Abstract
Objective
The aim of this study to evaluate the effects of autologous conditioned serum (ACS) on the healing of transected rat Achilles tendons via the assessment of biomechanical and histological parameters.
Methods
The study was conducted on 45 male Sprague–Dawley rats. Five rats were used as donors for ACS preparation. Animals were randomly assigned to the experimental or control group. In both groups, the Achilles tendon was cut transversally and then sutured. In the placebo control and ACS-treated groups, saline or ACS, respectively, was injected into the repair zone three times after surgery. Ten rats from each group (ACS group, n = 20; control group, n = 20) were euthanized at days 15 and 30 after surgery for histopathological (n = 5) and biomechanical (n = 5) testing. The histopathological findings were interpreted using the Bonar and Movin scales. Tendon remodelling was evaluated via the immunohistochemical staining of collagen type 3. Biomechanical effects were assessed by tensile testing.
Results
The Bonar and Movin scale scores were significantly better in the ACS-treated group on both day 15 (p = 0.003 and p = 0.003, respectively) and day 30 (p = 0.005 and p = 0.004, respectively). The immunohistochemical density of collagen type 3 was significantly lower in the ACS-treated group on day 30 (p = 0.018). The type 1/3 collagen ratios of the groups were similar on days 15 and 30, as determined by Sirius Red staining (p = 0.910 and p = 0.133, respectively). In the biomechanical assessment results, the ACS-treated group's maximum load to failure values were significantly higher on day 15 (p = 0.049).
Conclusion
Injection of ACS had a positive effect on the histopathological healing of rat Achilles tendons on days 15 and 30 and on biomechanical healing on day 15. ACS treatment contributed to lowering the collagen type 3 density by day 30. According to our study, ACS may be favourable for the treatment of human Achilles tendon injuries and tendinopathies.
Keywords: Autologous conditioned serum, Achilles tendon rupture, Rats, Animal model, Growth factors
Introduction
The Achilles tendon is the strongest, largest tendon in the body.1 However, it commonly ruptures in middle-aged men who exercise.2 The incidence of tendon rupture is estimated as 18/10 000.3, 4
Along with surgical and conservative methods for Achilles tendon rupture treatment, novel treatment methods have been developed due to the establishment of new biological approaches. Numerous articles have shown that individual growth factors are useful for tendon healing in animal models.4 Such growth factors include vascular endothelial growth factor (VEGF), transforming growth factor (TGF) β −1, platelet-derived growth factor, insulin-like growth factor-1, basic fibroblast growth factor (FGF-2) and bone morphogenic proteins (BMP-12 and BMP-13).5, 6, 7, 8, 9, 10, 11
Due to mechanical stress in tendons, interleukin 1 (IL-1) expression upregulates and stimulates the release of cytokines, which play a role in inflammation. This pathway is a potential contributor to existing inflammation in tendinopathy, and the inhibition of such a pathway may be useful for tendinopathy treatment.12 IL-1RA is a natural competitive inhibitor of IL-1; by inhibiting the signal pathway, it prevents the inflammatory cascade.
Autologous conditioned serum (ACS), which is used for the treatment of osteoarthritis and similar inflammatory diseases, is an injectable agent rich in endogenous IL-1RA. Meijer et al reported that contact between the blood and small glass spheres allowed a rapid, strong increase in the synthesis of many anti-inflammatory cytokines, including IL-1RA.13 ACS is also rich in anti-inflammatory cytokines, such as IL-4, IL-10 and IL-13; furthermore, its tumour necrosis factor (TNF)- α, FGF-2, VEGF and hepatocyte growth factor (HGF) values are high.14, 15
The aim of the present experimental study is to examine whether local ACS treatment implemented after tendon surgery would be useful for healing over a 4-week period. Our hypothesis is that ACS administration will have positive immunohistochemical, histopathological and biomechanical effects on the healing of Achilles tendons.
