Abstract
Introduction
The aim of this study was to evaluate whether delphinidin is cytoprotective or cytotoxic in osteosarcoma cell lines, and to elucidate the underlying mechanisms.
Materials and methods
The present study investigated whether apoptosis or autophagy is induced by delphinidin in human osteosarcoma cell lines. Delphinidin was used as the antioxidant, along with two autophagy inhibitors: 3-methyladenine and bafilomycin A1. Cell viability and known autophagic markers, such as LC3-II expression, were evaluated. Reactive oxygen species (ROS) formation and cell cycle analysis were also investigated.
Results
Delphinidin showed concentration-dependent cytotoxicity to osteosarcoma cell. Delphinidin is closely associated with apoptotic cell death mechanisms and pathways related to ROS accumulation. In addition, we observed delphinidin-induced autophagosome formation and increasing levels of LC3-II conversion. However, in spite of delphinidin induced autophagy, the cytotoxic effects induced in the osteosarcoma cells may not be operating via autophagic cell death mechanisms.
Conclusions
Delphinidin compromises the cellular protective mechanisms by inhibiting autophagy, permitting ROS to accumulate and finally enhance apoptotic cell death. Our results indicate that delphinidin may play a critical role as a chemotherapeutic agent by preventing the development and progression of osteosarcoma cells.
Keywords: Delphinidin, Cytotoxicity, Autophagy, Apoptosis, Osteosarcoma
Introduction
Osteosarcoma is one of the most common non-hematologic, primary, malignant bone tumors in children. Osteosarcoma is seen more frequently in younger individuals, and the incidence is slightly higher in males. Although the 5-year survival rate has increased from 10% to 70%, the prognosis for osteosarcoma is poor.1
Recently, natural compounds such as cyanidin, delphinidin, malvidin and pelargonidin have been used as therapeutics for several diseases.2 These natural compounds promote the function of modern drug treatments, with few side effects. Many studies have shown that anthocyanins suppress the growth of certain cancers, because of its oxidative stress-based cytotoxic effect or antioxidant activity.3, 4 For example, Yun et al suggest that delphinidin could have potential in inhibiting colon cancer growth.5 And Lim et al suggested that delphinidin plays a critical role as a new chemotherapeutic agent to prevent the development of human ovarian clear cell carcinoma.4 Delphinidin (phytochemicals), a natural compound of anthocyanins, is a specific class of polyphenols. It has shown beneficial effects on cancer cell, such as potent antioxidant, anti-inflammatory, anti-mutation, and anti-neovascularization. Recent researches concerning cancer therapy revealed that delphinidin is correlated to apoptotic or autophagic cell death in several cancers.3, 5, 6
In general, cell death is most commonly associated with apoptosis, but it can also occur through other mechanisms, such as autophagy.7, 8 Apoptosis is a mechanism for self-destruction or suicide regulated by programmed cellular signaling pathways, and is characterized by stereotypical morphological changes.9 Thus, it is important to induce cell apoptosis in osteosarcoma therapies. Contrary to apoptosis, autophagy is a homeostatic mechanism and an important process in all cells, for the removal of damaged mitochondria and misfolded proteins. Autophagy is considered to bypass apoptosis; however, excess autophagy induction is known to trigger autophagy-related apoptosis. Thus, understanding the role of apoptosis and autophagy in cancer treatment is critical, since many anti-cancer therapies have been shown to activate these mechanisms.
Reactive oxygen species (ROS) are highly reactive forms of molecular oxygen, and are generally derived during the normal metabolism of oxygen.10 ROS acts as physiological regulators of normal cell proliferation and differentiation at low levels, but excessive levels of ROS damages the DNA and proteins, leading to cell apoptosis.11 In addition, ROS is also closely associated with programmed cell death, such as autophagy. By regulating these mechanisms, ROS have the potential of either cytoprotective or cytotoxic effects. Some authors have reported that ROS can initiate autophagy, thus having a cytoprotective effect in several types of cancers.11, 12 Conversely, other studies have reported that the cytotoxic effect of anthocyanidins in cancer cells could result from ROS accumulation.3, 12, 13 Thus, although it has not been determined if delphinidin has a therapeutic effects on human osteosarcoma, it is rationale to investigate the ability of ROS to act as a mediator of a cancer-blocking agent or cancer-suppressing agent.
