Abstract
Diarylpropionitrile (DPN) is an estrogen receptor-β-specific agonist that has been linked to neuroprotection, preserving cognitive function with age, the suppression of anxiety-like behaviors, inhibition of cancer growth, and other positive properties. We hypothesized that DPN may have pro-longevity properties. DPN was administered via feed at a dose corresponding to approximately 3 mg/kg/day to ovariectomized female mice beginning at 7 months of age. Mice were followed for the duration of their lifespans while monitoring body mass, aspects of behavior, learning, memory, and frailty. DPN-treated mice gained more body mass over the first 2 years of age (17 months of the study). A test of voluntary running behavior at 24 months of age behavior revealed no deficits in DPN-treated mice, which were as likely as control mice to engage in extended bouts of wheel running, and did so at higher average speeds. DPN administration had anxiolytic-like effects when measured using an elevated plus maze at 9 months of age. A mouse frailty index was used to assess age-related changes. The correlation between age and frailty differed between control and DPN-treated mice. Overall, dietary DPN administration had some beneficial effects on the aging phenotype of ovariectomized female mice with few significant detrimental effects.
Electronic supplementary material
The online version of this article (10.1007/s11357-018-0038-7) contains supplementary material, which is available to authorized users.
Keywords: Estrogen receptor beta, Diarylpropionitrile, Aging, Lifespan, Frailty, Anxiety
Introduction
In addition to their role as regulators of female reproductive physiology, estrogens have effects on mammalian energy metabolism (Rettberg et al. 2014), stress physiology (Green and McCormick 2016), cognition and behavior (Ervin et al. 2015), neuroprotection (Arnold and Beyer 2009), cancer cell growth (Liao et al. 2015), and possibly also aging (Vasconsuelo et al. 2013; Lejri et al. 2018). 17-β-Estradiol (E2) modulates cellular activities acutely and chronically via estrogen receptor (ER)-mediated and independent pathways. Two classical ERs, ERα and ERβ, function as transcription factors regulating the expression of hundreds of genes. In many instances, ERα and ERβ stimulate opposing activities.
The study of ERα- and ERβ-mediated events has benefited from the development of selective ER agonists with specificity for one or the other ER. Diarylpropionitrile (DPN) was developed by Meyers et al. (2001) as an ERβ-selective ligand. DPN has a 70-fold higher binding affinity for ERβ than ERα and stimulates a 170-fold stronger transcriptional activity with ERβ. DPN has since been used to elucidate ERβ-mediated effects on a wide range of cellular and organismal biology that are not directly related to reproductive physiology. ERβ is widely expressed in the rodent brain and DPN has many effects on learning, memory, and the manifestation of anxiety-like behaviors in mice and rats (Walf and Frye 2005; Oyola et al. 2012). ERβ regulates mitochondrial function and DPN has multiple effects on bioenergetics and metabolism (Rettberg et al. 2014). DPN also inhibits growth of various cancers (Pravettoni et al. 2007; Motylewska et al. 2009; Mancuso et al. 2011; Giroux et al. 2011; Yakimchuk et al. 2014). Overall, it is clear that DPN can elicit a multitude of beneficial effects in mammals via ERβ.
Given its neuroprotective and anti-cancer activities, we hypothesized that DPN may have pro-longevity effects. We tested this hypothesis in ovariectomized female mice, as a model of postmenopausal female humans (Brinton 2012). ERβ agonists have been studied as potential therapeutics for symptoms of menopause and can ameliorate some of the negative physiological and neurological aspects of ovariectomy in mice (e.g., Zhao et al. 2011). Female mice were ovariectomized at 6 months of age and began receiving DPN supplemented chow at 7 months of age, continuing until death. Food intake and body mass were recorded throughout the study. During the first 2–2.5 years of life, effects of DPN on anxiety-like behaviors, learning and memory, and voluntary running behavior were assessed. A mouse frailty index (Whitehead et al. 2013) was used to assess signs of deterioration associated with aging. Finally, survivorship was analyzed using Online Application for Survival Analysis 2 (OASIS 2) (Han et al. 2016), an online tool for statistical analysis of lifespan-related parameters. While there was no statistically significant increase in mean, median, or maximal lifespan associated with dietary DPN supplementation, there were effects on various behaviors.
Materials and methods
Mouse husbandry
Fifty ovariectomized CD-1 female mice (retired breeders) were obtained from Charles River at approximately 6 months of age. They were maintained in the animal care facility at Brock University on regular chow for 1 month prior to the initiation of the study on May 1, 2015. At that time, the mice were divided into two groups. Mice in the control group (n = 24) were fed regular chow ad libitum, and those in the DPN group (n = 26) were fed regular chow supplemented with DPN as described below. Mice were housed initially at 3–4 per cage (at later time points following deaths of littermates, some mice were housed 1–2 per cage) in pressurized individually ventilated cages made of soft silicon with a floor area of 501 cm2. Cages were supplied with HEPA-filtered air using the Smart flow air handling unit (Techniplast, SealSafe Plus, CA). Individual mice were identified using ear notching. All procedures were approved by the Animal Care and Use Committee at Brock University, in compliance with CCAC guidelines.