Material and methods
Local ethics committee approval for animal experimentation was obtained on 08.06.2015 (no. 2015/26). Forty-five 12-month-old adult male Sprague–Dawley rats with a mean body weight of 400–450 g, including five rats as ACS donors, were used in the study. The animals were kept five rats to a cage at a temperature of 22 °C under a 12-h: 12-h light–dark cycle. They were fed ad libitum with standard rat feed and had free access to water.
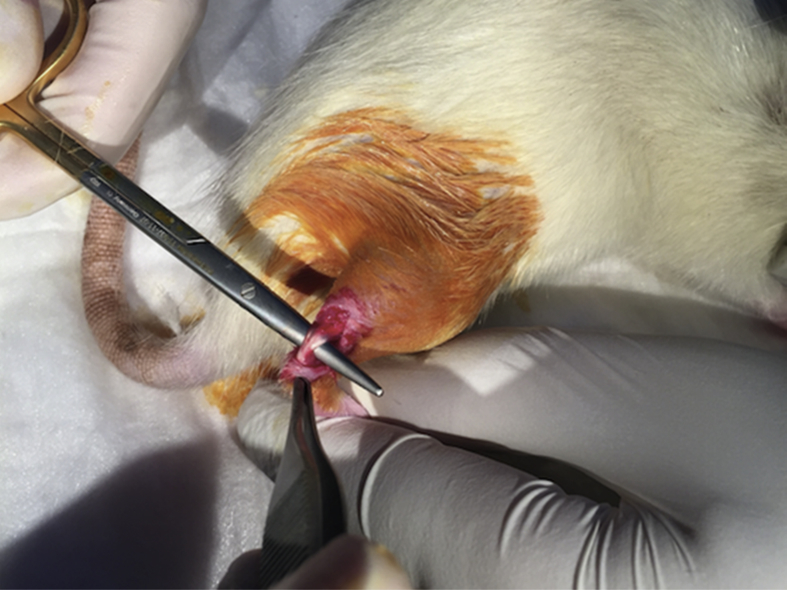
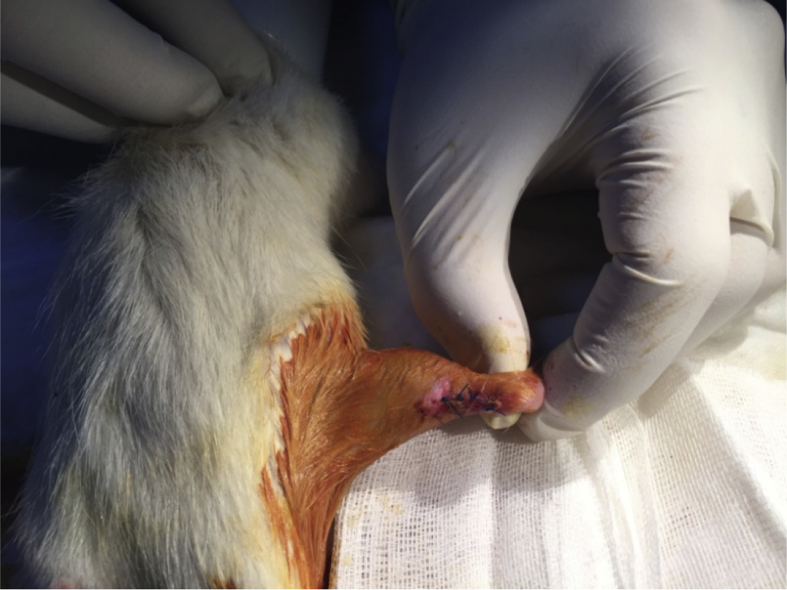
Forty rats were divided into two groups, with group 1 as the control group (n = 20) and group 2 as the ACS group (n = 20). Prophylactic gentamycin (8 mg/kg) was administered to the rats 30 min prior to the surgical procedure. The surgery was initiated with administration of inhaled anaesthesia, which started with 4% isoflurane (Forane) as an induction dose and continued with 2% as a maintenance dose. The posterior side of the right cruris was shaved, iodine was applied under aseptic surgical conditions and the area was covered using a sterile green cover; a standard posterior longitudinal incision of 2 cm was applied, and the Achilles tendon was revealed (Fig. 1). A complete transverse incision was performed using a no. 11 scalpel (Plusmed, Turkey) at 4–5 mm on the proximal side of the Achilles tendon–calcaneus junction. The end of the Achilles tendon was non-traumatically resutured using the modified Kessler method PDO II 4/0 (BOZ, Turkey). The incision site was sutured with four 3/0 propylene (Dogsan, Turkey) sutures placed at equal distances by under sterile conditions, and dressing was applied with povidone iodine (BatticonR, Adeka, Turkey; Fig. 2). No immobilisation method was applied to the rats during the postoperative period.