In the current study, we investigated whether delphinidin triggers apoptotic or autophagic cell death in a human osteosarcoma cell line (U2OS). Cell death is associated with apoptosis or autophagy, however, it is not proven as to through which pathway delphinidin induces cell death of osteosarcoma cell in previous studies. The purpose is to evaluate whether delphinidin are cytoprotective or cytotoxic in osteosarcoma cell lines, and to further elucidate the associated mechanisms. We hypothesized that delphinidin may be helpful for improving osteosarcoma treatments via the apoptotic or autophagic cell death mechanism, and the combination of delphinidin may increase the incidence of therapeutic reactions.
Materials and methods
Cell and chemicals
The human osteosarcoma derived U2OS cell line was prepared (Korean Cell Line Bank, Seoul, Korea), and delphinidin was obtained from Extrasynthese (Genay, France). U2OS cells were cultured in DMEM/F-12 (Gibco, Grand Island, N.Y, USA) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Gibco), 2 mM l-glutamine, and 1% Antibiotic-Antimycotic drug (Gibco); incubation was in humidified 5% CO2 atmosphere at 37 °C. Delphinidin was dissolved in dimethyl sulfoxide (DMSO, Sigma Aldrich, St Quentin Fallavier, France) which was used for vehicle control in all assays. An enhanced chemiluminescence (ECL) detection system was purchased from Amersham (Arlington Heights, IL). All other chemicals that are not indicated here were procured from Sigma Chemical Co. (St. Louis, MO).
Determination of cell viability
The effect of delphinidin on cell viability was determined by an MTT (3-[4, 5-dimethyldiazole-2-yl]-2,5-diphenyl tetrazoliumbromide) assay. The cells were plated in 96-well microtiter plates, at 1 × 104 cells per well in 200 μL of complete culture medium, and treated with the desired concentrations of delphinidin (0–200 μM) for 48 h at 37 °C in a humidified chamber. MTT (5 mg/ml in PBS; diluted in 10 ml of serum free media) was added to each well and incubated for further 2 h, after which the plate was centrifuged at 1000 rpm for 5 min at 4 °C. The culture medium was removed from the wells by aspiration, and the resultant formazan crystals were dissolved in 200 μL DMSO. The absorbance was recorded on automated microplate reader EL 311 (Bio-Tek Instruments, Winooski, VT) at a wave length of 540 nm. The effect of delphinidin on cell survival was assessed as percent cell viability, where vehicle-treated cells were considered as 100% viable.
ROS production using by FACS analysis
The intracellular ROS generation induced by delphinidin was assessed using FACS and a confocal microscope. The measurement of intracellular ROS production using FACS (Cytomics FC 500, Beckman, CA, USA) was performed as follows. U2OS cells were seeded in each well of a 6-well cell culture plate. The cells were incubated with DCF-DA (2′, 7′-Dicholrofluorescin diacetate) solution (5uM, Sigma, St. Louis, MO, USA) for 15 min at 37 °C; they were then washed, and resuspended in PBS. Intracellular ROS products were measured using FACS. Data were analyzed using CXP software (Beckman, CA, USA.
Western blotting, flow cytometry
To validate whether delphinidin can promote autophagy activation, we also observed autophagosome formation using western blotting analysis. In addition, to investigate the effects of delphinidin on the distribution of cells in the cell cycle, we performed DNA cell cycle analysis using flow cytometry. Detailed methods were indicated at Appendix 1.
Confocal microscopy
In each well of a 24-well plate, 50 μL green fluorescent protein (GFP)-LC3-labeled transfected osteosarcoma cell lines were cultured to subconfluent density; the cells were maintained in a 37 °C humidified incubator for 16 h. The sample was cultured on a cover slide, washed with PBS, and fixed with 3% formaldehyde. It was then permeabilized with 0.5% triton-X100 and blocked in 1% BSA solution. Analysis under confocal microscopy (Olympus FV1000, OLYMPUS, Tokyo, Japan) was performed while cells were under reaction with primary and FITC or TRITC-labeled secondary antibody. GFP-fluorescence was analyzed by confocal microscopy, immediately after fixation of cell.
Statistical analyses
Each experiment was performed at least three times, and representative data were reported. All statistical analyses were performed via one-way ANOVA with the exception of the cell-viability analysis for different concentrations of delphinidin. That cell-viability analysis was performed using repeated measure. Differences with a probability of less than 0.05 were considered statistically significant. All statistical analysis was done using SPSS 18.0 for Windows (SPSS, Chicago, IL, USA).