Experimental diets
Diarylpropylnitrile (DPN) was purchased from Tocris Bioscience (Bristol, UK) and mixed with Teklad global 14% protein rodent maintenance diet from Envigo (powdered chow) to form whole pellets that were provided to mice ad libitum. Control food was prepared by adding 225–250 mL of distilled water to 260 g of powdered chow. The DPN diet was prepared identically, except that 13 mg DPN was added per 260 g of powdered chow. Fresh food was provided to all cages daily, and at that time, the amount of food eaten per cage over the previous 24 h was recorded.
Mouse frailty index
Starting at approximately 16 months of age, each mouse was examined several times a week and a “frailty index” (modified from Whitehead et al. 2013) was scored (Table S1). The mouse frailty index is a numerical point scale used to gauge the onset and extent of age-related anatomical, physiological, and neurological issues. We assigned a value of 0 (absent), 0.5 (moderate), or 1 (severe) for each of the following traits: alopecia, fur color, whisker loss, coat condition, tumor presence, distended abdomen, kyphosis, tail stiffening, gait disorders, tremor, overall body condition, vestibular disturbance, corneal opacity, eye discharge/swelling, microphthalmia, vision loss, menace reflex, nasal discharge, intestinal malocclusions, rectal prolapse, vaginal/uterine prolapse, diarrhea, respiratory disturbance, grimace, piloerection, body temperature, and body weight. Mice either died of natural causes or were censored upon reaching endpoint according to the rules established by the Canadian Council on Animal Care (CCAC). Upon death, all mice were necropsied and major organs (including lumps/masses) were fixed in formaldehyde for further examination.
Analysis of survivorship
Survivorship data were analyzed using the OASIS 2 (Han et al. 2016).
Elevated plus maze
An elevated plus maze (EPM) was constructed from PVC in-house according to Leo and Pamplona (2014). Each mouse was tested once, at 9 months of age. Tests were initiated by placing the mouse in a closed arm and run for 5 min in an empty room illuminated by low fluorescent light while being video recorded from a ceiling mounted camera. Time spent in closed arms, open arms, center of the EPM, and each zone crossing event were all scored manually by a researcher blind to experimental condition. Between each use, the maze and escape cage were thoroughly cleaned with Virox.
Open field test
At 10, 12, and 20 months of age (following 3, 5, and 13 months of experimental diets, respectively), all mice underwent open field testing under red light in 50 cm × 50 cm boxes. Each test lasted 30 min. Between each use, the boxes were thoroughly cleaned with Virox. Behavior was recorded with a Sony digital video camera mounted to the ceiling and connected to SMART VIDEO Tracking Software (Panlab, Harvard Apparatus). Data were imported into Microsoft Excel for analysis. Time spent, distance traveled, and velocity in the perimeter (peripheral) zone and the central zone were measured.
Barnes maze
A Barnes maze (Rosenfeld and Ferguson 2014) was constructed in-house from a 1.3-cm-thick and 120-cm-diameter circular PVC slab, which was placed on a stool 89 cm above ground level. Twenty equally spaced 4.5 cm holes were made around the perimeter, each 2.5 cm from the edge. An escape cage was constructed from a regular mouse cage that contained black paper on the outside to keep the inside of the escape cage dark. A ramp from the maze surface into the escape cage was constructed of laminated card board. Lines were drawn to divide the PVC slab into four quadrants: target (containing escape cage), positive, negative, and opposite. Between each use, the maze and escape cage were thoroughly cleaned with Virox. Mice were tested at 24, 26, and 28 months of age. During an initial habituation phase, each mouse was put inside a clear beaker and guided around the maze for 30 s, ending with the escape hole leading to the escape cage. Mice were given 3 min to enter the escape hole and nudged in if they did not. They were then given 1 min to explore the escape cage. Twenty-four hours after the habituation phase, mice were allowed to explore the maze freely for 2 min, and if the mouse found the escape cage within that time, it was allowed to explore it for 1 min before being returned to their home cage. Forty-eight hours later, a test phase was conducted with the Barnes maze in the same location and orientation as during habituation phases. During the test phase, each mouse was given 5 min to explore the maze and find the escape hole. The amount of time spent per quadrant was recorded, along with total time taken to find the escape hole.
Mouse single wheel chamber
Mouse single activity wheel chambers (Model 80820, Lafayette Instrument Co.) were used to evaluate spontaneous voluntary running behavior in the mice at age 24 months. Each chamber was 35 cm long × 23 cm wide × 20 cm high. The running wheel was 5.7 cm wide and12.7 cm diameter with a run distance of 40 cm/revolution and < 3 g wheel drag. The wheel support frame was equipped with an infrared sensor that logged each revolution using a datalogger and software made in-house by Tom McDonald (Technical Services, Brock University). Each mouse was placed in the cage for 5 min during a training phase before testing began. During the testing phase, mice were housed in the wheel cage for 23.5 h and fed ad libitum during that time. Wheel cages were cleaned with Virox and bedding changed between each use.
Results
Since it was impractical to administer daily injections of DPN over an entire lifespan, and surgical implantation of a DPN delivery system would have needed to be done repeatedly with unknown effects on aging and lifespan, we administered DPN in mouse chow, based on the approach of Patisaul et al. (2009). Starting at 7 months of age, mice were fed ad libitum either regular chow or chow supplemented with DPN (50 mg/kg chow). Throughout the study, food consumption per cage was determined daily and an average value per individual mouse was calculated (Fig. 1a). Mice consumed approximately 3.8 mg chow per day and therefore ingested on average ~ 0.15 mg DPN daily. Mouse body mass averaged ~ 48 g over the course of the study. Therefore, each mouse consumed on average approximately 3 mg/kg/day of DPN, which is equal to the dosage described by Patisaul et al. (2009) that corresponded to plasma DPN levels of 1–10 ng/mL.