Fig. 1.
Exploration of the Achilles tendon.
Fig. 2.
Postoperative Achilles tendon with sutures at equal distances.
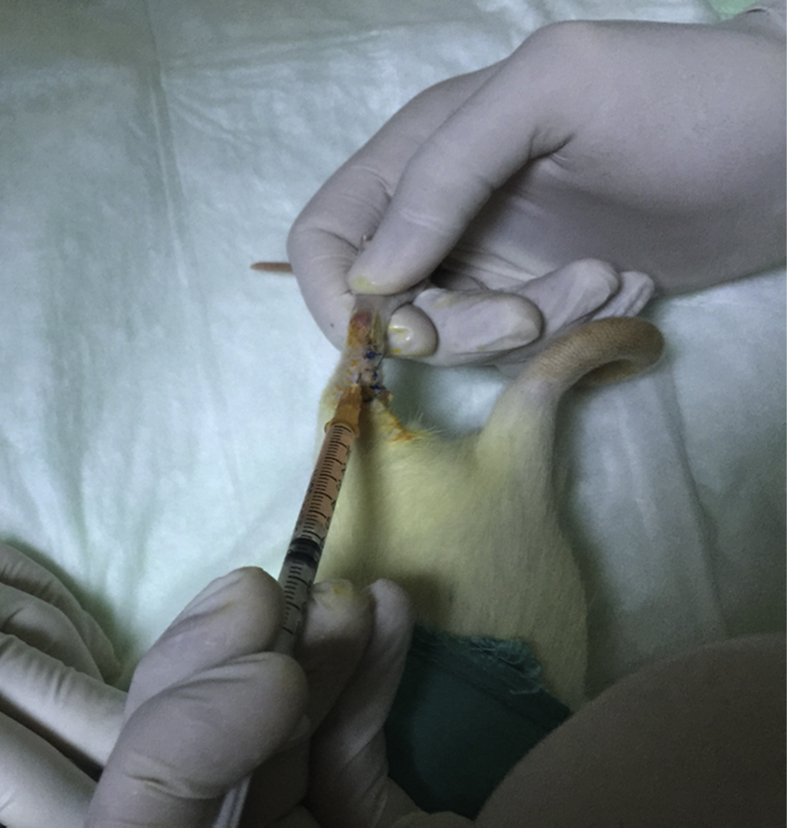
The five rats included in the donor group were decapitated after the collection of 5–6 cc of blood under anaesthesia. The blood samples collected from the donor group were transferred into special Orthokine (Orthogen AG, Düsseldorf, Germany) injectors2 containing glass spheres, with a surface area of 21 mm, under a temperature of 37 °C. The samples were centrifuged using a centrifugation device (Megafuge, Kendro, Germany) at a rate of 3500 rpm for 10 min; following this, concentrated serum was collected in 0.2-mL quantities and kept at −20 °C. The samples were melted and brought up to room temperature before injection, and they were injected into the surgery area of the Achilles tendon at 170 uL using a 1-mL insulin injector. This dose was calculated according to the dose used in a previous study based on the rats' body weight.16 Each dose injection was applied to sutures 2 and 3 (Fig. 3).
Fig. 3.
Autologous conditioned serum (ACS) administration after surgery.
On days 15 and 30, 10 rats from each group were euthanised under anaesthesia for histopathological (n = 5) and biomechanical (n = 5) testing. The right Achilles tendon and one part of the calcaneus was removed with the femur condyle. Care was taken to leave the plantaris tendon in place during the removal of the Achilles tendon to prevent the biomechanical measurements from being affected. For the biomechanical analyses, the left Achilles tendons of all rats in the control and ACS groups were also removed.