Results
Anti-proliferative effects of delphinidin on osteosarcoma cell viability
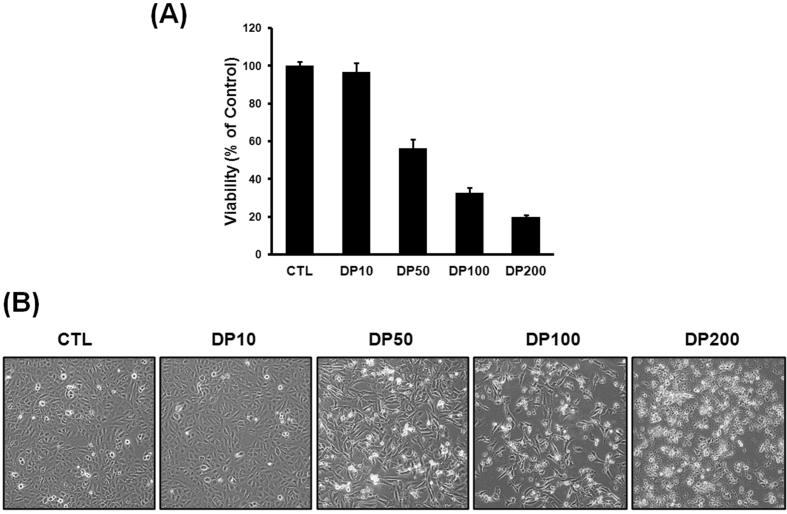
Delphinidin was cytotoxic to osteosarcoma cells in a concentration-dependent manner, with maximum effect between of 10–50 μg/ml (Fig. 1-A). By increasing the delphinidin concentrations, the in cell viability was 96%, 55%, 32%, and 22%, at 10, 50, 100, and 200 μg/ml respectively, when compared to untreated controls (p < 0.001). Microscopic analysis revealed the presence of vacuoles and damaged cell morphology, which are associated with the suppression of cell growth and proliferation in osteosarcoma cells, indicating an active process of cell death (Fig. 1-B).
Fig. 1.
Cell viability was assessed by MTT assays. (A) Cell viability decreased by exposure to delphinidin in a concentration-dependent manner. (B) Microscopic analysis demonstrated the presence of vacuoles and damaged cell morphology significantly increased in a concentration-dependent manner. These findings are associated with the suppression of cell growth and proliferation in osteosarcoma cell. Each results represents mean ± standard deviation of three independent experiments (p < 0.001).
ROS production in U2OS cell line
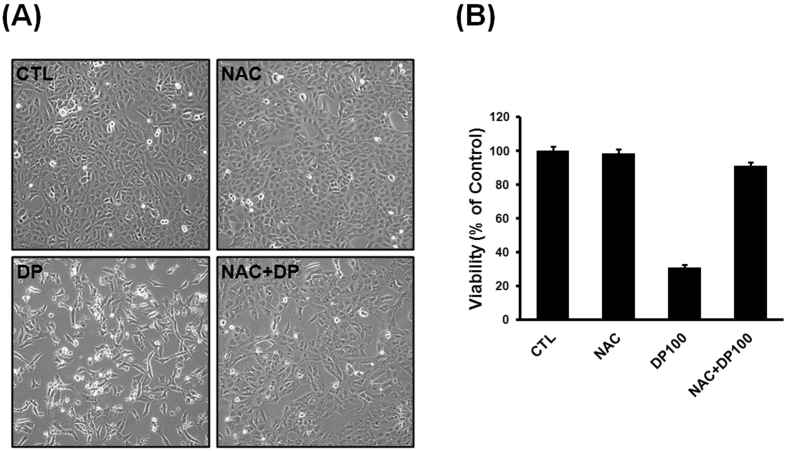
As reported in Fig. 2-A, B, when cells were treated with delphinidin alone, we observed decreased cell viability compare to untreated cells (p < 0.001). However, when cells were pretreated with NAC and delphinidin, reduction in the viability of osteosarcoma cells was not observed. Therefore, we considered delphinidin-related cell death is closely associated with the accumulation of ROS, and delphinidin acts as anti-cancer agent, independent of its anti-oxidant capacity.
Fig. 2.