Fig. 1.
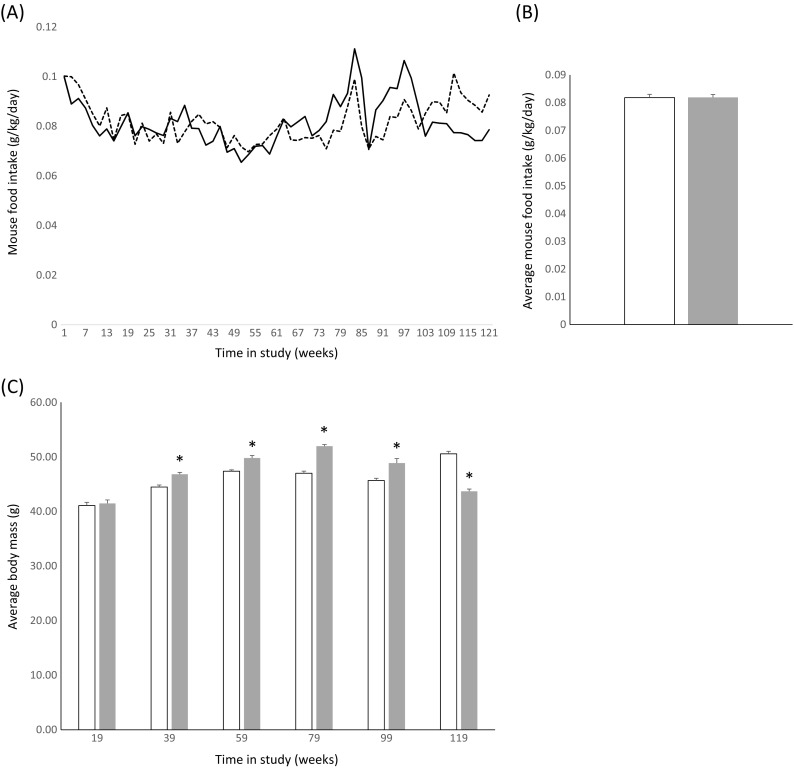
Increased food consumption and body mass in DPN-treated mice. a Average daily food consumption over the first 2 years of the study (corresponding to mouse ages 7–31 months). Regression lines for control (solid) and DPN-treated (dashed) are shown. b Average daily food consumption of control (open bars) and DPN-treated (filled bars) mice over the course of the study. c Average body mass of control (open bars) and DPN-treated (filled bars) mice calculated over 20-week periods. Where shown, error bars represent SEM. * = p < 0.05 (Student’s t test)
Mice receiving the DPN diet consumed the same amount of chow per unit body mass on average as control mice (Fig. 1a, b). Nonetheless, DPN-fed mice gained more weight, with greater average body mass as measured over each 20-week interval from week 21 to week 99 (Fig. 1c). Interestingly, this difference was reversed toward the end of the study, with surviving DPN-fed mice having lower average body masses than surviving control mice.
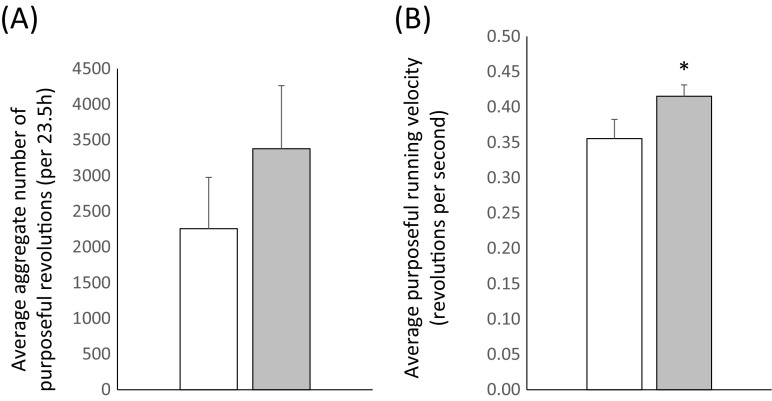
The higher average body mass of DPN-fed mice could be explained by reduced caloric expenditure due to reduced voluntary physical activity. Mice were housed in cages without running wheels. However, voluntary running behavior was evaluated in all mice at 24 months of age to determine possible effects of DPN treatment on propensity to exercise and on performance while exercising. Each healthy mouse (15 control and 17 DPN-fed) spent 23.5 h in a single wheel activity cage during which the duration of continuous movement and number of revolutions were recorded for each running event. Purposeful running was considered to be those individual running events involving > 30 wheel revolutions, since fewer revolutions appeared to be associated with more exploratory behavior. The total aggregate wheel rotations and mean velocity for all purposeful running events were calculated. The aggregate number of revolutions in 23.5 h, for all purposeful running events of > 30 rotations, was highly variable but did not differ between control and DPN mice (Fig. 2a). However, DPN mice ran with greater velocity on average (Fig. 2b). Thus, there was no evidence for reduced activity levels or running performance in DPN-fed mice. Rather, in this test, DPN-fed mice appeared more interested in engaging in active running.