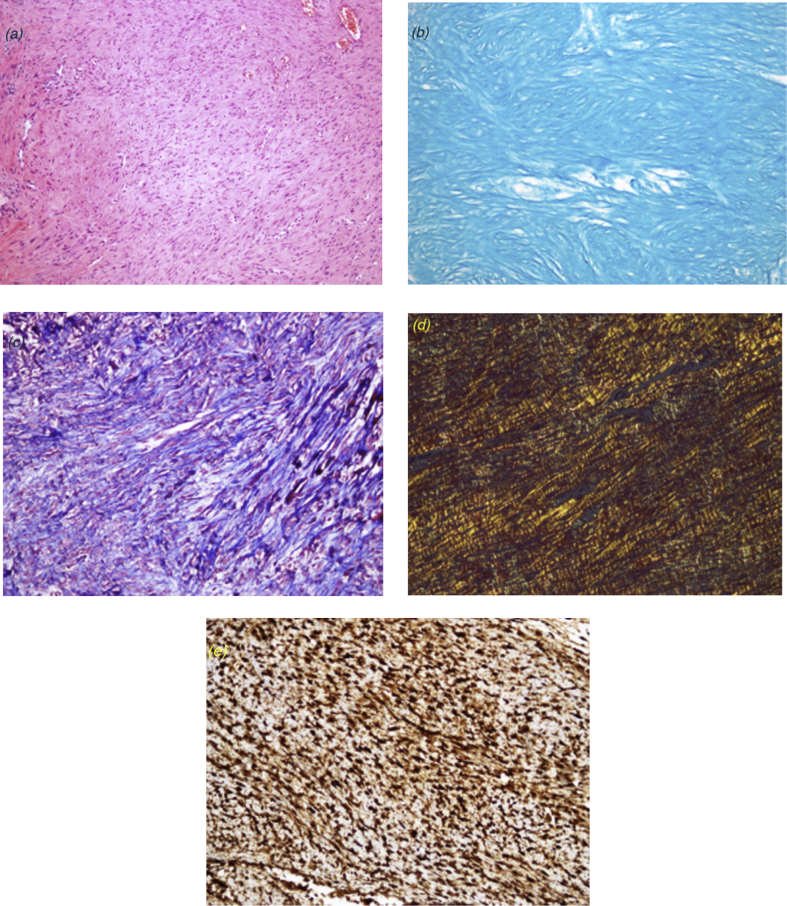
Samples were obtained from the tendon repair area for histopathological evaluation. Tendon tissue samples were fixed using 10% neutral formaldehyde solution and kept in 5% formic acid. After the histopathological preparation processes, the materials were embedded in paraffin blocks and sliced. The sections were stained with haematoxylin and eosin (H&E), Masson's trichrome and alcian blue (pH 2.5; Fig. 4a–c). The samples were examined using a BX51TF (Olympus, Tokyo, Japan) light microscope. Bonar's semi-quantitative score and Movin's semi-quantitative grading scale were used for evaluation.
Fig. 4.
Histological effect of autologous conditioned serum (ACS) (a) Control group, day 15 (haematoxylin and eosin [H&E], 100 × magnification). The nuclei in tenocytes are round and slightly enlarged, while the cytoplasm is less detectable. More than two capillary clusters are observed in each of the 10 large magnification areas. Significant dissociation and total loss of the alignment are observed in the collagen fibres. (b) Autologous conditioned serum (ACS) group, day 15 (alcian blue staining, 100 × magnification). Abundant mucin presence is noted. (c) ACS group, day 15 (trichrome staining, 100 × magnification). Marginal loss of collagen fibre bundles and fibre dissociation are observed. (d) ACS group, day 30 (Sirius Red staining, 200 × magnification). Type 1/3 collagen staining pattern (type 1 collagen fibres are thicker and red; type 3 collagen is thinner, opaque and green). (e) Control group, day 30 (200 × magnification). The type 3 collagen staining pattern is observed via immunohistochemical staining.