Delphinidin-induced ROS production in osteosarcoma cell line. (A, B) Cells were treated with delphinidin alone, we observed decreased cell viability compare to untreated cells. When cells were pretreated with NAC and delphinidin, reduction of the viability of osteosarcoma cells was not observed. Each results represents mean ± standard deviation of three independent experiments (p < 0.001).
Delphinidin-induced autophagosome formation
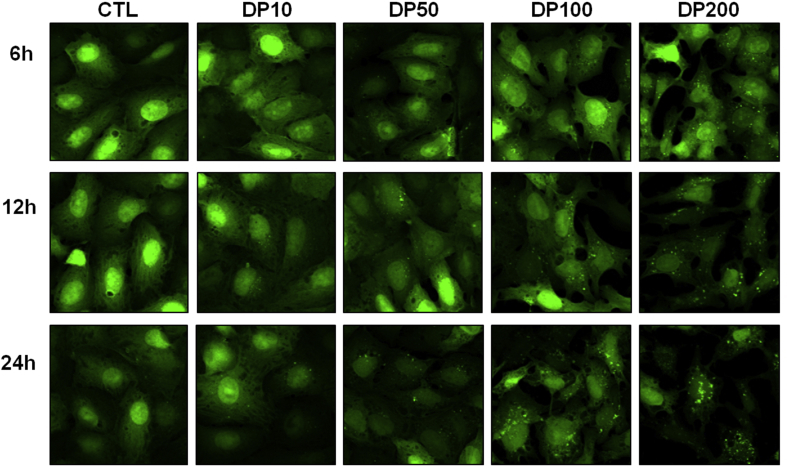
We observed that the delphinidin-induced LC3-II, one of the marker proteins of autophagosome, was observed as a spotted localization, or so-called puncta. After autophagy induction, the puncta, which indicate an autophagosome, were counted after 6 h, 12 h, and 24 h of treatment (Fig. 3). These results clearly demonstrate that delphinidin significantly induces autophagosome formation in a concentration-dependent and a time-dependent manner (p < 0.001).
Fig. 3.
Delphinidin-induced autophagosome formation. LC3-II expression was induced in osteosarcoma cell line by exposure to various concentrations and time-dependent manners. Each results represents mean ± standard deviation of three independent experiments (p < 0.001).
Delphinidin-induced autophagy activation
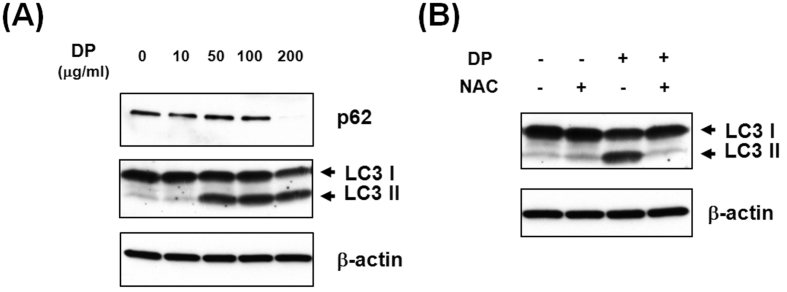
As reported in Fig. 4-A, to verify whether delphinidin can promote autophagosome formation using western blot, the degradation of p62 (one of the cargo proteins) and conversion of LC3-II protein (an autophagy marker) had increased in a concentration-dependent manner (p < 0.001). However, when cells were pretreated with NAC, we observed a reduction of LC3-II protein conversions (Fig. 4-B).
Fig. 4.
Delphinidin-induced autophagy activation. (A) LC3-II expression was increased by exposure to delphinidin in a concentration-dependent manner, and degradation of p62, one of the cargo proteins, had increased. β-actin was used as a loading control. Each results represents mean ± standard deviation of three independent experiments (p < 0.001). (B) When cells were pretreated with NAC, a reduction of LC3-II protein conversion was found.