Fig. 2.
Long-term DPN treatment affects running wheel behavior at 24 months of age. Purposeful running behavior, defined as events involving wheel rotations of > 30, was evaluated in single wheel activity chambers over a 23.5-h period. a No differences between control and DPN-treated mice were observed for aggregate number of revolutions. b DPN-treated mice ran with greater average velocity. Error bars represent SEM. * = p < 0.05 (Student’s t test). n = 15 (control) and 17 (DPN). Only mice that were considered healthy at the time of testing were included
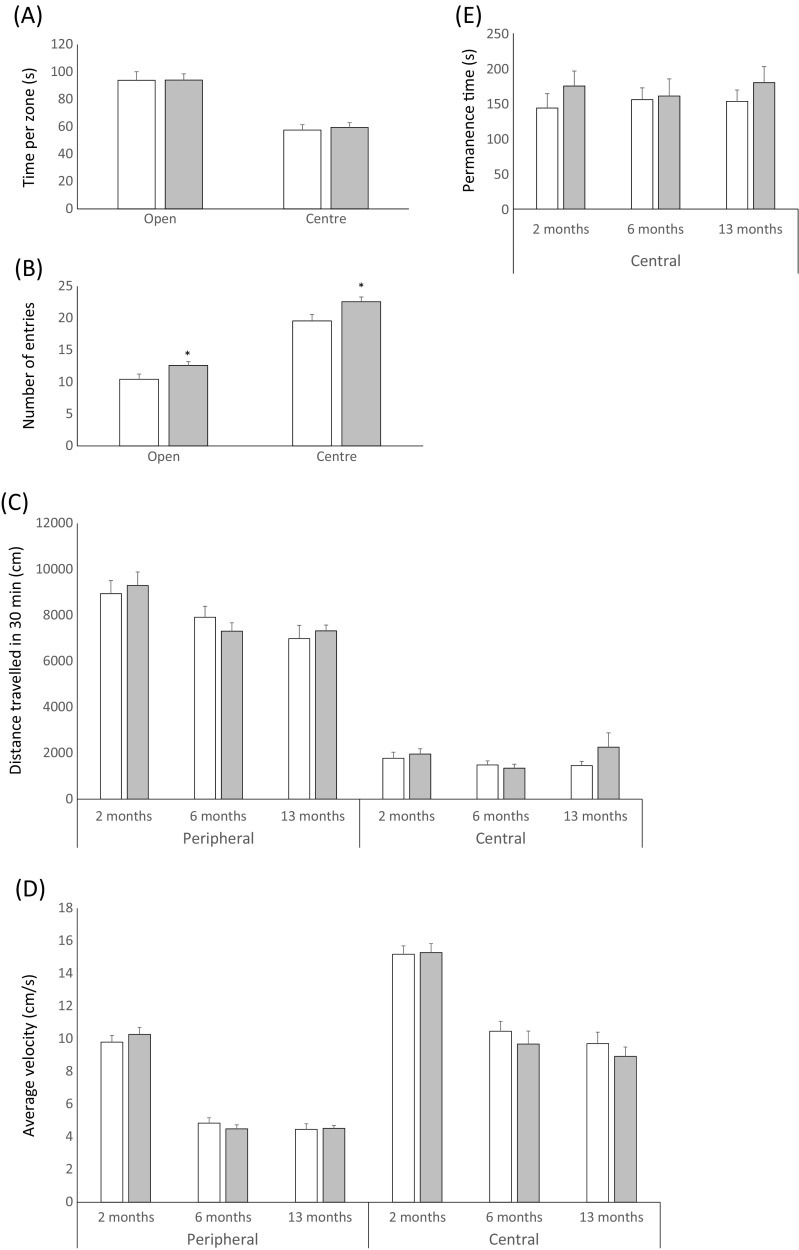
ERβ is expressed throughout the brain where its activity has been associated with beneficial effects on anxiety, learning, and memory. DPN’s effects on rodent aversive behaviors have been characterized using the elevated plus maze, open field box, and other methods. However, these effects have been measured in studies using relatively short durations of DPN administration, with the longest duration of chronic DPN treatment that we are aware of being 29 days (Sarvari et al. 2016). We evaluated the potential anxiolytic effects of dietary DPN administration 2 months following the initiation of DPN supplementation using the EPM assay, which measures the relative amount of time spent in the center and open arms as well as the number of entries into center and open arms to evaluate anxiety. Though DPN treatment had no effect on time spent in the open arms or center (Fig. 3a), it did increase the number of open arm and center entries (Fig. 3b), behaviors consistent with reduced anxiety (reviewed in Campos et al. 2013; Bourin 2015). While many investigators believe the EPM should be used on maze-naïve mice and therefore only once, other indirect assays of anxiety-like behavior such as the open field test can be used repeatedly (Campos et al. 2013). The open field test assesses anxiety as the aversion to the central zone of a square box. Mice were subjected to open field testing following 2, 6, and 13 months of experimental diet. No significant differences were found in either total distance traveled (Fig. 3c) or average velocity (Fig. 3d), suggesting that the greater mean body mass of DPN-fed mice did not significantly affect mobility. There was also no effect of DPN treatment on central zone permanence time (Fig. 3e) at any specific time point, though there was a trend toward DPN-fed mice spending more time in the central zone at all time points (two-way ANOVA, p = 0.08 for main effect of treatment). Taken together, these results are consistent with an anxiolytic effect of DPN at 2 months but this may not be sustained over longer durations of treatment.