Bonar's scale includes the analysis of the following components: 1) tenocytes, 2) ground substance, 3) collagen and 4) vascularity. Each variable was scored on a 4-point scale of 0–3, as follows: 0, normal; 1, slightly abnormal; 2, abnormal; and 3, markedly abnormal. The samples were scored according to whether there was a significant abnormal appearance. The total score varied between 0 (normal tendon) and 12 (severest abnormality).17 Movin's semi-quantitative scale includes analysis of eight variables, as follows: 1) fibre structure, 2) fibre arrangement, 3) rounding of the nuclei, 4) regional variations in cellularity, 5) increased vascularity, 6) decreased collagen stainability, 7) hyalinisation and 8) glycosaminoglycan (GAG) content. The first seven variables were evaluated using the slides stained with H&E, while the eighth variable – the GAG content – was examined using the slides stained with alcian blue (pH 2.5). Each variable was scored between 0 and 3, as follows: 0, normal; 1, slightly abnormal; 2, abnormal; and 3, markedly abnormal. The total semi-quantitative histological score varied between 0 (normal tendon) and 24 (severest abnormality).17
Evaluation of the type 1/3 collagen staining pattern by Sirius Red was performed using an Olympus BX51 polarised light microscope under 400 × magnification (Fig. 4d). Type 3 collagen staining was evaluated using an Olympus BX51 light microscope under 400 × magnification (Fig. 4e).
Achilles tendon samples were kept at −20 °C and defrosted to room temperature on the study day; measurements were performed by connecting the Achilles tendons from the origo and insertion sides. The pulling test was applied to measure the longitudinal axis strength, and this was carried out using a Mecmesin Multitest 5-i mechanical test device (Mecmesin, Slinfold, West Sussex, United Kingdom) and mechanical test software (Emperor, Mecmesin). Unnotched grasper jaws, which were specifically designed and manufactured for the study, were employed (Fig. 5). The tests were performed with a 10 mm/min pulling rate for each sample. Loading values measured with a sensitivity of 0.1% by the device's load cell were entered into the software (Emperor), and force values (F) were obtained; the tests were terminated after the detection of a force decrease and rupture of the sample. The highest force value among the data obtained was determined as the maximum strength value, Fmax.
Fig. 5.
Mecmesin mechanical test device and specially designed unnotched grasper jaws, which hold the tendon.
In the present study, statistical analyses were performed using the Number Cruncher Statistical System (NCSS) 2007 statistical software (UT, USA). Definitive statistical values of the data included the mean, standard deviation, median, and lowest and highest values. Distribution of the variables was measured by using the Kolmogorov Kolmogorov–Simirnov test. The Mann–Whitney U test was used for the analysis of quantitative data.
Results
The ACS group had significantly lower scores than the control group at days 15 and 30 on both the Bonar and Movin scales (p = 0.003, p = 0.005 and p = 0.003, p = 0.004, respectively; Table 1, Table 2). The immunohistochemical type 3 collagen ratios were similar in the ACS and control groups at day 15 (p = 1.000). The type 1 and type 3 collagen staining ratios under Sirius Red staining were similar in the ACS and control groups (p = 0.910). However, immunohistochemical type 3 collagen staining in the ACS group was significantly lower at day 30 (p = 0.018). There was no significant difference in Sirius Red staining for collagen 1 and 3 at day 30 (p = 0.133; Table 3, Table 4).
Table 1.
Statistical analysis of the groups according to Bonar scoring.
| Histopathology Bonar scale | Control group |
ACS group |
p | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Day 15 | 11.0 ± 0.0 | 11 (11–11) | 10.0 ± 0.0 | 10 (10–10) | 0.003a |
| Day 30 | 7.0 ± 0.0 | 7 (7–7) | 5.6 ± 0.5 | 6 (5–6) | 0.005a |
| p | 0.003 | 0.005 | |||
p<0.05.
Table 2.