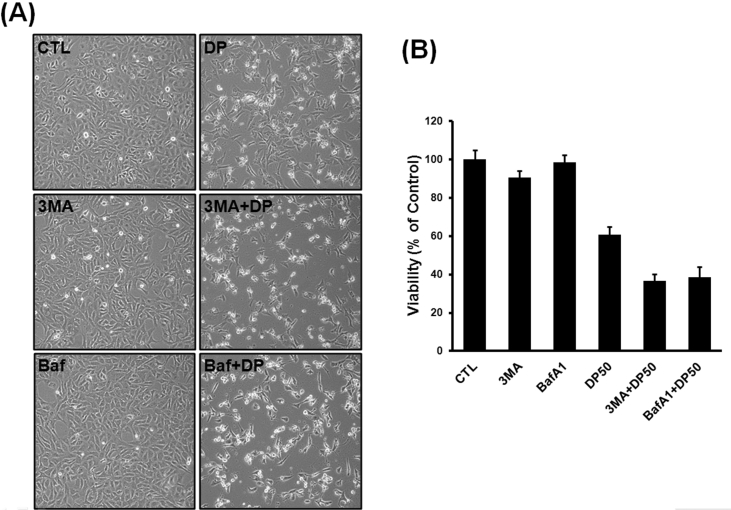
Effects of delphinidin and autophagy inhibitors on osteosarcoma cell viability
As presented in Fig. 5, in cells pretreated with delphinidin and autophagy inhibitors (3-MA and bafilomycin A1), the effects of autophagy inhibitors reinforced the delphinidin-induced cell death. These results suggest that blocking autophagy by inhibitors might strengthen the antitumor efficacy of delphinidin. In other words, despite delphinidin-induced autophagy, delphinidin-induced cytotoxic effects to osteosarcoma cells may not act via autophagic cell death mechanisms. Thus, the combined therapy of delphinidin and autophagy inhibitors has synergic effects on cancer cell death, and combination therapy should be considered.
Fig. 5.
Effects of delphinidin and autophagy inhibitors on osteosarcoma cell viability (A) Cells were treated with autophagy inhibitor alone, reduction of the viability of osteosarcoma cells was not observed. Cells were treated with delphinidin alone, we observed decreased cell viability compare to untreated cells. In addition, when cells were pretreated with delphinidin and autophagy inhibitor, we observed more decreased cell viability compared to delphinidin-treated cells. Each results represents mean ± standard deviation of three independent experiments (p < 0.001).
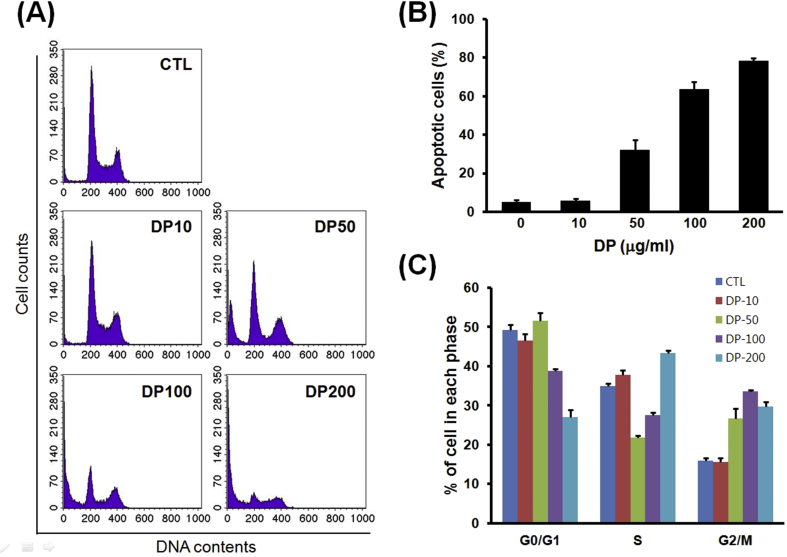
Effects of delphinidin on cell-cycle progression of osteosarcoma cells
To further evaluate the potential mechanisms of delphinidin-induced cell-cycle arrest and apoptosis, cells were incubated with different concentration of delphinidin for 24 h, and protein levels were determined. Results are shown in Fig. 6, which indicate that a proportion of sub-G1 DNA content (considered as apoptotic cell death) increased in a concentration-dependent manner. In addition, on the basis of cell cycle analysis, whereas the number of cells in G0/G1 phase decreased, the number of cells in G2/M phase increased in a concentration-dependent manner.
Fig. 6.
Effects of delphinidin on cell-cycle progression of osteosarcoma cell. In flow cytometry and cell cycle analysis, a proportion of sub-G1 DNA content increased in a concentration-dependent manner. Whereas the number of cells in G0/G1 phase had decreased, the number of cells in G2/M phase had increased in a concentration-dependent of delphinidin. Each results represents mean ± standard deviation of three independent experiments (p < 0.001).