Fig. 3.
Long-term DPN treatment has subtle anxiolytic effects in mice. The potential anxiolytic effect of continuous dietary administration of DPN was evaluated using a single elevated plus maze trial (a, b; 9-month-old mice in the study for 2 months) and serial open field tests (c–e; mice aged 9–20 months in the study for 2–13 months). a No difference in zone time between control and DPN-treated mice. b Increased zone entries in DPN-treated mice. c–e No difference in c total distance traveled in central and peripheral zones, d average velocity in each zone, or e permanence time in the central zone between control and DPN-treated mice at each time point. n = 23–26. Error bars represent SEM. * = p < 0.05 (Student’s t test) for individual comparisons. Note that p = 0.08 (ANOVA) for the main effect of DPN treatment on permanence time in central zone
Spatial learning and memory was evaluated in old mice (28 months of age) by measuring performance in the Barnes maze, which is compromised during natural aging (e.g., Barreto et al. 2010). In the 5-min test, DPN-treated mice spent significantly more time in the target quadrant (where the escape hole is located) than control mice (Fig. 4a). The latency time to find the escape hole was also shorter in DPN-treated mice (Fig. 4b). Both results are consistent with superior spatial learning and memory ability in older DPN-treated mice.
Fig. 4.
Long-term DPN treatment benefits spatial learning and memory at 28 months of age. Spatial learning and memory were tested using a Barnes maze. DPN-treated mice a spent more time in the target quadrant and b took less time to locate and investigate the escape hole than controls. n = 7 (control) and 14 (DPN). Open bars = control; filled bars = DPN-treated. Error bars represent SEM. * = p < 0.05 (t test)
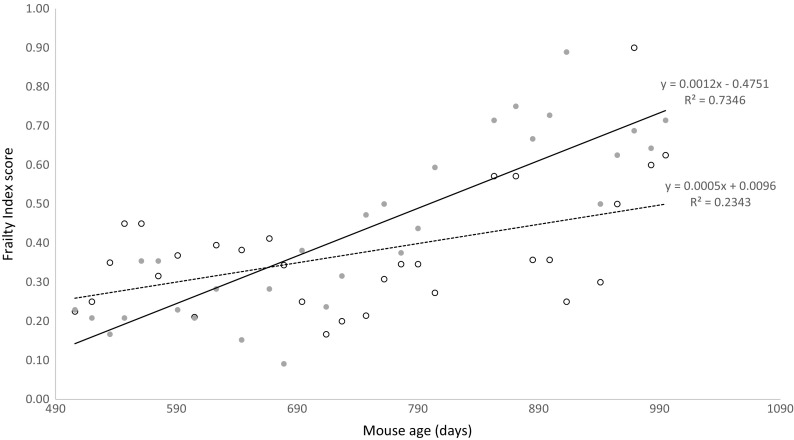
From 17 months of age (corresponding to one full year in the study) to 33 months of age, mice were monitored for signs of aging and frailty approximately every 2 weeks (Fig. 5), using a frailty index modified from Whitehead et al. (2013) that takes physical and behavioral observations into account to calculate a numerical score (Table S1). The mean frailty indices averaged over 17 to 33 months of age were 0.43 ± 0.03 for control mice, versus 0.62 ± 0.02 for DPN-fed mice, which were not significantly different (p = 0.13; t test). However, the regression lines describing the relationships between mouse age and frailty index differed between control and DPN-fed mice (p < 0.05; Fisher r-to-z transformation), indicating different relationships between age and frailty in control versus DPN-fed mice.
Fig. 5.
Long-term DPN treatment affects mouse frailty index. Relationship between age and average calculated frailty index differed in control (open symbols, dashed line) and DPN-treated (filled symbols, solid line) mice. Equations and goodness of fit of linear regression lines are shown
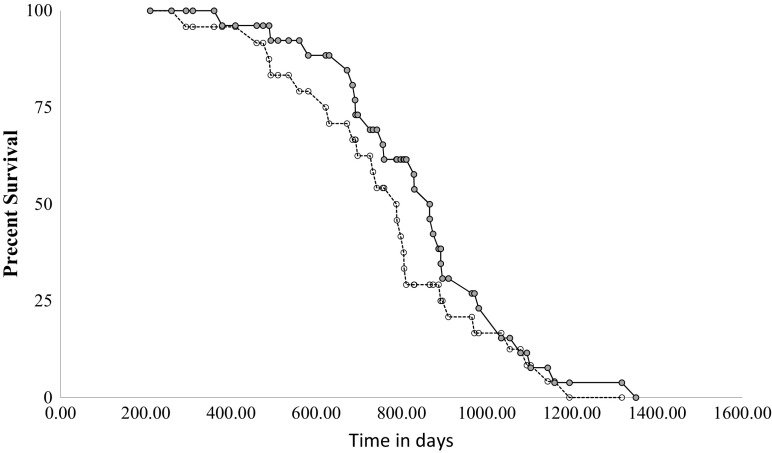
There were no differences in survivorship parameters between control and DPN-treated mice (Fig. 6). Mean, median, and maximum lifespans of control and DPN-treated groups were compared using the online lifespan analysis tool OASIS 2 (Han et al. 2016). The mean lifespan of control mice was 769 days (681–857, 95% confidence interval) versus 849 days (769–929, 95% confidence interval) for DPN-fed mice. Similarly, the median lifespan of control mice was 789 days (685–806, 95% confidence interval) versus 866 (756–892, 95% confidence interval) for DPN-treated mice, which corresponds to approximately 9 human years, using the conversion factor of 9 mouse days per human year in reproductively senescent mice (Dutta and Sengupta 2016). There were no statistically significant differences in any parameter (mean, median, maximum lifespan) between experimental groups. The age in days at which control and DPN-treated groups reached each survivorship quartile is shown in Table 1. Control mice reached each survivorship quartile earlier than DPN-fed mice, though again these differences were not statistically significant.
