. doi: 10.1021/bi982292a. [DOI] [PubMed] [Google Scholar]
- 135.Kim P.K.M., Zamora R., Petrosko P., Billiar T.R. The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol. 2001;1:1421–1441. doi: 10.1016/s1567-5769(01)00088-1. [DOI] [PubMed] [Google Scholar]