Discussion
The most important finding of the present study is delphinidin compromises the cellular protective mechanisms by inhibiting autophagy. Furthermore, delphinidin induced human osteosarcoma cell death closely associated with apoptotic cell death. In the current study, we investigated the effects of delphinidin on apoptotic or autophagic cell death in a human osteosarcoma cell line (U2OS). The authors hypothesized that delphinidin may be helpful for improving osteosarcoma treatment by inducing apoptosis or autophagy, as the combination of delphinidin may increase the incidence of therapeutic reactions. This study demonstrated that delphinidin triggers apoptotic cell death, and not autophagic cell death, in U2OS cells via the ROS-related pathways, and the suppression of osteosarcoma cells after treating with delphinidin was observed. Therefore, this study supports the hypothesis that delphinidin-induced apoptotic cell death can provide a theoretical evidence of a new insight for treatment, as therapeutic agents of osteosarcoma.
The current study demonstrated that ROS-induced cell death in osteosarcoma cells was reduced by antioxidants, such as NAC. When cells were pretreated with delphinidin alone, significant reduction of cell viability was observed. However, when cells were pretreated with delphinidin and NAC, the viability of cancer cells was similar to those of control group. These results implicate that delphinidin-induced cell death is significantly associated with the ROS-related pathway. ROS serve as signaling molecules in a variety of cellular processes, including growth, differentiation, adhesion, and programmed cell death. Previous studies have shown that ROS play an initial role in drug or chemical induced apoptotic pathways in human cancer cells.3, 11 Cvorovic et al reported that ROS accumulation triggers the apoptotic cell death in colon cancer cells.3 In addition, some authors have suggested that ROS production promotes genome instability, thus furthering oncogenic transformation and cancer progression.14 Although anthocyanins have been proven to be strong antioxidants, based on our study, delphinidin acts as an anti-cancer agent, independently of its anti-oxidant property. These differences of the effect of anthocyanidins on osteosarcoma cell death are not yet completely clarified, and the ROS-related pathway leading to osteosarcoma cell death is not fully elucidated because of complex signaling pathways. According to some studies, at high levels, ROS are deleterious to cells, leading to programmed cell death. However, at low levels, ROS serve as signaling molecules, by oxidizing factors in a variety of pathways that lead to growth and survival.15 Due to this different-faced role of ROS, a better understanding of the regulation and modulation of the ROS-related pathway might provide new insights into cancer treatment and prevention.
As reported in our results, increasing concentrations of delphinidin decreased the cell viability of osteosarcoma cells, when compared to untreated controls. In addition, we found that delphinidin induces autophagosome formation and LC3-II protein conversion in a concentration-dependent manner. Cell death is most commonly associated with apoptosis, but it can also occur through other mechanism, including autophagy.7, 16 However, the role of autophagy in cancer is controversial and still not completely clarified. In general, autophagy is considered to bypass apoptosis, and it is constitutively active at low levels in order to preserve cellular homeostasis. Conversely, under some stressful conditions, excess autophagy induction has been shown to trigger autophagy-related apoptosis.17 Considering our results, delphinidin induces autophagosome formation and LC3-II conversion; the autophagosome formation being the hallmark of autophagy. However, when cells were pretreated with delphinidin and autophagy inhibitors such as 3-MA and bafilomycin A1, we verified that the effects of autophagy inhibitors might reinforce the delphinidin-induced cell death. These results indicate that delphinidin-induced autophagy is cytoprotective to osteosarcoma cells, and inhibition of autophagy due to autophagy inhibitors can cause synergetic effect to suppress osteosarcoma tumor cells. Thus, we concluded that delphinidin closely associates with induction of autophagy activation; however, delphinidin-induced autophagy has a as cytoprotective effect on osteosarcoma cells. Delphinidin-induced osteosarcoma cell death might be achieved through different mechanisms.