Fig. 6.
Survivorship curves for control and DPN-treated mice. Twenty-four control mice (open symbols, dotted line) and 26 DPN-treated mice (filled symbols, solid line) were included in the study
Table 1.
Age (in days) at mortality quartiles
| 25% | 50% | 75% | 100% | |
|---|---|---|---|---|
| Control | 622 | 789 | 892 | 1194 |
| DPN treated | 692 | 866 | 981 | 1350 |
All mice were necropsied at time of death and gross observations were made. Since DPN has been found to inhibit growth of some tumors, we took note of tumors observed during necropsy, recording the sites and numbers of visible tumors. In addition, since long-term effects of DPN have not been studied previously, we made notes of other obvious anomalies during necropsies. The frequencies of all observations made are listed in Table S2. There were no statistically significant differences between experimental groups for any individual observation (p > 0.05; Fisher’s exact test).
Discussion
We hypothesized that DPN would affect mouse aging and lifespan. ERβ-selective agonists have beneficial effects on aging-associated pathologies like neurodegeneration and cancer, and recent studies have demonstrated the ability of the 17-β-estradiol enantiomer, 17-α-estradiol, to extend median and maximum lifespans of male mice (Strong et al. 2016). Our hypothesis was tested in ovariectomized female mice since this removes the confounding effects of natural estrogen cycles while also being a model of post-menopausal aging in human females. ERβ-specific agonists have the potential to reverse some negative correlates of post-menopausal aging (Bansal and Chopra 2015). We found that while DPN-treatment, beginning at age 7 months (1 month following ovariectomy), did not extend mouse lifespan as predicted, it did have significant effects on some important aging-associated deficits in brain function and apparent health, without evidence of adverse side effects.
Inclusion of DPN in the diet-affected weight gain over the first 2 years of the study, from age 7 to 31 months of age. Mice eating chow supplemented with DPN ate more on average (3.84 versus 3.75 g/day; 2.4%), though this difference was absent when standardized to body mass (Fig. 1a, b). From 20 to 99 weeks in the study (approximately 50 to 149 weeks of age), the average body mass of DPN-fed mice remained greater than controls. Ovariectomy is associated with weight gain in female mice (e.g., Hong et al. 2009, reviewed in Eckel 2011), which can be reversed by treatment with 17β-estradiol or PPT (Hamilton et al. 2016). Though ERα agonists can prevent post-ovariectomy weight gain, there is less information available regarding the effects of ERβ agonists like DPN. Wegorzewska et al. (2008) showed no effect of 21 days of DPN treatment on post-ovariectomy weight gain in rats, while PPT significantly reduced weight gain. To the best of our knowledge, our study of DPN treatment is by far of the longest duration, and the effect of DPN on body weight appeared to manifest only after about 20 weeks of dietary supplementation.
Our data do not allow insight into the mechanism(s) of weight gain. Since body mass-adjusted food intake was equal in control and DPN-fed mice, it appears that the difference between control and DPN-fed mice could have been in daily energy expenditure. When given the opportunity of voluntarily running in wheel cages (home cages did not contain running wheels), however, DPN-fed mice were as likely to engage in bouts of active running as controls and did so at higher average velocities (Fig. 2). On the other hand, DPN feeding was associated with anxiolytic-like effects (Fig. 3), based on the elevated plus maze results following 2 months of dietary DPN administration and a similar trend (not statistically significant) in three rounds of open field box testing, where marginally more time spent in the central zone by DPN-treated mice. It is possible that this apparent anxiolytic effect of DPN might include reductions in spontaneous behaviors that consume energy. In humans, for example, apparent “fidgeting” behavior can contribute measurably to daily energy expenditure (Levine et al. 2000). Unanimously, individuals in daily contact with the mice in this study observed that DPN-fed mice appeared “calmer,” though the value of this anecdotal observation is unclear. Further study, perhaps using the method of Bains et al. (2016), will be required to assess the possibility that DPN-fed mice have lower daily energy expenditures due to some subtle behavioral differences not captured by our measurements.
Effects of DPN treatment on voluntary running activity as assessed at 24 months of age were consistent with DPN’s reported cardioprotective effects (Nikolic et al. 2007). Although there was no difference in the number of purposeful running events between DPN-fed and control mice, average running velocity was faster in the DPN-fed group (0.415 versus 0.355 rev/s during purposeful running events). This is further surprising given the slightly greater body mass of DPN-treated mice. This effect could be physiological; ERβ and DPN have beneficial effects on the cardiovascular system that could improve running performance in old mice (Barros and Gustafsson 2011).