Statistical analysis of the groups according to Movin scoring.
| Histopathology Movin scale | Control group |
ACS group |
p | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Day 15 | 20.0 ± 0.0 | 20 (20–20) | 18.0 ± 0.0 | 18 (18–18) | 0.003a |
| Day 30 | 13.0 ± 0.0 | 13 (13–13) | 11.2 ± 0.4 | 11 (11–12) | 0.004a |
| p | 0.003a | 0.004a | |||
p<0.05.
Table 3.
Statistical analysis of the groups according to the collagen 3 ratios shown by sirius red staining.
| Type 3 collagen % | Control group |
ACS group |
p | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Day 15 | 78.0 ± 8.4 | 80 (70–90) | 77.0 ± 4.5 | 80 (70–80) | 0.910 |
| Day 30 | 46.0 ± 11.4 | 50 (30–60) | 34.0 ± 11.4 | 30 (20–50) | 0.133 |
| p | 0.008a | 0.008a | |||
p<0.05.
Table 4.
Statistical analysis of the groups According to collagen 3 ratios shown by immunohistochemical staining.
| Type 3 collagen % | Control group |
ACS group |
p | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Day 15 | 78.0 ± 4.5 | 80 (70–80) | 78.0 ± 4.5 | 80 (70–80) | 1.000 |
| Day 30 | 54.0 ± 11.4 | 50 (40–70) | 38.0 ± 4.5 | 40 (30–40) | 0.018a |
| p | 0.006 | 0.005 | |||
p<0.05.
The Biomechanical results revealed a significantly higher Fmax value on day 15 in the ACS group (p = 0.049). Fmax values on day 30 were similar between groups (p = 0.127; Table 5).
Table 5.
Statistical analysis of the groups from biomechanical tests According to the Fmax value.
| Fmax | Control group |
ACS group |
p | ||
|---|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | ||
| Day 15 ruptured | 21.4 ± 1.9 | 21.4 (19.4–23.3) | 36.1 ± 8.8 | 36.3 (27.2–44.7) | 0.049a |
| Day 30 ruptured | 32.0 ± 8.2 | 33.1 (23.3–39.5) | 33.8 ± 2.8 | 34.3 (30.7–36.3) | 0.827 |
| Day 15 healthy | 58.5 ± 2.3 | 58 (56–61) | 53.0 ± 5.3 | 56 (47–56) | 0.127 |
| Day 30 healthy | 49.3 ± 2.0 | 50 (47–51) | 46.1 ± 4.0 | 48 (41–49) | 0.275 |
p<0.05.
Discussion
Problematic treatment complications are common after Achilles tendon ruptures. Since endogenous repair is not flawless, some symptoms may persist and result in delayed return to exercise.4 Hence, there is increasing interest in novel treatment methods, including biological approaches, to treat these injuries18, 19. ACS treatment has been found to be useful in tendinopathies.12 In our study, which considered the effects of ACS treatment after Achilles tendon rupture repair, the results demonstrated that ACS was effective in histopathological healing at day 15, and this effect was shown to continue at day 30. However, while the biomechanical effect was significant at day 15, no significance could be shown at day 30. Moreover, while no significant immunohistochemical difference in the collagen 3 ratio was shown at day 15, the ratio was significantly lower at day 30. With such results, using the ACS treatment, we could show a significant effect of the high collagen 1 ratio on tendon stiffness and strength at day 30 at the earliest. ACS treatment increased the expression of collagen 1; however, a quantitatively significant result could be obtained at day 30.
Our findings are in agreement with Majewski et al’s study, which involved biomechanical, histological and immunohistological evaluations of the outcomes of ACS treatment administered after Achilles tendon surgery.4 In their study, which was performed on 80 rats, useful effects of ACS administration on the collagen composition, histological appearance and mechanical strength of tendon regeneration were shown. Majewski et al reported an increase in type 1 collagen and decrease in type 3 collagen in the ACS treatment group. They also found that ACS enhanced the expression of collagen mRNA and collagen deposition, which affect tendon resistance and collagen fibre maturation. Furthermore, they reported that significant effects on tendon remodelling appeared in week 8, although there was no significant difference at week 4. We could not see a significant difference between the ACS and control groups using Sirius Red staining in the present study. Since our study period was 4 weeks long, we think that significant results would be obtained for a longer period. We obtained faster and more significant results using H&E staining. Moreover, we demonstrated significantly better Bonar and Movin scores in the ACS group than in the control group at both days 15 and 30.