To investigate the effects of delphinidin on the distribution of cells in the cell cycle, we performed DNA cell cycle analysis. We observed that delphinidin treatment resulted in a concentration-dependent accumulation of cells in the sub-G1 and G2/M phase of cell cycle at different concentrations. These results indicate that cell growth inhibition by delphinidin might be due to cell cycle arrest in G2/M phase, and delphinidin-induced cell death associated with apoptotic pathways. A number of anthocyanins have been shown to inhibit apoptosis and cell cycle progression of various cancer cells. Induction of apoptosis and/or inhibition of cell proliferation are highly correlated with the activation of intracellular signaling pathways, leading to arrest of the cell cycle.18 In accordance with previous studies, our study demonstrated that delphinidin inhibited cell growth and cell cycle progression in cancer cell lines in a concentration-dependent manner. Since several studies have suggested that cell cycle arrest is the primary determinant in the induction of cell death, the inhibition of cell cycle has been regarded as a target for the treatment of cancer therapy.18, 19, 20 Bucher et al reported that G2/M accumulation prevents cancer cells from repairing DNA damage, compelling them into M phase; thus, the G2 check point is an attractive therapeutic target for cancer therapy.20 Collectively, on the basis of the result of the very study and the current study, delphinidin forces growth inhibitory effects on osteosarcoma cells by arresting in the G2/M phase of cell cycle. Therefore, our data demonstrates that delphinidin arrests osteosarcoma cells in G2/M phase via modulating cell cycle. Furthermore, delphinidin-induced G2/M phase arrest is critical for suppression of cell proliferation, leading to cell growth inhibition and possible apoptotic cell death.
According to these various aforementioned mechanisms, delphinidin act as an anti-cancer agent through ROS induced apoptotic cell death mechanisms (excluding autophagy) in a human osteosarcoma cell. Although there are many studies concerning this topic, namely the benefits of delphinidin to cancer cell suppression, there are no studies regarding the effects of delphinidin on osteosarcoma cell suppression. Therefore, in this respect, our study is valuable and provides preclinical evidence of using delphinidin as a chemotherapeutic agent.
Despite the many benefits of the present study, we have several limitations. Although we verified delphinidin-induced osteosarcoma cell death is closely associated with apoptotic cell death, the autophagic abilities of various concentrations of delphinidin were not evaluated fully. Because of involvement of complex signaling pathways, the response of cancer cells to the autophagic stimulus, whether it can trigger cell death or cell survival, is not yet completely elucidated. Since autophagy might have different effects in tumors at different stages of progression, a better understanding of the regulation and modulation of the autophagic pathway is therefore needed, and further researches are encouraged. In addition, recent studies suggest that delphinidin inhibits the growth of human tumor cell line by shutting off the epidermal growth-factor receptor downstream signaling cascade, AKT and ERK 1/2 MAPK signaling pathways, vascular endothelial growth factor (VEGF) induced-mitochondrial biogenesis and Akt activation.4, 21, 22 Thus, in order to completely elucidate osteosarcoma cell death mechanism due to delphinidin, not only apoptotic/autophagic cell death, we also need to understand the various inhibitory mechanisms of cancer cell proliferation.
In conclusion, delphinidin compromises cellular protective mechanisms, permitting ROS to accumulate and finally cause apoptotic cell death. Our results indicate that delphinidin may play a critical role as a chemotherapeutic agent to prevent development and progression of osteosarcoma cells. Therefore, the present study provides a novel insight into delphinidin with respect to its inhibitory functions in the progression of osteosarcoma, as a new chemotherapeutic regimen.
Footnotes
This work was supported by Biomedical Research Institute Fund “GNUH CRF 2010-003” from the Gyeongsang National University Hospital.
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Appendix 1.
1. Western blotting
In order to extract all the protein, the cells were lysed for 30 min in 1 mL RIPA lysis buffer solution (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P40, 0.5% sodium deoxycholate and 1 mM EDTA) containing a 10 μL protease inhibitor cocktail (Sigma). The digested cells were then sonicated and centrifuged at 13,000g for 15 min at 4 °C to remove the insoluble debris. Thirty micrograms of the protein was resolved on 10% SDS-polyacrylamide gel, after which it was electrophoretically transferred onto a nitrocellulose membrane, using the semidry technique. After blocking for 2 h with 3% bovine serum albumin (BSA) in a TBS-T buffer solution (10 mM Tris, 150 mM NaCl and 0.1% Tween-20), the membrane was incubated with primary antibodies against p62, LC3, and β-actin (Sigma–Aldrich, St Louis, MO, USA). After washing thrice with PBST, the membrane was incubated with peroxidase-conjugated secondary antibody for 2 h, and it was again washed thrice with PBST. Protein was identified with the ECL detection system.