In addition to behavior and physiology, ERβ and DPN have complicated roles in learning and memory functions of the brain (Bean et al. 2014). We chose the Barnes maze to evaluate spatial learning and memory to avoid undue psychological and physiological stresses associated with some other maze paradigms, such as the Morris water maze. Old mice perform poorly on Barnes maze testing compared to younger mice (Barreto et al. 2010). In our study, old mice (28 months of age) that had received dietary DPN for 21 months performed better in the Barnes maze, spending more time in the target quadrant and taking less time to reach the escape cage. These results are consistent with some previous rodent studies showing that DPN, at the right dose and timing of administration, can improve spatial memory in rats (Jacome et al. 2010).
We did observe different correlations between age and frailty index in control and DPN-fed mice, suggesting differences in the apparent “rate of aging” between the two groups. While DPN improved overall mouse condition early in life, this seemed to occur at the expense of late-life frailty. We observed no obvious difference between control and DPN-treated mice at necropsy, so the underlying reason for this difference is at this time unknown.
Despite the improvements in some aspects of behavior and cognitive performance in ovariectomized female mice, DPN treatment did not affect lifespan. DPN-treated mice took longer to reach each survivorship quartile (Table 1) and the longest-lived DPN-treated mouse lived for almost 3 years 9 months. However, effects on mean, median, and maximal lifespans were not statistically significant. With larger experimental group sizes, it is possible that differences would be detectable. In addition, it is possible that higher (or lower) doses of DPN might exert statistically significant effects. And finally, we did not study intact female mice or male mice. It will be interesting in future to determine how DPN may affect these populations, particularly since some ER-interacting compounds (e.g., 17α-estradiol) extend lifespan of males (e.g., Strong et al. 2016).
In summary, dietary DPN administration to ovariectomized female mice initiated at 7 months of age had effects on feeding, anxiety-like behavior, spatial learning/memory, and running behavior. DPN treatment seemed to affect the “rate of aging,” as measured by frailty, but did not alter lifespan and nor did it affect the incidence of abnormalities observed at necropsy. Future studies will be necessary to understand more completely the effects of long-term DPN treatment on mouse physiology, behavior, and aging.
Electronic supplementary material
(DOCX 16 kb)
Funding
This work was supported in part by a Natural Sciences and Engineering Research Council of Canada Discovery Grant to JAS.
Compliance with ethical standards
All procedures were approved by the Animal Care and Use Committee at Brock University, in compliance with CCAC guidelines.
References
- Arnold S, Beyer C. Neuroprotection by estrogen in the brain: the mitochondrial compartment as presumed therapeutic target. J Neurochem. 2009;110:1–11. doi: 10.1111/j.1471-4159.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- Bains RS, Cater HL, Sillito RR, Chartsias A, Sneddon D, Concas D, Keskivali-Bond P, Lukins TC, Wells S, Acevedo Arozena A, Nolan PM, Armstrong JD. Analysis of individual mouse activity in group housed animals of different inbred strains using a novel automated home cage analysis system. Behav Neurosci. 2016;10:1–12. doi: 10.3389/fnbeh.2016.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal S, Chopra K. Differential role of estrogen receptor modulators in depression-like behavior and memory impairment in rats with postmenopausal diabetes. Menopause. 2015;22:1117–1124. doi: 10.1097/GME.0000000000000435. [DOI] [PubMed] [Google Scholar]
- Barreto G, Huang T-T, Giffard RG. Age-related defects in sensorimotor activity, spatial learning and memory in C57BL/6 mice. J Neurosurg Anesthesiol. 2010;22:214–219. doi: 10.1097/ANA.0b013e3181d56c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros RPA, Gustafsson J-A. Estrogen receptors and the metabolic network. Cell Metab. 2011;14(3):289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Bean LA, Ianov L, Foster TC (2014) Estrogen Receptors, the Hippocampus, and Memory. Neuroscientist 20(5):534–545 [DOI] [PMC free article] [PubMed]
- Bourin M. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin Neurosci. 2015;17:295–303. doi: 10.31887/DCNS.2015.17.3/mbourin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD. Translational animal models of human menopause: challenges and emerging opportunities. Endocrinology. 2012;153:3571–3578. doi: 10.1210/en.2012-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr Suppl. 2013;2:S101–S111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin KS, Lymer JM, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Giroux V, Bernatchez G, Carrier JC. Chemopreventive effect of ERβSelective agonist on intestinal tumorigenesis in Apc(Min/+) mice. Mol Carcinog. 2011;50(5):35969. doi: 10.1002/mc.20719. [DOI] [PubMed] [Google Scholar]