Although no immunohistochemical difference in collagen 3 density was observed on day 15, we detected significantly lower levels of collagen 3 on day 30. We think that ACS is effective in bringing about collagen remodelling, but the remodelling outcomes, which present in the late term, are significant at week 4 and later.
Like our findings showing a positive effect of ACS on tendon rupture repair, beneficial effects of ACS have been shown for the treatment of muscle injuries, tendinopathy and osteoarthritis in animal and human studies.16,20, 21, 22, 23 The immunohistochemical results we obtained are comparable to the results of one study considering the effect of single-dose ACS on tendon healing in racehorses with tendinopathy. The immunohistochemical analysis of type 1 and type 3 collagen quantities demonstrated an earlier, more permanent healing in tendinopathy of the superficial digital flexor tendon with a single dose of intralesional ACS injection in comparison to placebo control group. Transient flattened morphology of the nuclei of tenocytes in tendons treated with ACS and increase in expression of type 1 collagen are indicators of reduced proliferation and increased differentiation in this cell type. Furthermore, the healing effect of ACS treatment in the experimental group compared with the control group was explained by the early reduction of limping.20
The results of the study should be viewed in the context of its limitations. The first limitation is that, due to the experimental design, a minimum number of subjects, sufficient for statistical validity was included in the present study. A second limitation is the lack of clinical and functional results. Finally, the Achilles rupture was created deliberately in the present study, which did not allow demonstration of the presumptive degeneration that occurs before rupture.
Conclusion
Our study findings showed that postoperative administration of ACS is biomechanically and histochemically beneficial for the healing of transected rat Achilles tendons. The biomechanical effects of ACS may enhance tendon strength recovery. The histopathological and immunohistochemical effects may accelerate tendon remodelling. Further experimental and clinical studies for determining dose and period should be conducted. Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Contributor Information
Erdinç Genç, Email: erdincgenc@hotmail.com.
Ozan Beytemur, Email: beytemur@yahoo.com.
Serdar Yuksel, Email: Serdar84yuksel@gmail.com.
Yılmaz Eren, Email: Yilmazeren55@gmail.com.
Aysel Çağlar, Email: ayselkaracaglar@gmail.com.
Bedri Onur Küçükyıldırım, Email: kucukyil@yildiz.edu.tr.
Mehmet Akif Güleç, Email: akifgulec@yahoo.com.
References
- 1.Maquirriain J. Achilles tendon rupture: avoiding tendon lengthening during surgical repair and rehabilitation. Yale J Biol Med. 2011;84(3):289–300. [PMC free article] [PubMed] [Google Scholar]
- 2.Inglis A.E., Sculco T.P. Surgical repair of ruptures of the tendo Achillis. Clin Orthop Relat Res. 1981;156(3):160–169. [PubMed] [Google Scholar]
- 3.Daghino W., Enrietti E., Sprio A.E. Subcutaneous Achilles tendon rupture: a comparison between open technique and mini-invasive tenorrhaphy with Achillon® suture system. Injury. 2016;47(11):2591–2595. doi: 10.1016/j.injury.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Majewski M., Ochsner P.E., Liu F. Accelerated healing of the rat Achilles tendon in response to autologous conditioned serum. Am J Sports Med. 2009;37(11):2117–2125. doi: 10.1177/0363546509348047. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F., Liu H., Stile F. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plast Reconstr Surg. 2003;112(6):1613–1619. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- 6.Wang X.T., Liu P.Y., Tang J.B. Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. J Hand Surg. 2004;29(5):884–890. doi: 10.1016/j.jhsa.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Drissi H., Lomri A., Lasmoles F. Skeletal unloading induces biphasic changes in insulin-like growth factor-I mRNA levels and osteoblast activity. Exp Cell Res. 1999;251(2):275–284. doi: 10.1006/excr.1999.4539. [DOI] [PubMed] [Google Scholar]