2. Flow cytometry
Cells were separated, washed with PBS, and resuspended in 0.5 ml 0.1% glucose solution, after which they were fixed with 70% cold ethanol at 4 °C for 2 h. Cells were then washed with PBS and reacted with RNase A (250 μg/ml), followed by propidium (50 μg/ml). The cell cycle was then analyzed with fluorescence-activated cell sorting (FACS) caliber (Becton Dickinson, San Jose, CA). The sub-G1, G0/G1 phase, and G2/M phase were calculated to estimate the effect of delphinidin treatment on distribution of cells in the different phases of the cell cycle.
References
- 1.Bacci G., Longhi A., Versari M., Mercuri M., Briccoli A., Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–1161. doi: 10.1002/cncr.21724. [DOI] [PubMed] [Google Scholar]
- 2.Kong J.M., Chia L.S., Goh N.K., Chia T.F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 3.Cvorovic J., Tramer F., Granzotto M., Candussio L., Decorti G., Passamonti S. Oxidative stress-based cytotoxicity of delphinidin and cyanidin in colon cancer cells. Arch Biochem Biophys. 2010;501:151–157. doi: 10.1016/j.abb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Lim W., Jeong W., Song G. Delphinidin suppresses proliferation and migration of human ovarian clear cell carcinoma cells through blocking AKT and ERK1/2 MAPK signaling pathways. Mol Cell Endocrinol. 2016;422:172–181. doi: 10.1016/j.mce.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Yun J.M., Afaq F., Khan N., Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, induces apoptosis and cell cycle arrest in human colon cancer HCT116 cells. Mol Carcinog. 2009;48:260–270. doi: 10.1002/mc.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuyuki S., Fukui S., Watanabe A., Akune S., Tanabe M., Yoshida K. Delphinidin induces autolysosome as well as autophagosome formation and delphinidin-induced autophagy exerts a cell protective role. J Biochem Mol Toxicol. 2012;26:445–453. doi: 10.1002/jbt.21443. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Yang Z., Li Y. Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment. Oncotarget. 2016;7:44763–44778. doi: 10.18632/oncotarget.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S.H., Kim J.H., Chi G.Y. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol Lett. 2012;212:252–261. doi: 10.1016/j.toxlet.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Cherukuri D.P., Nelson M.A. Role of reactive oxygen species (ROS) and JNKs in selenite-induced apoptosis in HepG2 cells. Cancer Biol Ther. 2008;7:697–698. doi: 10.4161/cbt.7.5.6088. [DOI] [PubMed] [Google Scholar]
- 11.Scherz-Shouval R., Shvets E., Fass E., Shorer H., Gil L., Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng R., Ni H.M., Wang S.Y. Cyanidin-3-rutinoside, a natural polyphenol antioxidant, selectively kills leukemic cells by induction of oxidative stress. J Biol Chem. 2007;282:13468–13476. doi: 10.1074/jbc.M610616200. [DOI] [PubMed] [Google Scholar]
- 13.Duan Y., Ke J., Zhang H., He Y., Sun G., Sun X. Autophagic cell death of human hepatoma G2 cells mediated by procyanidins from Castanea mollissima Bl. Shell-induced reactive oxygen species generation. Chem Biol Interact. 2014;224:13–23. doi: 10.1016/j.cbi.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Mathew R., Karp C.M., Beaudoin B. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macip S., Igarashi M., Berggren P., Yu J., Lee S.W., Aaronson S.A. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–8585. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galluzzi L., Maiuri M.C., Vitale I. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 17.Ling Y.H., Aracil M., Zou Y. PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase. Clin Cancer Res. 2011;17:5353–5366. doi: 10.1158/1078-0432.CCR-10-1948. [DOI] [PubMed] [Google Scholar]
- 18.Hafeez B.B., Siddiqui I.A., Asim M. A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res. 2008;68:8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeulen K., Van Bockstaele D.R., Berneman Z.N. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucher N., Britten C.D. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98:523–528. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meiers S., Kemeny M., Weyand U., Gastpar R., von Angerer E., Marko D. The anthocyanidins cyanidin and delphinidin are potent inhibitors of the epidermal growth-factor receptor. J Agric Food Chem. 2001;49:958–962. doi: 10.1021/jf0009100. [DOI] [PubMed] [Google Scholar]
- 22.Duluc L., Jacques C., Soleti R., Andriantsitohaina R., Simard G. Delphinidin inhibits VEGF induced-mitochondrial biogenesis and Akt activation in endothelial cells. Int J Biochem Cell Biol. 2014;53:9–14. doi: 10.1016/j.biocel.2014.03.030. [DOI] [PubMed] [Google Scholar]