- Green MR, McCormick CM. Sex and stress steroids in adolescence: gonadal regulation of the hypothalamic-pituitary-adrenal axis in the rat. Gen Comp Endocrinol. 2016;234:110–116. doi: 10.1016/j.ygcen.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Hamilton DJ, Minze LJ, Kumar T, Cao TN, Lyon CJ, Geiger PC, Hsueh WA, Gupte AA. Estrogen receptor alpha activation enhances mitochondrial function and systemic metabolism in high-fat-fed ovariectomized mice. Physiol Rep. 2016;4(17):e12913. doi: 10.14814/phy2.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Lee D, Lee H, Kim D, Son HG, Yang J-S, Lee S-J V, Kim S (2016) OASIS 2: online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 7(35):56147–56152 [DOI] [PMC free article] [PubMed]
- Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female, and ovariectomized female mice. Nutr J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacome LF, Gautreaux C, Inagaki T, Mohan G, Alves S, Lubbers LS, Luine V (2010) Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem 94(4):488–498 [DOI] [PMC free article] [PubMed]
- Lejri I, Grimm A, Eckert A. Mitochondria, estrogen and female brain aging. Front Neurosci. 2018;10:124. doi: 10.3389/fnagi.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo LM, Pamplona FA. Elevated plus maze test to assess anxiety-like behavior in the mouse. Bio Protocol. 2014;4:1–8. doi: 10.21769/BioProtoc.1211. [DOI] [Google Scholar]
- Levine JA, Schleusner SJ, Jensen MD. Energy expenditure of non-exercise activity. Am J Clin Nutr. 2000;72:1451–1454. doi: 10.1093/ajcn/72.6.1451. [DOI] [PubMed] [Google Scholar]
- Liao T-L, Tzeng C-R, Yu C-L, Wang Y-P, Kao S-H. Estrogen receptor-β in mitochondria: implications for mitochondrial bioenergetics and tumorigenesis. Ann NY Acad Sci. 2015;1350:52–60. doi: 10.1111/nyas.12872. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Leonardi S, Giardullo P, Pasquali E, Borra F, Stefano ID, Prisco MG, Tanori M, Scambia G, Majo VD, Pazzaglia S, Saran A, Gallo D. The estrogen receptor beta agonist diarylpropionitrile (DPN) inhibits medulloblastoma development via anti-proliferative and pro-apoptotic pathways. Cancer Lett. 2011;308:197–202. doi: 10.1016/j.canlet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Meyers MH, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Motylewska E, Stasikowska O, Melen-Mucha G. The inhibitory effect of diarylpropionitrile, a selective agonist of estrogen receptor beta, on the growth of MC38 colon cancer line. Cancer Lett. 2009;276:68–73. doi: 10.1016/j.canlet.2008.10.050. [DOI] [PubMed] [Google Scholar]
- Nikolic I, Liu D, Bell JA, Collins J, Steenbergen C, Murphy E. Treatment with an estrogen receptor-beta-selective agonist is cardioprotective. J Mol Cell Cardiol. 2007;42:769–780. doi: 10.1016/j.yjmcc.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Oyola MG, Portillo W, Reyna A, Foradori CD, Kudwa A, Hinds L, Handa RJ, Mani SK. Anxiolytic effects and neuroanatomical targets of estrogen receptor-β (ERβ) activation by a selective ERβ agonist in female mice. Endocrinology. 2012;153(2):837–846. doi: 10.1210/en.2011-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Burke KT, Hinkle RE, Adewale HL, Shea D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm Behav. 2009;55(2):319–328. doi: 10.1016/j.yhbeh.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravettoni A, Mornati O, Martini PG, Marino M, Colciago CF, Motta M, Negri-Cesi P. Estrogen receptor beta (ERbeta) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol Cell Endocrinol. 2007;263:46–54. doi: 10.1016/j.mce.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Rettberg JR, Yao J, Brinton RD. Estrogen: a master regulator of bioenergetic systems in the brain and body. Front Neuroendocrinol. 2014;35:8–30. doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CS, Ferguson SA. Barnes maze testing strategies with small and large rodent models. J Vis Expt. 2014;84:e51194. doi: 10.3791/51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvari M, Kallo I, Hrabovszky E, Solymosi N, Rodolosse A, Liposits Z. Long-term estrogen receptor beta agonist treatment modifies the hippocampal transcriptome in middle-aged ovariectomized rats. Front Cell Neurosci. 2016;10:149. doi: 10.3389/fncel.2016.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhaes JP, Martinez PA, McCord JM, Miller BF, Muller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconsuelo A, Milanesi L, Boland R. Actions of 17β-estradiol and testosterone in the mitochondria and their implications in aging. Ageing Res Rev. 2013;12:907–917. doi: 10.1016/j.arr.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariectomized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- Wegorzewska IN, Walters K, Weiser MJ, Cruthirds DF, Ewell E, Larco DO, Handa RJ, Wu TJ. Postovariectomy weight gain in female rats is reversed by estrogen receptor alpha agonist propylpyrazoletriol. Am J Obstet Gynecol. 2008;199:67.e1–67.e5. doi: 10.1016/j.ajog.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Whitehead JC, Hildebrand BA, Sun M, Rockwood MR, Rose RA, Rockwood K, Howlett SE. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2013;69(6):621–632. doi: 10.1093/gerona/glt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimchuk K, Hasni MS, Guan J, Chao MP, Sander B, Okret S. Inhibition of lymphoma vascularization and dissemination by estrogen receptor b agonists. Blood. 2014;123(13):2054–2061. doi: 10.1182/blood-2013-07-517292. [DOI] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Schneider LS, Brinton RD. Estrogen receptor β-selective phytoestrogenic formulation prevents physical and neurological changes in a preclinical model of human menopause. Menopause. 2011;18:1131–1142. doi: 10.1097/gme.0b013e3182175b66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 16 kb)