- 8.Thomopoulos S., Das R., Sakiyama-Elbert S. bFGF and PDGF-BB for tendon repair: controlled release and biologic activity by tendon fibroblasts in vitro. Ann Biomed Eng. 2010;38(2):225–234. doi: 10.1007/s10439-009-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forslund C., Rueger D., Aspenberg P. A comparative dose–response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and-3 for tendon healing in rats. J Orthop Res. 2003;21(4):617–621. doi: 10.1016/S0736-0266(03)00010-X. [DOI] [PubMed] [Google Scholar]
- 10.Longo U.G., Lamberti A., Maffuli N. Tissue engineered biological augmentation for tendon healing: a systematic review. Br Med Bull. 2011;98(1):31–59. doi: 10.1093/bmb/ldq030. [DOI] [PubMed] [Google Scholar]
- 11.Aspenberg P., Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 12.Berkoff D.J., Kallianos S.A., Eskildsen S.M. Use of an IL1-receptor antagonist to prevent the progression of tendinopathy in a rat model. J Orthop Res. 2015;34(4):616–622. doi: 10.1002/jor.23057. [DOI] [PubMed] [Google Scholar]
- 13.Evans C.H., Chevalier X., Wehling P. Autologous conditioned serum. Phys Med Rehabil Clin. 2016;27(4):893–908. doi: 10.1016/j.pmr.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Darabos N., Trsek D., Miklic D. Comparison of double-bundle anterior cruciate ligament reconstruction with and without autologous conditioned serum application. Knee Surg Sports Traumatol Arthrosc. 2014;24(10):1–8. doi: 10.1007/s00167-014-3457-8. [DOI] [PubMed] [Google Scholar]
- 15.Rutgers M., Saris D.B., Dhert W.J. Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Research and Therapy. 2010;12(3):1. doi: 10.1186/ar3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright-Carpenter T., Klein P., Schaeferhoff P. Treatment of muscle injuries by local administration of autologous conditioned serum: a pilot study on sportsmen with muscle strains. Int J Sports Med. 2004;25(8):588–593. doi: 10.1055/s-2004-821304. [DOI] [PubMed] [Google Scholar]
- 17.Maffulli N. Current concepts review – rupture of the Achilles tendon. J Bone Joint Surg Am. 1999;81(7):1019–1036. doi: 10.2106/00004623-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Yüksel S., Adanır O., Gültekin M.Z. Effect of platelet-rich plasma for treatment of Achilles tendons in free-moving rats after surgical incision and treatment. Acta Orthopaedica et Traumatologica Turcica. 2014;49(5):544–551. doi: 10.3944/AOTT.2015.15.0028. [DOI] [PubMed] [Google Scholar]
- 19.López-Nájera D., Rubia-Zaragoza M., Sopena-Juncosa J.J. Effects of plasma rich in growth factors (PRGF) on biomechanical properties of Achilles tendon repair. Knee Surg Sports Traumatol Arthrosc. 2015;24(12):1–8. doi: 10.1007/s00167-015-3725-2. [DOI] [PubMed] [Google Scholar]
- 20.Geburek F., Lietzau M., Beineke A. Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies. Stem Cell Res Therapy. 2015;6(1):1. doi: 10.1186/s13287-015-0115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang K.A., Raijmakers N.J., van Arkel E.R. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage. 2008;16(4):498–505. doi: 10.1016/j.joca.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Baltzer A.W., Ostapczuk M.S., Stosch D. A new treatment for hip osteoarthritis: clinical evidence for the efficacy of autologous conditioned serum. Orthop Rev. 2013;5(2):13. doi: 10.4081/or.2013.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heisterbach P.E., Todorov A., Flückiger R. Effect of BMP-12, TGF-β1 and autologous conditioned serum on growth factor expression in Achilles tendon healing. Knee Surg Sports Traumatol Arthrosc. 2012;20(10):1907–1914. doi: 10.1007/s00167-011-1772-x. [DOI] [PubMed] [Google Scholar]